Tetrafluorosubstituted Metal Phthalocyanines: Study of the Effect of the Position of Fluorine Substituents on the Chemiresistive Sensor Response to Ammonia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of MPcF4 and Deposition of Their Films
2.2. Investigation of the Chemiresistive Sensor Response
2.3. Quantum-Chemical Calculations
3. Results
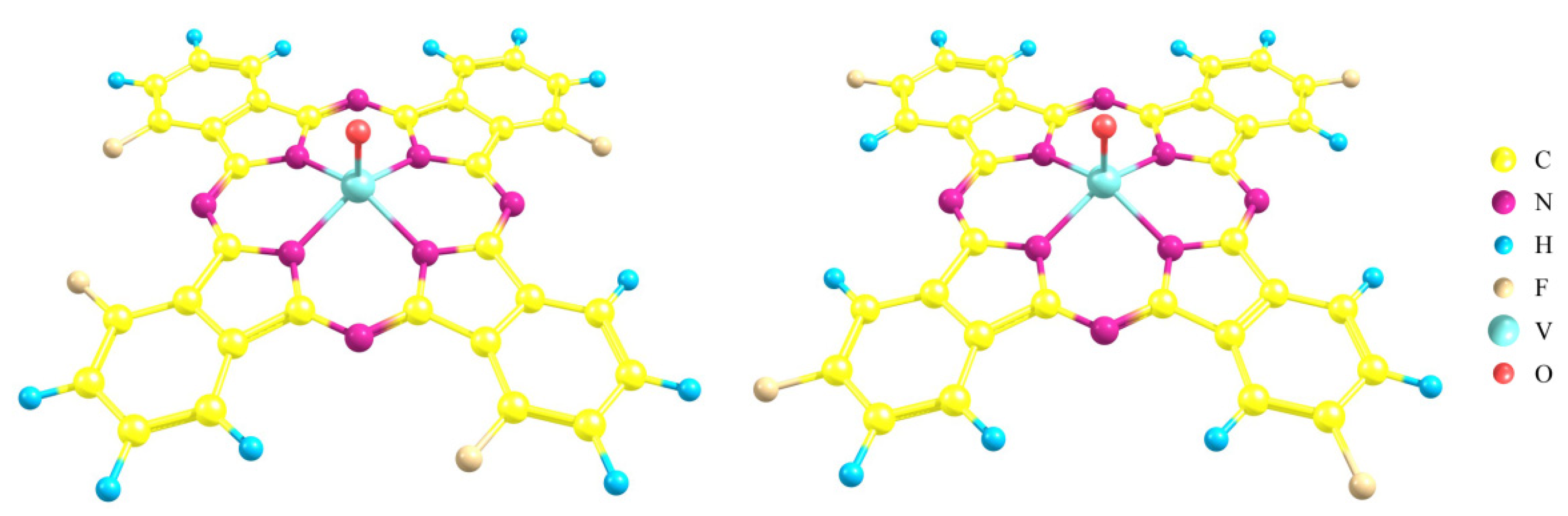
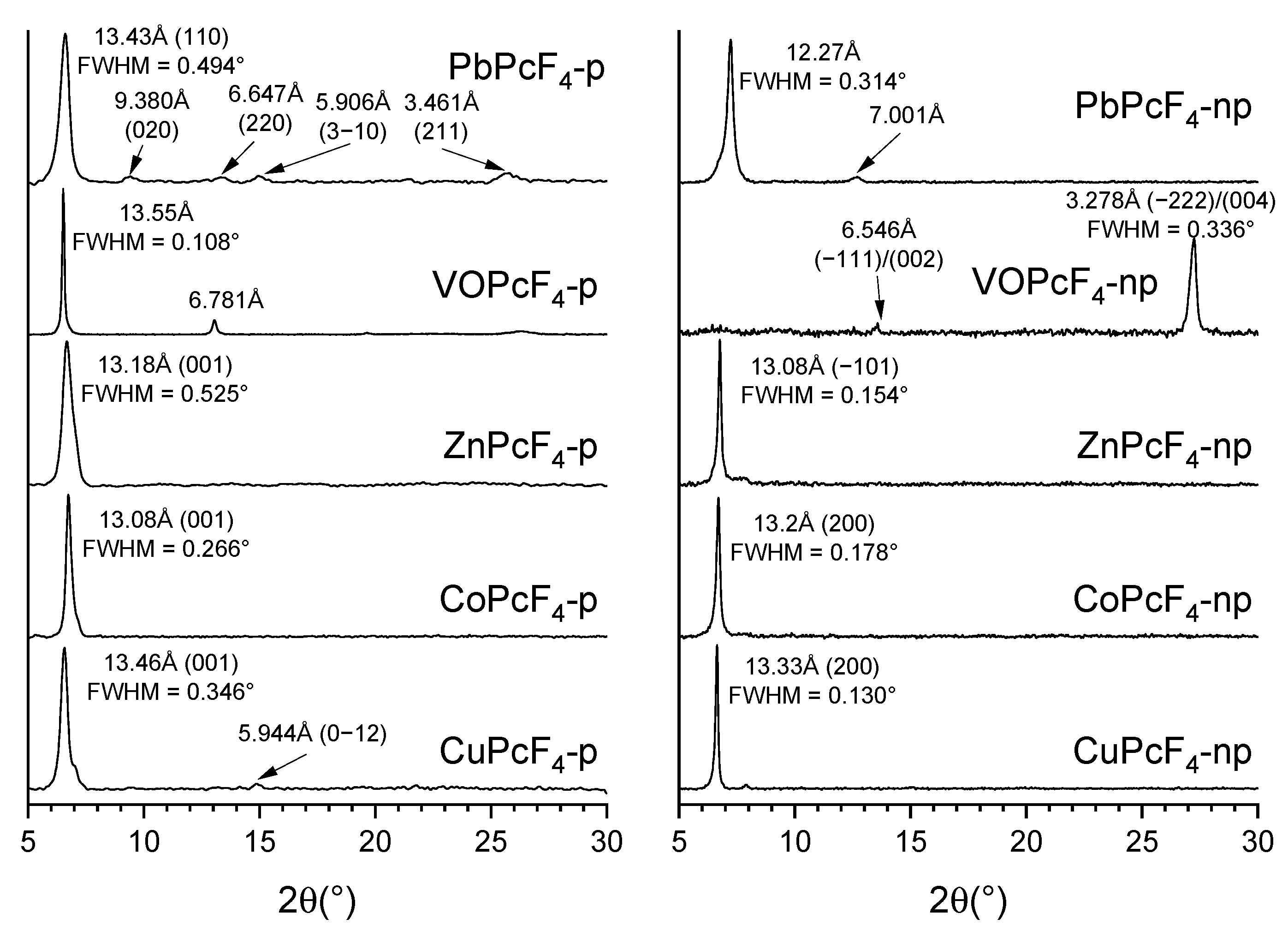
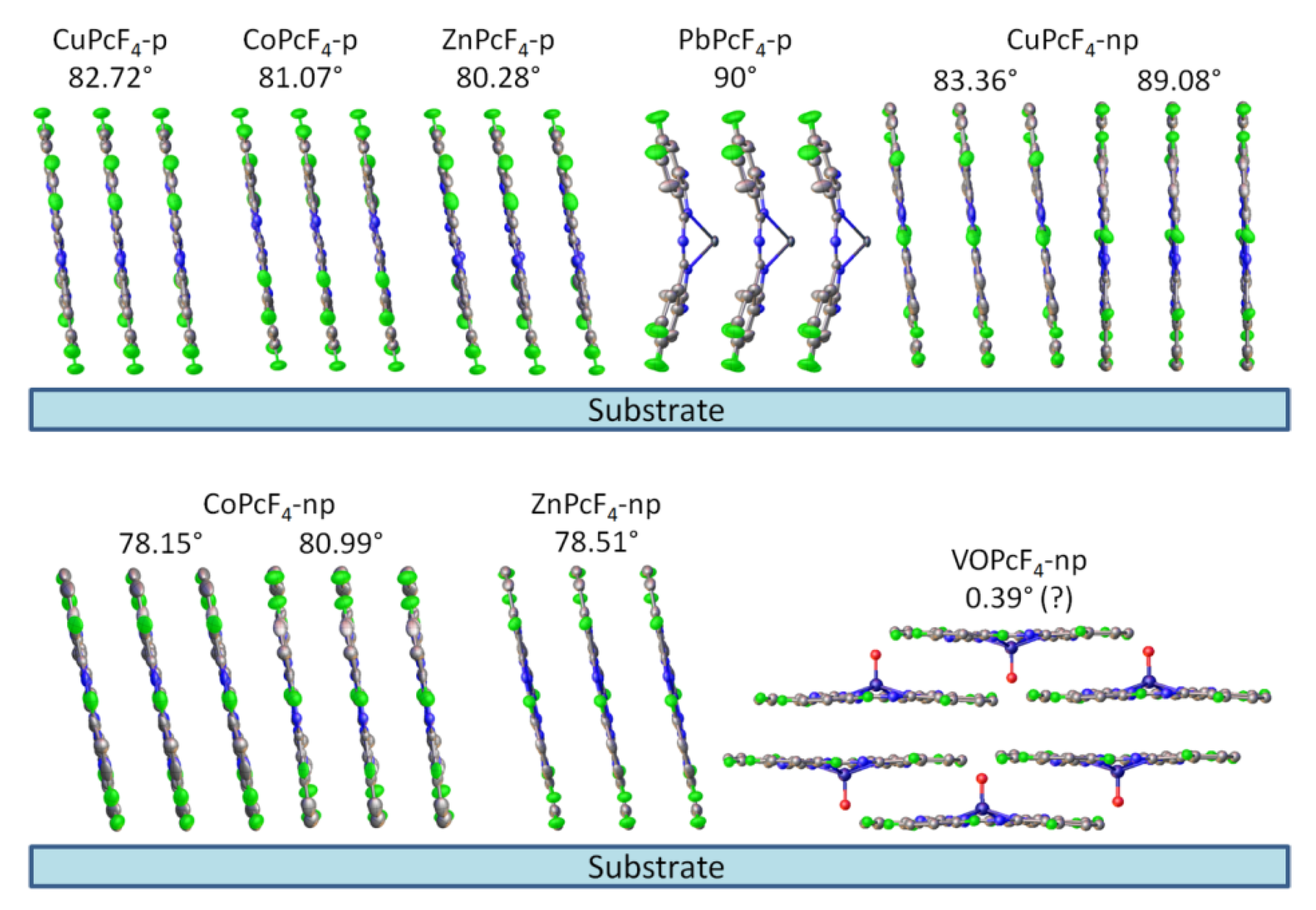
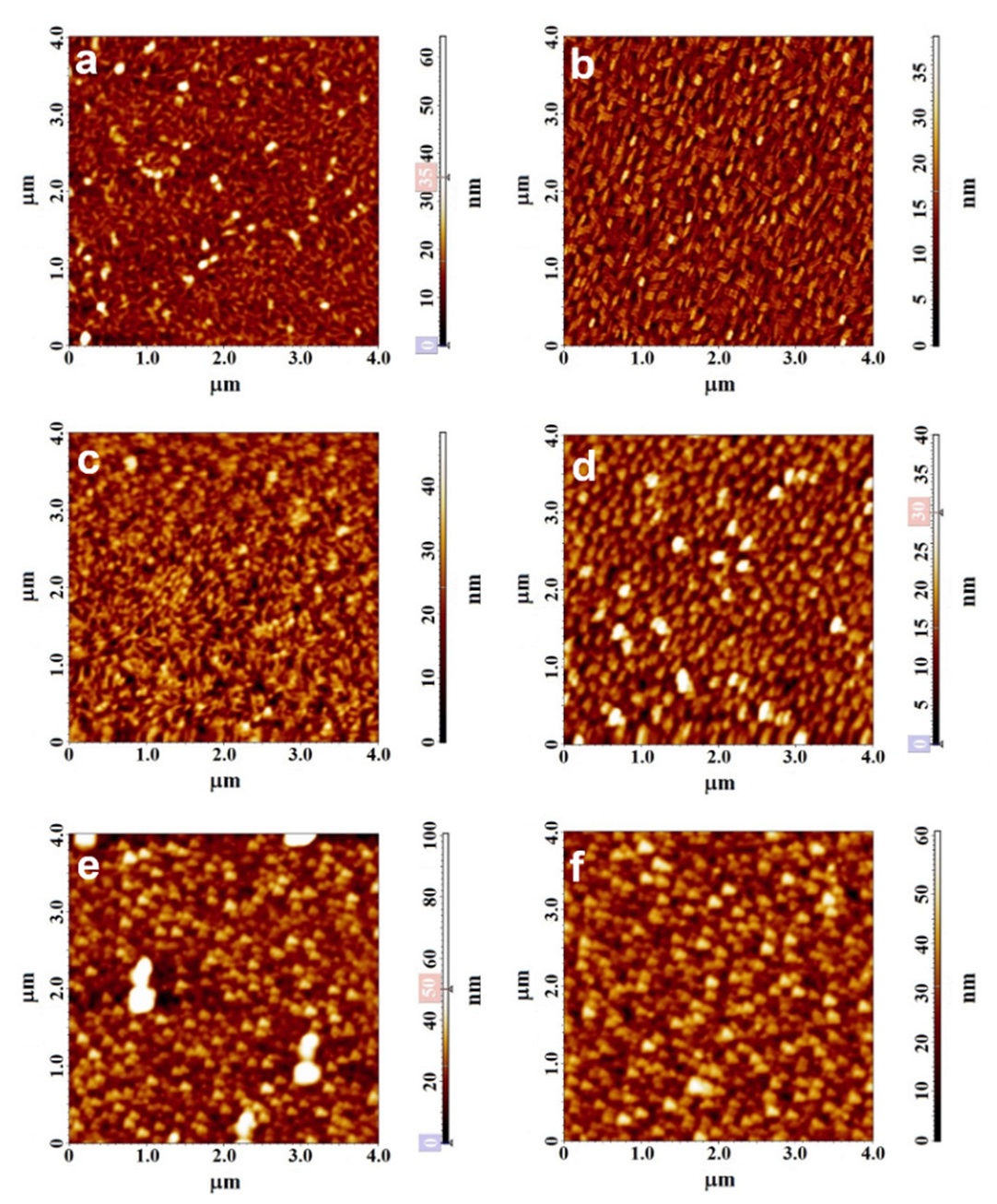
3.1. Characterization of MPcF4 Films
3.2. Sensor Properties of MPcF4 Films
3.2.1. Comparative Study of the Sensor Response of MPcF4-p and MPcF4-np Films to Ammonia
3.2.2. Quantum-Chemical Modeling of the Interaction of the NH3 Molecule with Phthalocyanines
3.2.3. Detailed Study of the Sensor Characteristics of MPcF4-p (M = Co, VO) Films to Ammonia
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Zhang, Y.; Han, Q.; Xu, Y.; Hu, G.; Xing, H. The inflammatory injury of heart caused by ammonia is realized by oxidative stress and abnormal energy metabolism activating inflammatory pathway. Sci. Total Environ. 2020, 742, 140532. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Luo, X.; Wen, M.; Wang, C.; Qin, C.; Shao, J.; Gan, L.; Dong, R.; Jiang, H. Effect of acute ammonia toxicity on inflammation, oxidative stress and apoptosis in head kidney macrophage of Pelteobagrus fulvidraco and the alleviation of curcumin. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 248, 109098. [Google Scholar] [CrossRef] [PubMed]
- Vuppaladadiyam, A.K.; Antunes, E.; Vuppaladadiyam, S.S.V.; Baig, Z.T.; Subiantoro, A.; Lei, G.; Leu, S.Y.; Sarmah, A.K.; Duan, H. Progress in the development and use of refrigerants and unintended environmental consequences. Sci. Total Environ. 2022, 823, 153670. [Google Scholar] [CrossRef] [PubMed]
- Insausti, M.; Timmis, R.; Kinnersley, R.; Rufino, M.C. Advances in sensing ammonia from agricultural sources. Sci. Total Environ. 2020, 706, 135124. [Google Scholar] [CrossRef]
- Kwak, D.; Lei, Y.; Maric, R. Ammonia gas sensors: A comprehensive review. Talanta 2019, 204, 713–730. [Google Scholar] [CrossRef]
- Wyer, K.E.; Kelleghan, D.B.; Blanes-Vidal, V.; Schauberger, G.; Curran, T.P. Ammonia emissions from agriculture and their contribution to fine particulate matter: A review of implications for human health. J. Environ. Manage. 2022, 323, 116285. [Google Scholar] [CrossRef]
- Kumar, V.; Mirzaei, A.; Bonyani, M.; Kim, K.H.; Kim, H.W.; Kim, S.S. Advances in electrospun nanofiber fabrication for polyaniline (PANI)-based chemoresistive sensors for gaseous ammonia. TrAC Trends Anal. Chem. 2020, 129, 115938. [Google Scholar] [CrossRef]
- Brannelly, N.T.; Hamilton-Shield, J.P.; Killard, A.J. The Measurement of Ammonia in Human Breath and its Potential in Clinical Diagnostics. Crit. Rev. Anal. Chem. 2016, 46, 490–501. [Google Scholar] [CrossRef] [Green Version]
- Song, G.; Jiang, D.; Wu, J.; Sun, X.; Deng, M.; Wang, L.; Hao, C.; Shi, J.; Liu, H.; Tian, Y.; et al. An ultrasensitive fluorescent breath ammonia sensor for noninvasive diagnosis of chronic kidney disease and helicobacter pylori infection. Chem. Eng. J. 2022, 440, 135979. [Google Scholar] [CrossRef]
- Shetty, S.S.; Jayarama, A.; Bhat, S.; Karunasagar, I.; Pinto, R. A review on metal-oxide based trace ammonia sensor for detection of renal disease by exhaled breath analysis. Mater. Today Proc. 2022, 55, 113–117. [Google Scholar] [CrossRef]
