Fabric Phase Sorptive Extraction Combined with HPLC-UV for the Quantitation of Amphotericin B in Human Urine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents, Solutions and FPSE Membranes
2.2. HPLC Instrumentation and Conditions
2.3. FPSE Protocol
2.4. Method Validation
3. Results and Discussion
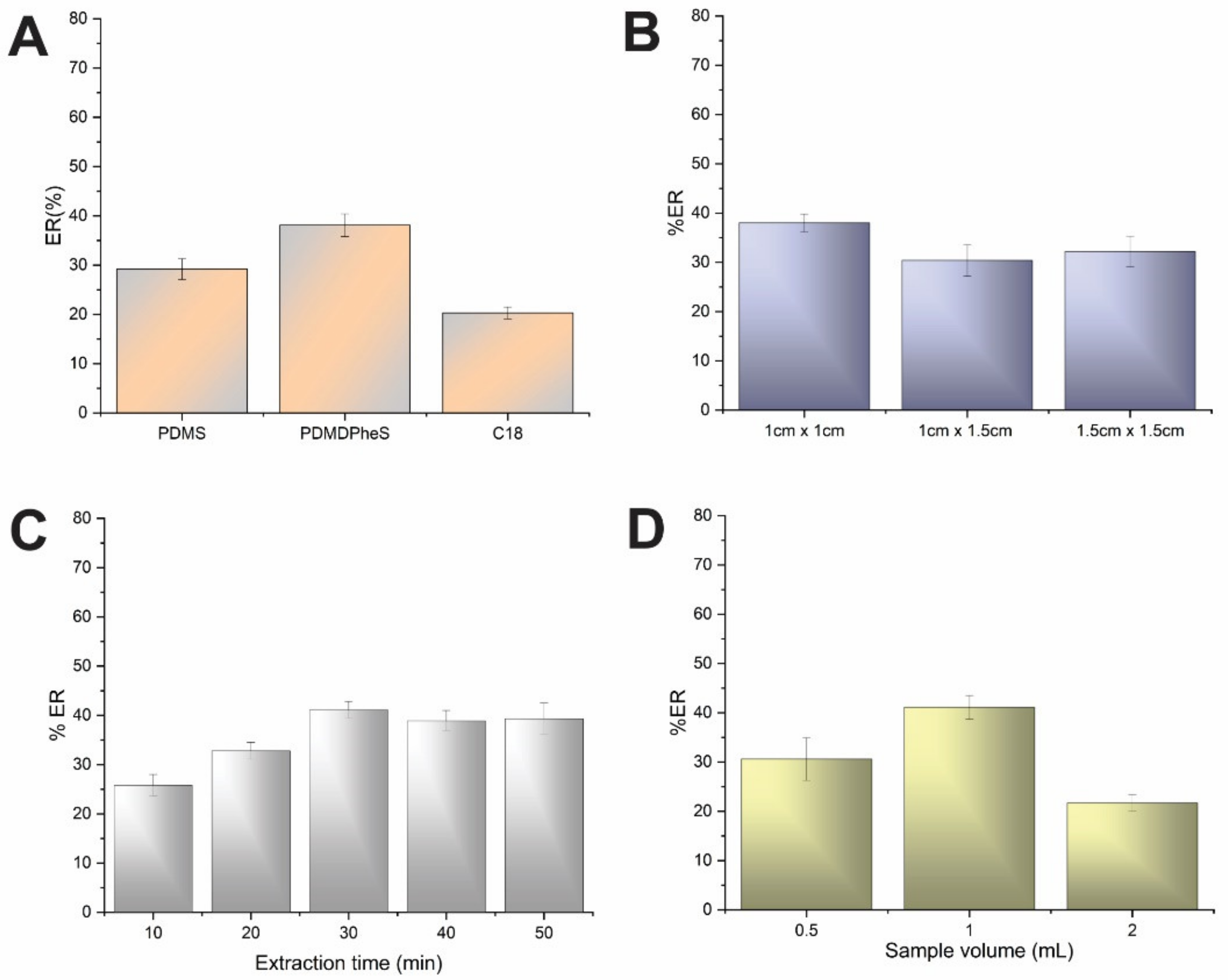
3.1. Effect of FPSE Sorbent Chemistry and Dimensions
3.2. Effect of Sample pH and Volume and Extraction Time
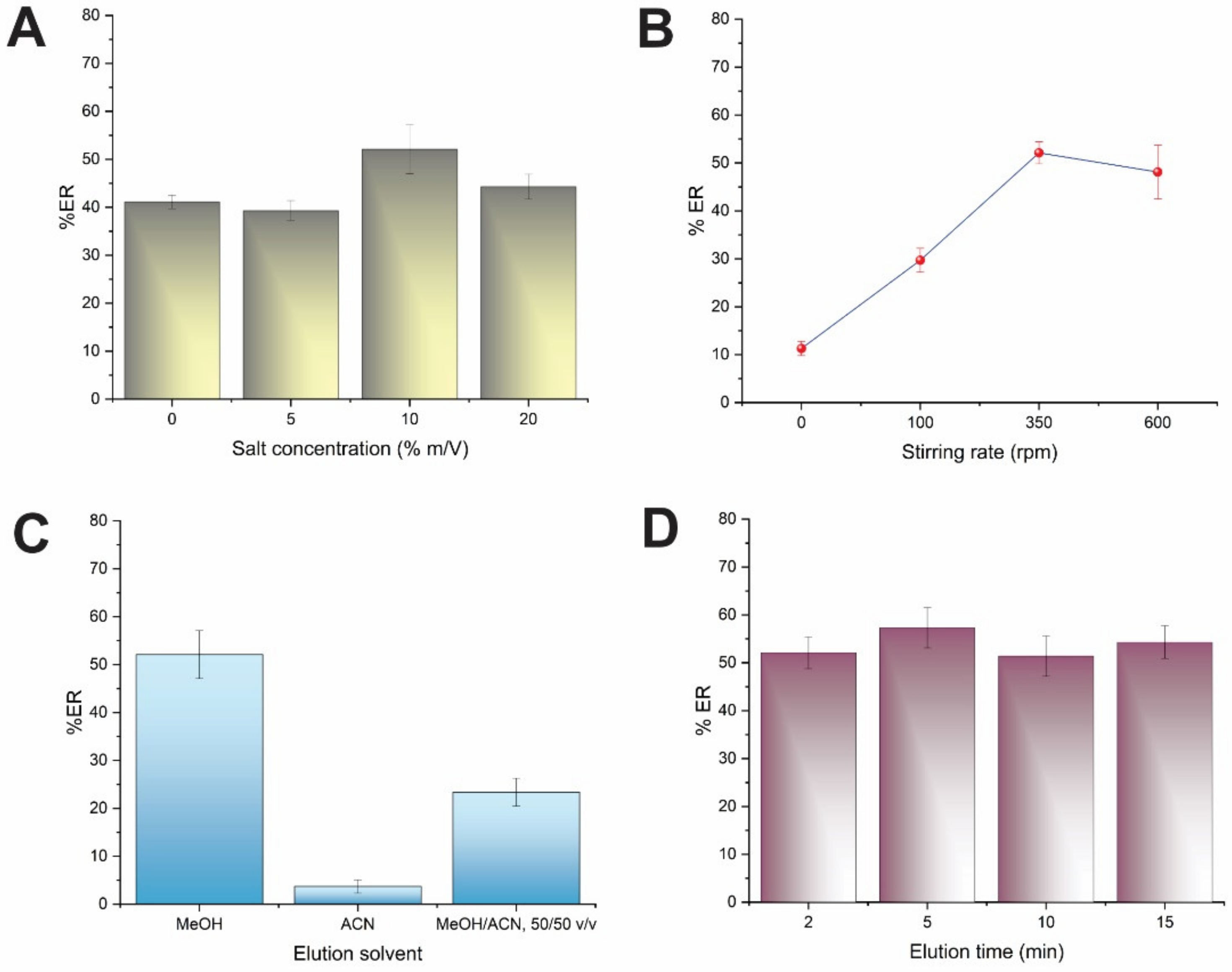