- Kim, K.-H.; Jahan, S.A.; Kabir, E. A review of breath analysis for diagnosis of human health. TrAC Trends Anal. Chem. 2012, 33, 1–8. [Google Scholar] [CrossRef]
- Wang, G.; Gao, J.; Sun, B.; He, D.; Zhao, C.; Suo, H. Enhanced ammonia sensitivity electrochemical sensors based on PtCu alloy nanoparticles in-situ synthesized on carbon cloth electrode. J. Electroanal. Chem. 2022, 922, 116721. [Google Scholar] [CrossRef]
- Tsai, J.-H.; Niu, J.-S.; Shao, W.-C.; Liu, W.-C. Characteristics of chemiresistive-type ammonia sensor based on Ga2O3 thin film functionalized with platinum nanoparticles. Sens. Actuators B Chem. 2022, 371, 132589. [Google Scholar] [CrossRef]
- Krishna, K.G.; Parne, S.; Pothukanuri, N.; Kathirvelu, V.; Gandi, S.; Joshi, D. Nanostructured metal oxide semiconductor-based gas sensors: A comprehensive review. Sens. Actuators A Phys. 2022, 341, 113578. [Google Scholar] [CrossRef]
- Ma, J.; Fan, H.; Li, Z.; Jia, Y.; Yadav, A.K.; Dong, G.; Wang, W.; Dong, W.; Wang, S. Multi-walled carbon nanotubes/polyaniline on the ethylenediamine modified polyethylene terephthalate fibers for a flexible room temperature ammonia gas sensor with high responses. Sens. Actuators B Chem. 2021, 334, 129677. [Google Scholar] [CrossRef]
- Ganesan, S.; Kalimuthu, R.; Kanagaraj, T.; Kulandaivelu, R.; Nagappan, R.; Pragasan, L.A.; Ponnusamy, V.K. Microwave-assisted green synthesis of multi-functional carbon quantum dots as efficient fluorescence sensor for ultra-trace level monitoring of ammonia in environmental water. Environ. Res. 2022, 206, 112589. [Google Scholar] [CrossRef]
- Kanaparthi, S.; Singh, S.G. Solvent-free fabrication of a room temperature ammonia gas sensor by frictional deposition of a conducting polymer on paper. Org. Electron. 2019, 68, 108–112. [Google Scholar] [CrossRef]
- Gao, R.; Ma, X.; Liu, L.; Gao, S.; Zhang, X.; Xu, Y.; Cheng, X.; Zhao, H.; Huo, L. In-situ deposition of POMA/ZnO nanorods array film by vapor phase polymerization for detection of trace ammonia in human exhaled breath at room temperature. Anal. Chim. Acta 2022, 1199, 339563. [Google Scholar] [CrossRef]
- Gai, S.; Wang, B.; Wang, X.; Zhang, R.; Miao, S.; Wu, Y. Ultrafast NH3 gas sensor based on phthalocyanine-optimized non-covalent hybrid of carbon nanotubes with pyrrole. Sens. Actuators B Chem. 2022, 357, 131352. [Google Scholar] [CrossRef]
- Kuprikova, N.M.; Klyamer, D.D.; Sukhikh, A.S.; Krasnov, P.O.; Mrsic, I.; Basova, T.V. Fluorosubstituted lead phthalocyanines: Crystal structure, spectral and sensing properties. Dye. Pigment. 2020, 173, 107939. [Google Scholar] [CrossRef]
- Klyamer, D.; Sukhikh, A.; Gromilov, S.; Krasnov, P.; Basova, T. Fluorinated metal phthalocyanines: Interplay between fluorination degree, films orientation, and ammonia sensing properties. Sensors 2018, 18, 2141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valli, L. Phthalocyanine-based Langmuir-Blodgett films as chemical sensors. Adv. Colloid Interface Sci. 2005, 116, 13–44. [Google Scholar] [CrossRef] [PubMed]
- Schöllhorn, B.; Germain, J.P.; Pauly, A.; Maleysson, C.; Blanc, J.P. Influence of peripheral electron-withdrawing substituents on the conductivity of zinc phthalocyanine in the presence of gases. Part 1: Reducing gases. Thin Solid Film. 1998, 326, 245–250. [Google Scholar] [CrossRef]
- Brinkmann, H.; Kelting, C.; Makarov, S.; Tsaryova, O.; Schnurpfeil, G.; Wöhrle, D.; Schlettwein, D. Fluorinated phthalocyanines as molecular semiconductor thin films. Phys. Status Solidi Appl. Mater. Sci. 2008, 205, 409–420. [Google Scholar] [CrossRef]
- Germain, J.P.; Pauly, A.; Maleysson, C.; Blanc, J.P.; Schöllhorn, B. Influence of peripheral electron-withdrawing substituents on the conductivity of zinc phthalocyanine in the presence of gases. Part 2: Oxidizing gases. Thin Solid Film. 1998, 333, 235–239. [Google Scholar] [CrossRef]
- Hesse, K.; Schlettwein, D. Spectroelectrochemical investigations on the reduction of thin films of hexadecafluorophthalocyaninatozinc (F16PcZn). J. Electroanal. Chem. 1999, 476, 148–158. [Google Scholar] [CrossRef]
- Shao, X.; Wang, S.; Li, X.; Su, Z.; Chen, Y.; Xiao, Y. Single component p-, ambipolar and n-type OTFTs based on fluorinated copper phthalocyanines. Dye. Pigment. 2016, 132, 378–386. [Google Scholar] [CrossRef]
- Kuzumoto, Y.; Matsuyama, H.; Kitamura, M. Partially fluorinated copper phthalocyanine toward band engineering for high-efficiency organic photovoltaics. Jpn. J. Appl. Phys. 2014, 53, 01AB03. [Google Scholar] [CrossRef]
- Bonegardt, D.; Klyamer, D.; Sukhikh, A.; Krasnov, P.; Popovetskiy, P.; Basova, T. Fluorination vs. Chlorination: Effect on the Sensor Response of Tetrasubstituted Zinc Phthalocyanine Films to Ammonia. Chemosensors 2021, 9, 137. [Google Scholar] [CrossRef]
- Klyamer, D.; Bonegardt, D.; Krasnov, P.; Sukhikh, A.; Popovetskiy, P.; Khezami, K.; Durmuş, M.; Basova, T. Halogen-substituted zinc(II) phthalocyanines: Spectral properties and structure of thin films. Thin Solid Film. 2022, 754, 139301. [Google Scholar] [CrossRef]
- Klyamer, D.D.; Sukhikh, A.S.; Gromilov, S.A.; Kruchinin, V.N.; Spesivtsev, E.V.; Hassan, A.K.; Basova, T.V. Influence of fluorosubstitution on the structure of zinc phthalocyanine thin films. Macroheterocycles 2018, 11, 304–311. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- Baerends, E.J.; Ellis, D.E.; Ros, P. Self-consistent molecular Hartree-Fock-Slater calculations I. The computational procedure. Chem. Phys. 1973, 2, 41–51. [Google Scholar] [CrossRef]
- Dunlap, B.I.; Connolly, J.W.D.; Sabin, J.R. On some approximations in applications of Xα theory. J. Chem. Phys. 1979, 71, 3396–3402. [Google Scholar] [CrossRef]
- Van Alsenoy, C. Ab initio calculations on large molecules: The multiplicative integral approximation. J. Comput. Chem. 1988, 9, 620–626. [Google Scholar] [CrossRef]
- Kendall, R.A.; Früchtl, H.A. The impact of the resolution of the identity approximate integral method on modern ab initio algorithm development. Theor. Chem. Acc. 1997, 97, 158–163. [Google Scholar] [CrossRef]
- Eichkorn, K.; Treutler, O.; Öhm, H.; Häser, M.; Ahlrichs, R. Auxiliary basis sets to approximate Coulomb potentials. Chem. Phys. Lett. 1995, 242, 652–660, Erratum in Chem. Phys. Lett. 1995, 242, 652–660. [Google Scholar] [CrossRef]
- Eichkorn, K.; Weigend, F.; Treutler, O.; Ahlrichs, R. Auxiliary basis sets for main row atoms and transition metals and their use to approximate Coulomb potentials. Theor. Chem. Acc. 1997, 97, 119–124. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2017, 8, 73–78. [Google Scholar] [CrossRef]
- Bühl, M.; Kabrede, H. Geometries of transition-metal complexes from Density-Functional Theory. J. Chem. Theory Comput. 2006, 2, 1282–1290. [Google Scholar] [CrossRef]
- Keith, T.A. AIMAll, version 19.10.12; TK Gristmill Software: Overland Park, KS, USA, 2019. Available online: aim.tkgristmill.com (accessed on 5 September 2022).
- Bonegardt, D.; Klyamer, D.; Krasnov, P.; Sukhikh, A.; Basova, T. Effect of the position of fluorine substituents in tetrasubstituted metal phthalocyanines on their vibrational spectra. J. Fluor. Chem. 2021, 246, 109780. [Google Scholar] [CrossRef]
- Boys, S.F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Simon, S.; Duran, M. How does basis set superposition error change the potential surfaces for hydrogen-bonded dimers? J. Chem. Phys. 1996, 105, 11024–11031. [Google Scholar] [CrossRef] [Green Version]
- Dunning, T.H. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Bader, R.F.W.; Essén, H. The characterization of atomic interactions. J. Chem. Phys. 1984, 80, 1943–1960. [Google Scholar] [CrossRef]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atom in Molecules: A Quantum Theory; Oxford University Press: Oxford, UK, 1994. [Google Scholar]
- Sukhikh, A.; Bonegardt, D.; Klyamer, D.; Basova, T. Effect of non-peripheral fluorosubstitution on the structure of metal phthalocyanines and their films. Dye. Pigment. 2021, 192, 109442. [Google Scholar] [CrossRef]
- Klyamer, D.D.; Sukhikh, A.S.; Trubin, S.V.; Gromilov, S.A.; Morozova, N.B.; Basova, T.V.; Hassan, A.K. Tetrafluorosubstituted Metal Phthalocyanines: Interplay between Saturated Vapor Pressure and Crystal Structure. Cryst. Growth Des. 2020, 20, 1016–1024. [Google Scholar] [CrossRef]
- Wisitsoraat, A.; Tuantranont, A.; Comini, E.; Sberveglieri, G.; Wlodarski, W. Characterization of n-type and p-type semiconductor gas sensors based on NiOx doped TiO2 thin films. Thin Solid Film. 2009, 517, 2775–2780. [Google Scholar] [CrossRef]
- Chia, L.S.; Hua Du, Y.; Palale, S.; See Lee, P. Interaction of Copper Phthalocyanine with Nitrogen Dioxide and Ammonia Investigation Using X-ray Absorption Spectroscopy and Chemiresistive Gas Measurements. ACS Omega 2019, 4, 10388–10395. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, V.; Klyamer, D.; Krasnov, P.; Kaya, E.N.; Kulu, I.; Tuncel Kostakoğlu, S.; Durmuş, M.; Basova, T. Hybrid materials based on pyrene-substituted metallo phthalocyanines as sensing layers for ammonia detection: Effect of the number of pyrene substituents. Sens. Actuators B Chem. 2023, 375, 132843. [Google Scholar] [CrossRef]
- Bushmarinov, I.S.; Lyssenko, K.A.; Antipin, M.Y. Atomic energy in the “Atoms in Molecules” theory and its use for solving chemical problems. Russ. Chem. Rev. 2009, 78, 283–302. [Google Scholar] [CrossRef]
- Bejaoui, A.; Guerin, J.; Aguir, K. Modeling of a p-type resistive gas sensor in the presence of a reducing gas. Sens. Actuators B Chem. 2013, 181, 340–347. [Google Scholar] [CrossRef]
- Chen, Y.; Yuan, M.; Zhang, Y.; Wang, X.; Ke, F.; Wang, H. One-pot synthesis of tin oxide/reduced graphene oxide composite coated fabric for wearable ammonia sensor with fast response/recovery rate. J. Alloy. Compds. 2023, 931, 167585. [Google Scholar] [CrossRef]
- Li, Q.; Xu, M.; Jiang, C.; Song, S.; Li, T.; Sun, M.; Chen, W.; Peng, H. Highly sensitive graphene-based ammonia sensor enhanced by electrophoretic deposition of MXene. Carbon 2023, 202, 561–570. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.; Chen, C.; Hu, Z.; Wang, J. Preparation and mechanism of high-performance ammonia sensor based on tungsten oxide and zinc oxide composite at room temperature. Curr. Appl. Phys. 2023, 45, 30–36. [Google Scholar] [CrossRef]
- Ridhi, R.; Gautam, S.; Saini, G.S.S.; Tripathi, S.K.; Rawat, J.S.; Jha, P. Study of the effect of orbital on interaction behaviour of SWCNT-metal phthalocyanines composites with ammonia gas. Sens. Actuators B Chem. 2021, 337, 129767. [Google Scholar] [CrossRef]
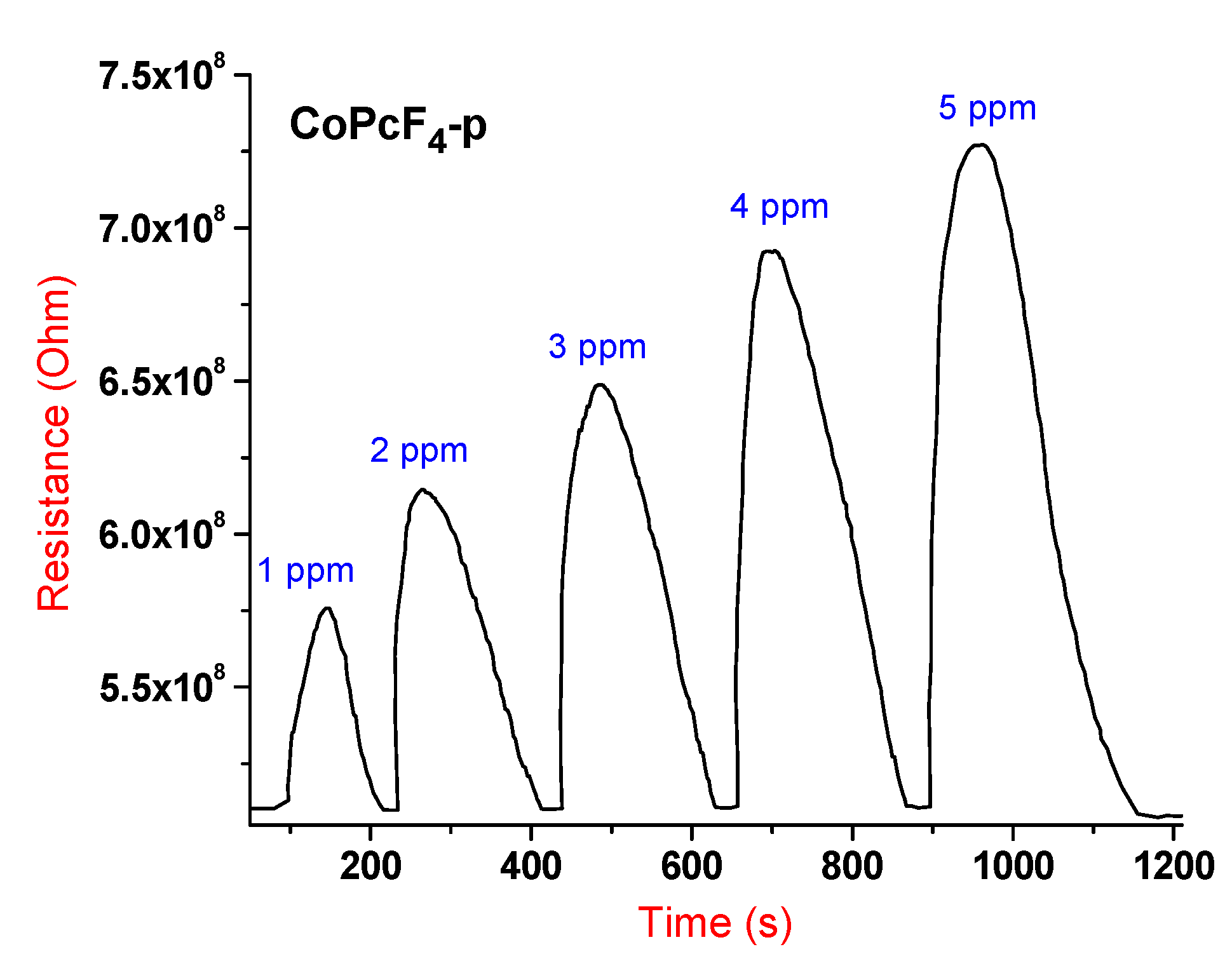
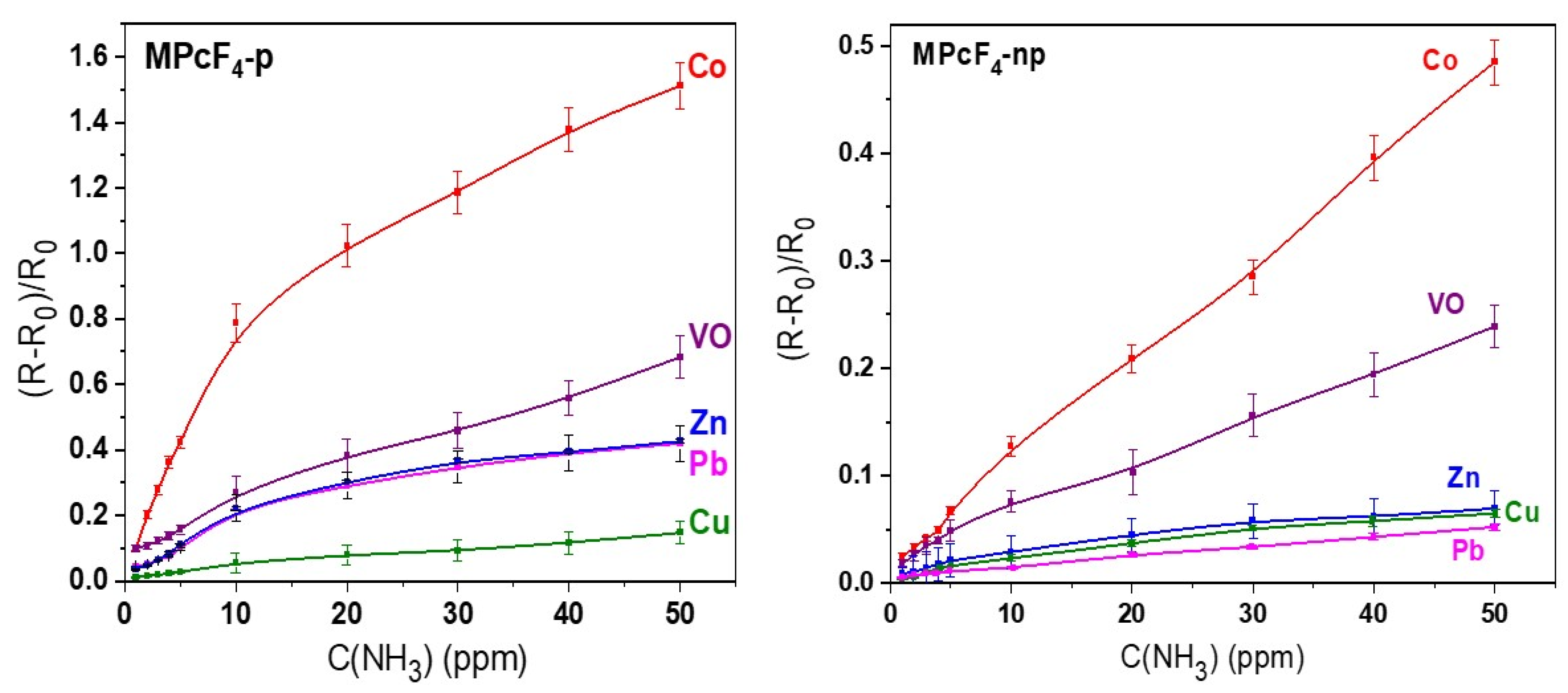
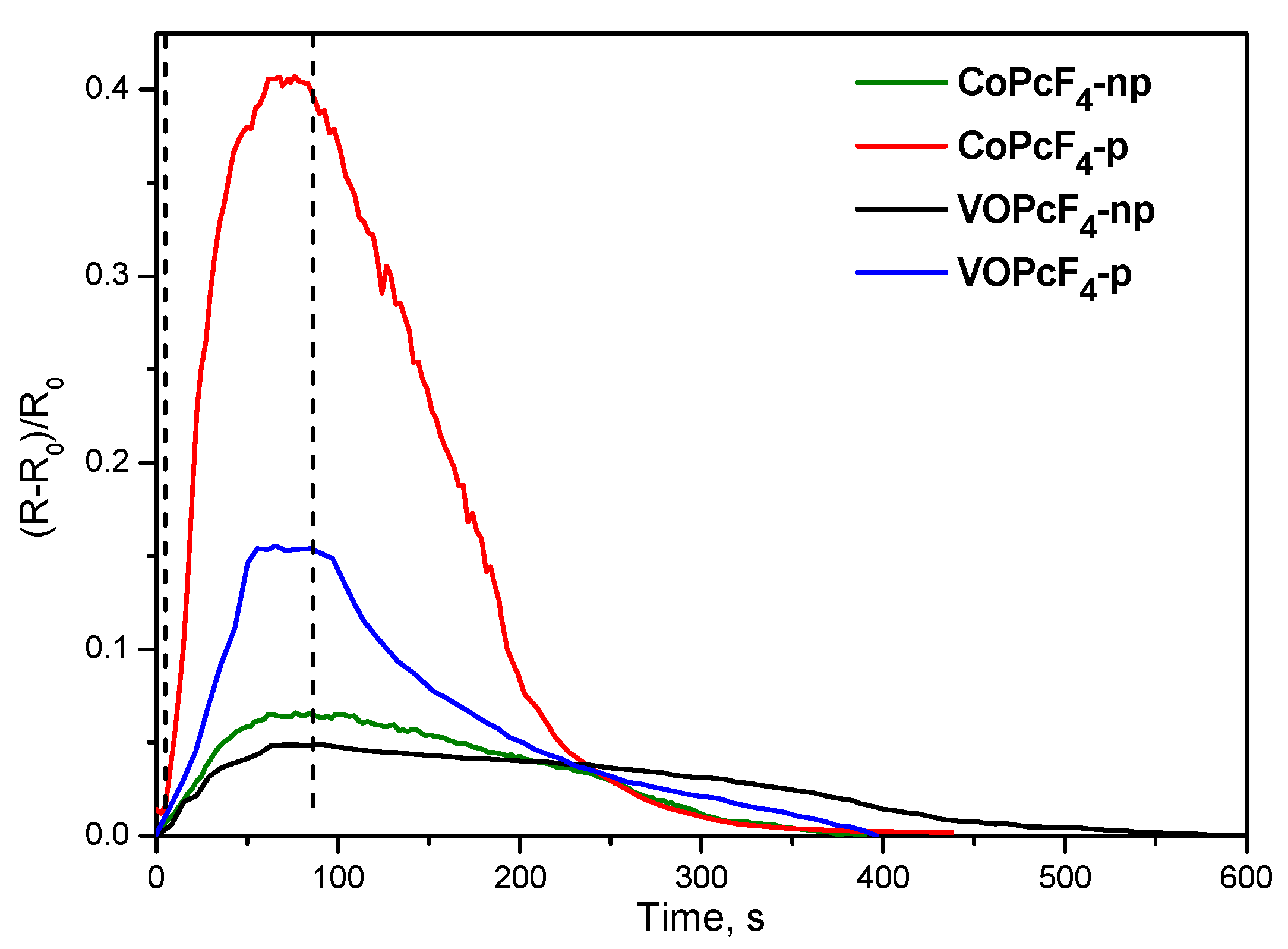
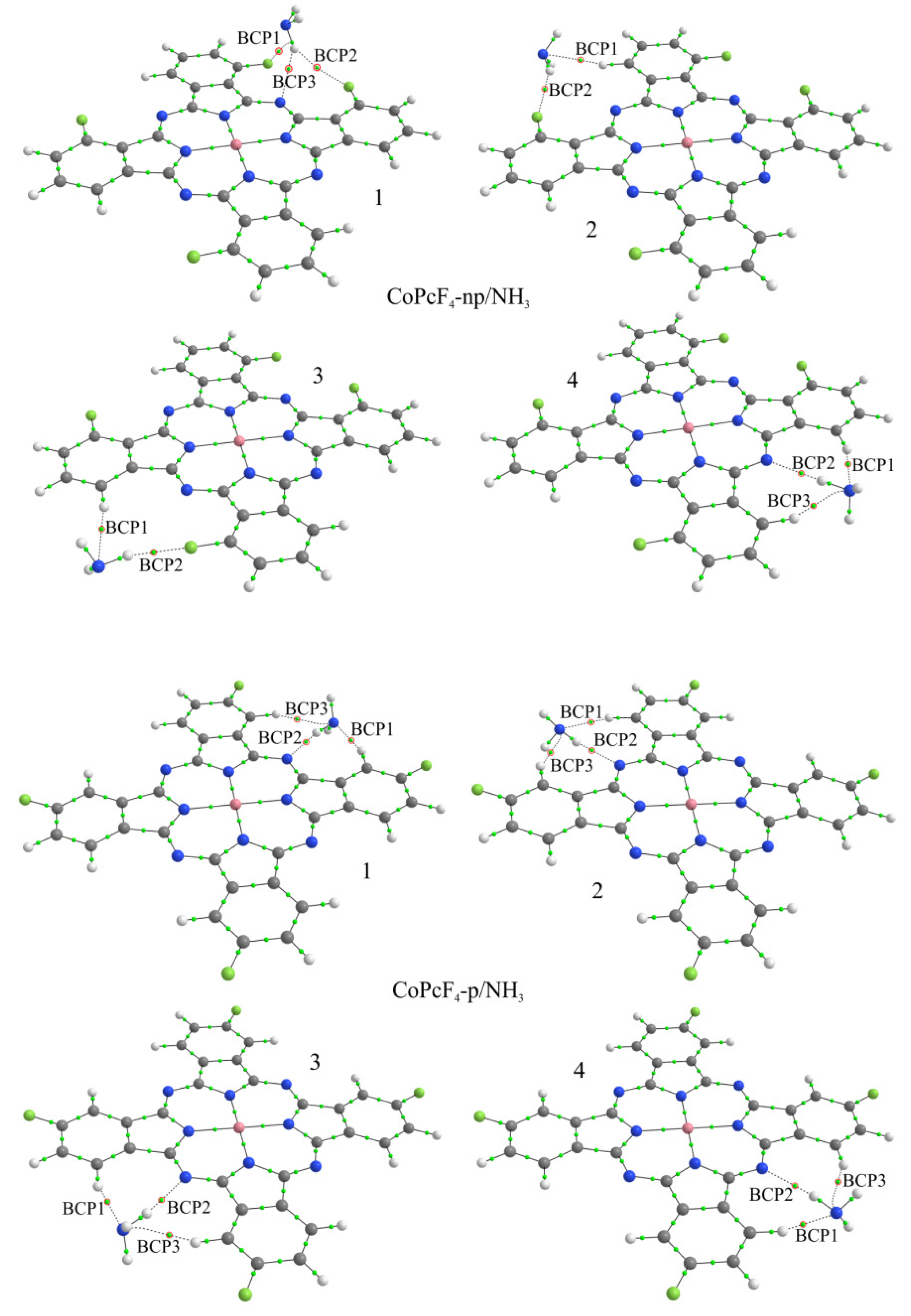
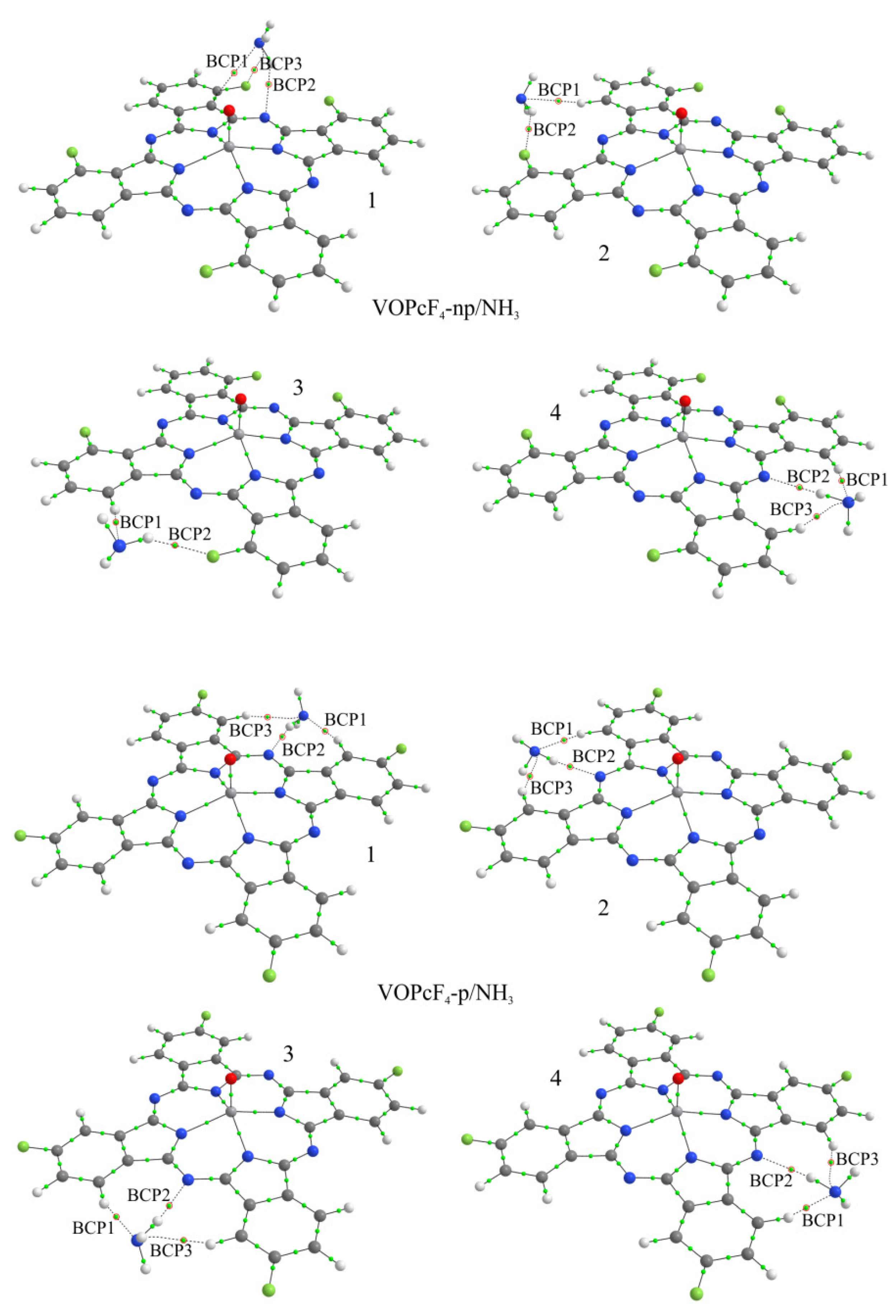
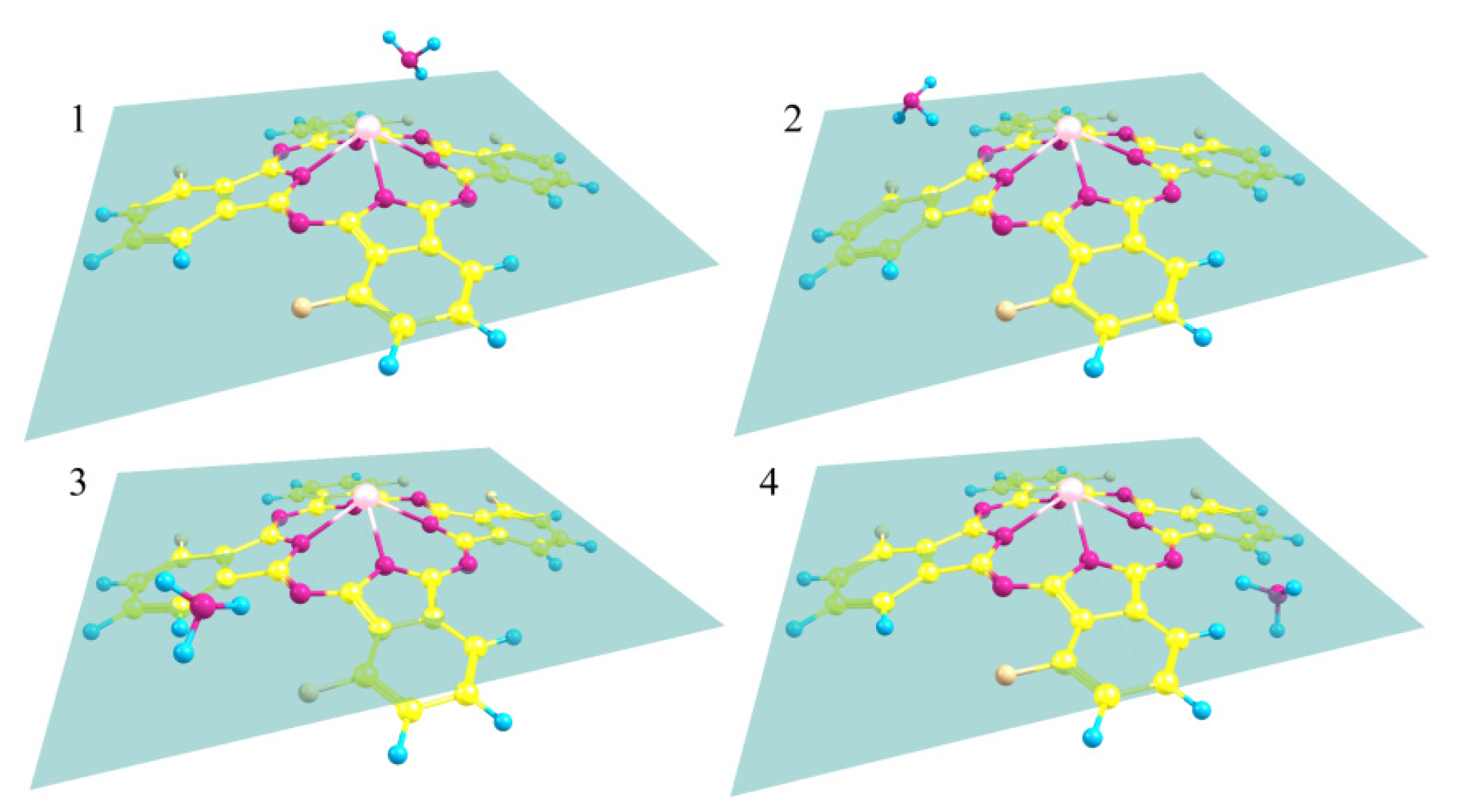

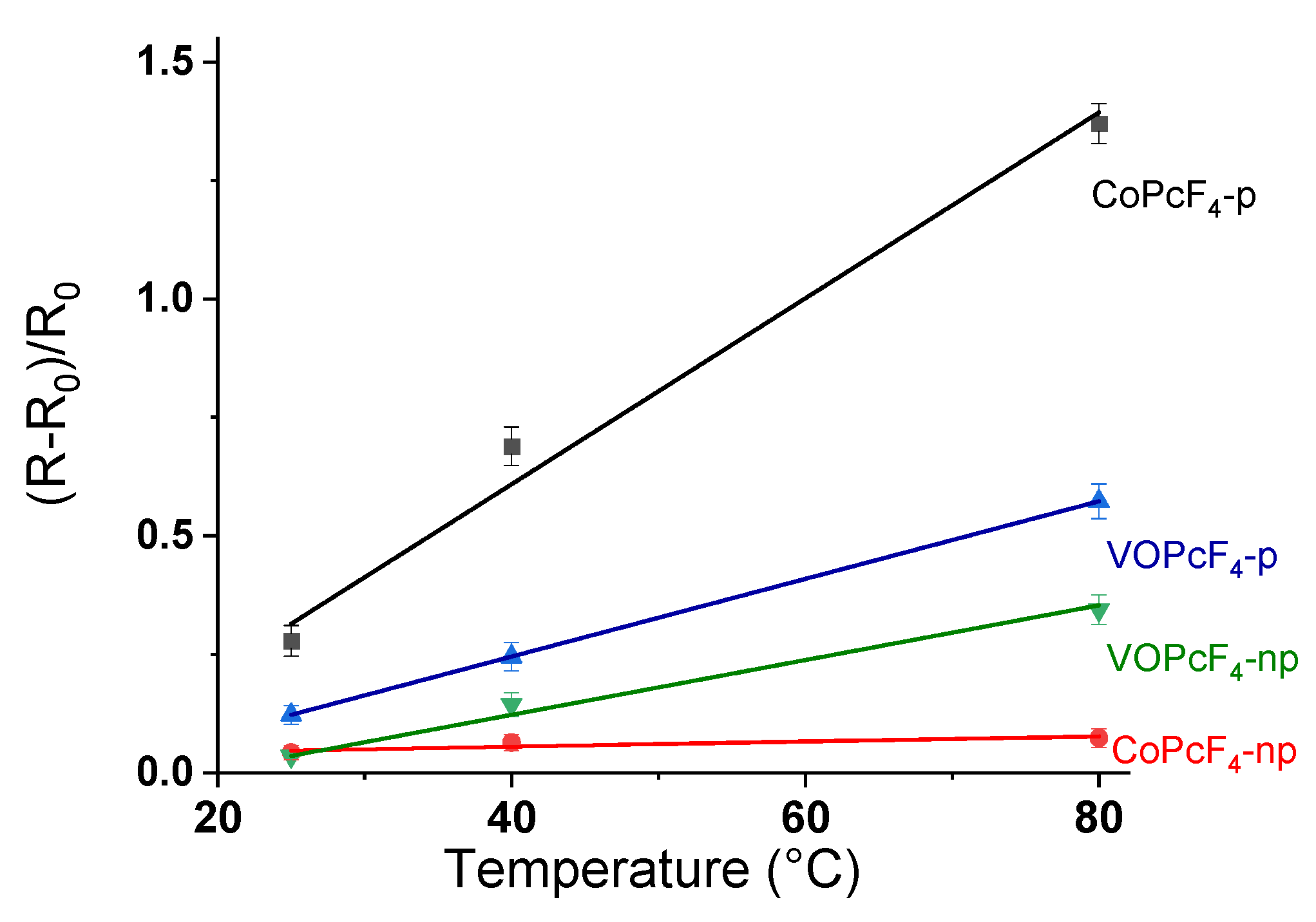
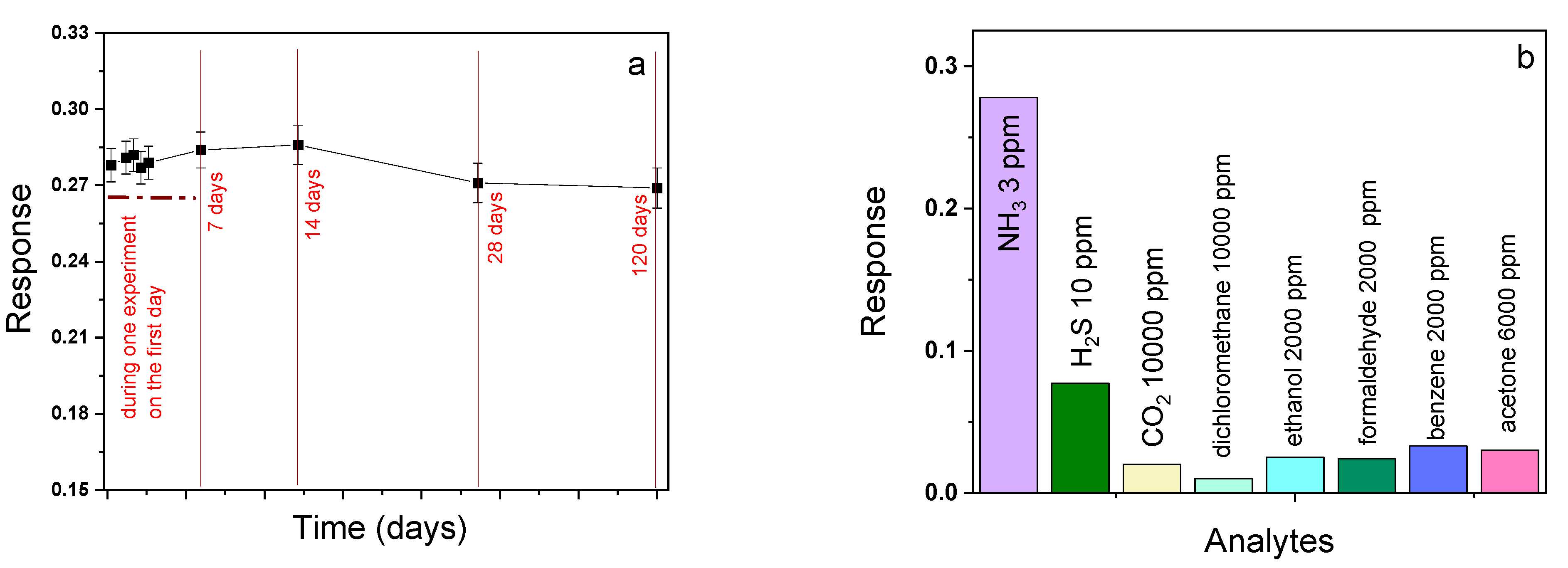
| Sensing Layer | LOD, ppm | Response Time, s (at 5 ppm) | Recovery Time, s (at 5 ppm) |
|---|---|---|---|
| CoPcF4-p | 0.01 | 55 | 215 |
| VOPcF4-p | 0.04 | 48 | 270 |
| ZnPcF4-p | 0.01 | 45 | 210 |
| PbPcF4-p | 0.65 | 40 | 250 |
| CuPcF4-p | 0.75 | 40 | 240 |
| CoPcF4-np | 0.11 | 60 | 215 |
| VOPcF4-np | 0.87 | 62 | 350 |
| ZnPcF4-np | 0.08 | 35 | 155 |
| PbPcF4-np | 1.82 | 40 | 110 |
| CuPcF4-np | 1.49 | 55 | 90 |
| Compound | Eb, eV | d, Å | BCP | Atoms * | ρ(r), e/Å3 | ∇2ρ(r), e/Å5 |
|---|---|---|---|---|---|---|
| CoPcF4-np/NH3-2 | 0.036 | 4.251 | 1 | H-N | 0.083 | 0.880 |
| 2 | F-H | 0.066 | 1.058 | |||
| CoPcF4-np/NH3-3 | 0.035 | 4.270 | 1 | H-N | 0.083 | 0.885 |
| 2 | F-H | 0.066 | 1.060 | |||
| CoPcF4-np/NH3-4 | 0.130 | 3.236 | 1 | H-N | 0.137 | 1.526 |
| 2 | N-H | 0.123 | 1.238 | |||
| 3 | H-N | 0.099 | 1.070 | |||
| CoPcF4-p/NH3-1 | 0.160 | 3.192 | 1 | H-N | 0.146 | 1.567 |
| 2 | N-H | 0.135 | 1.334 | |||
| 3 | H-N | 0.094 | 1.009 | |||
| CoPcF4-p/NH3-2 | 0.161 | 3.209 | 1 | H-N | 0.137 | 1.502 |
| 2 | N-H | 0.131 | 1.300 | |||
| 3 | H-N | 0.095 | 1.023 | |||
| CoPcF4-p/NH3-3 | 0.162 | 3.207 | 1 | H-N | 0.137 | 1.504 |
| 2 | N-H | 0.131 | 1.304 | |||
| 3 | H-N | 0.095 | 1.023 | |||
| CoPcF4-p/NH3-4 | 0.158 | 3.203 | 1 | H-N | 0.138 | 1.516 |
| 2 | N-H | 0.133 | 1.312 | |||
| 3 | H-N | 0.093 | 1.005 |
| Compound | Eb, eV | d, Å | BCP | Atoms * | ρ(r), e/Å3 | ∇2ρ(r), e/Å5 |
|---|---|---|---|---|---|---|
| VOPcF4-np/NH3-2 | 0.035 | 4.213 | 1 | H-N | 0.085 | 0.904 |
| 2 | F-H | 0.065 | 1.050 | |||
| VOPcF4-np/NH3-3 | 0.034 | 4.220 | 1 | H-N | 0.085 | 0.904 |
| 2 | F-H | 0.065 | 1.053 | |||
| VOPcF4-np/NH3-4 | 0.144 | 3.227 | 1 | H-N | 0.137 | 1.519 |
| 2 | N-H | 0.125 | 1.256 | |||
| 3 | H-N | 0.096 | 1.038 | |||
| VOPcF4-p/NH3-1 | 0.174 | 3.179 | 1 | H-N | 0.148 | 1.570 |
| 2 | N-H | 0.139 | 1.360 | |||
| 3 | H-N | 0.091 | 0.979 | |||
| VOPcF4-p/NH3-2 | 0.173 | 3.196 | 1 | H-N | 0.138 | 1.502 |
| 2 | N-H | 0.135 | 1.327 | |||
| 3 | H-N | 0.093 | 0.997 | |||
| VOPcF4-p/NH3-3 | 0.173 | 3.195 | 1 | H-N | 0.138 | 1.504 |
| 2 | N-H | 0.135 | 1.328 | |||
| 3 | H-N | 0.092 | 0.996 | |||
| VOPcF4-p/NH3-4 | 0.170 | 3.190 | 1 | H-N | 0.140 | 1.518 |
| 2 | N-H | 0.137 | 1.338 | |||
| 3 | H-N | 0.091 | 0.980 |
| Compound | NH3 Position | M = Cu | M = Zn | M = Pb |
|---|---|---|---|---|
| MPcF4-np/NH3 | 2 | 0.037 | 0.037 | - |
| 3 | 0.036 | 0.036 | - | |
| 4 | 0.145 | 0.151 | 0.144 | |
| MPcF4-p/NH3 | 1 | 0.172 | 0.179 | 0.173 |
| 2 | 0.172 | 0.179 | 0.172 | |
| 3 | 0.173 | 0.179 | 0.172 | |
| 4 | 0.169 | 0.176 | 0.169 |
| Material | Concentration Range, ppm | LOD, ppm | Response, % | Response/Recovery Time, s | Ref. |
|---|---|---|---|---|---|
| polyimide-SnO2/rGO | 50–800 | 15 | 5.16 (100 ppm) | 94/57 | [62] |
| MXene/Graphene composite | 0.5–100 | 0.056 | 25 (100 ppm) | 26/148 | [63] |
| ZnO and WO3·H2O composite | 2–100 | 0.76 | ~15 (10 ppm) | 2.4/1.2 | [64] |
| SWCNT-TiOPc | 5–50 | n/a | 1.76 (50 ppm) | 120 (fixed)/~40 | [65] |
| CoPcF4-p | 1–50 | 0.01 | 42 (5 ppm) | 55/215 | Our work |
| VOPcF4-p | 1–50 | 0.04 | 15 (5 ppm) | 48/270 | Our work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klyamer, D.; Bonegardt, D.; Krasnov, P.; Sukhikh, A.; Popovetskiy, P.; Basova, T. Tetrafluorosubstituted Metal Phthalocyanines: Study of the Effect of the Position of Fluorine Substituents on the Chemiresistive Sensor Response to Ammonia. Chemosensors 2022, 10, 515. https://doi.org/10.3390/chemosensors10120515
Klyamer D, Bonegardt D, Krasnov P, Sukhikh A, Popovetskiy P, Basova T. Tetrafluorosubstituted Metal Phthalocyanines: Study of the Effect of the Position of Fluorine Substituents on the Chemiresistive Sensor Response to Ammonia. Chemosensors. 2022; 10(12):515. https://doi.org/10.3390/chemosensors10120515
Chicago/Turabian StyleKlyamer, Darya, Dmitry Bonegardt, Pavel Krasnov, Alexander Sukhikh, Pavel Popovetskiy, and Tamara Basova. 2022. "Tetrafluorosubstituted Metal Phthalocyanines: Study of the Effect of the Position of Fluorine Substituents on the Chemiresistive Sensor Response to Ammonia" Chemosensors 10, no. 12: 515. https://doi.org/10.3390/chemosensors10120515
APA StyleKlyamer, D., Bonegardt, D., Krasnov, P., Sukhikh, A., Popovetskiy, P., & Basova, T. (2022). Tetrafluorosubstituted Metal Phthalocyanines: Study of the Effect of the Position of Fluorine Substituents on the Chemiresistive Sensor Response to Ammonia. Chemosensors, 10(12), 515. https://doi.org/10.3390/chemosensors10120515