3.3. Effect of Ionic Strength and Stirring Rate
3.4. Effect of the Elution Solvent, Volume and Time
3.5. Method Validation
3.6. Sample Stability
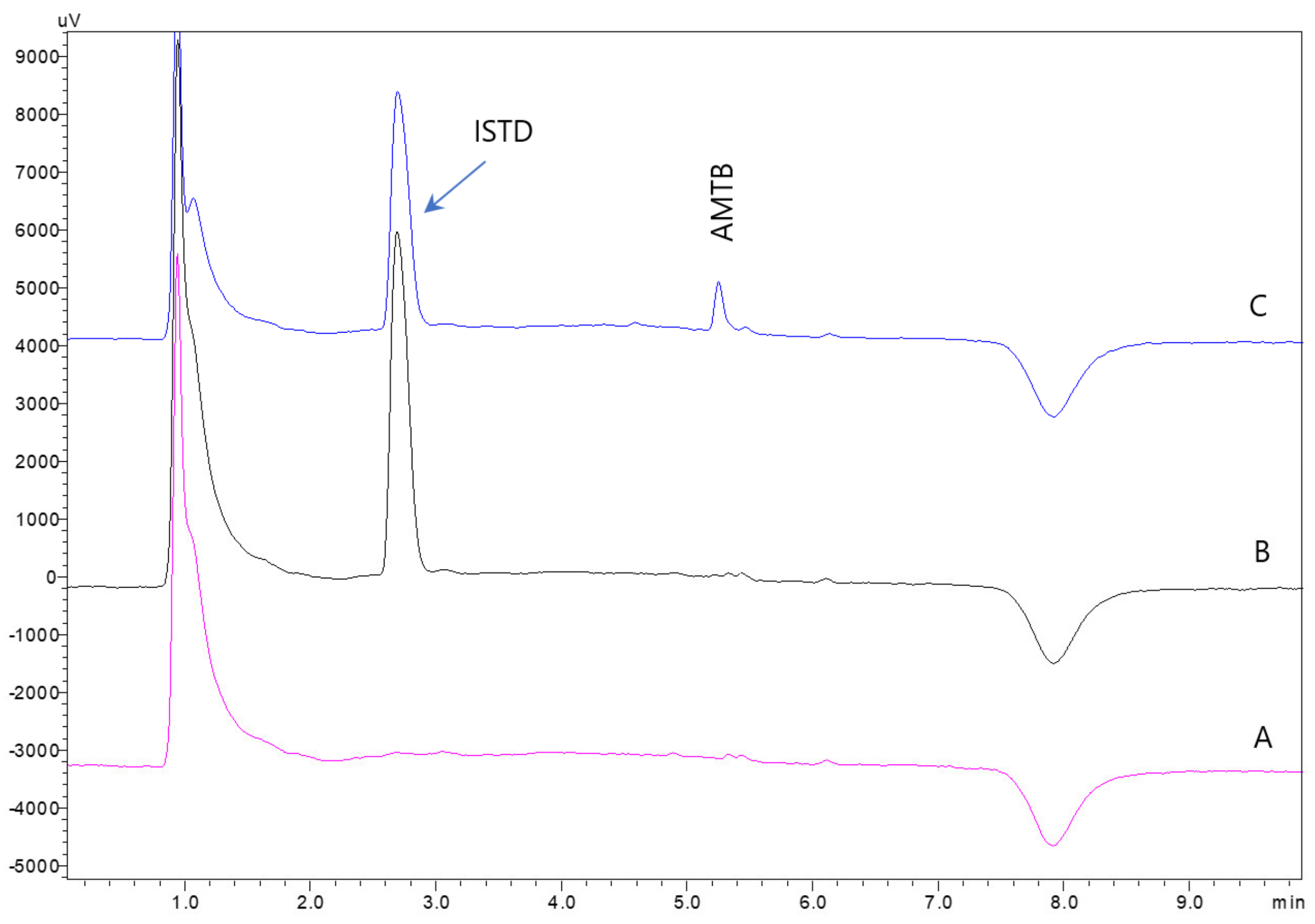
3.7. Application to Human Urine Samples
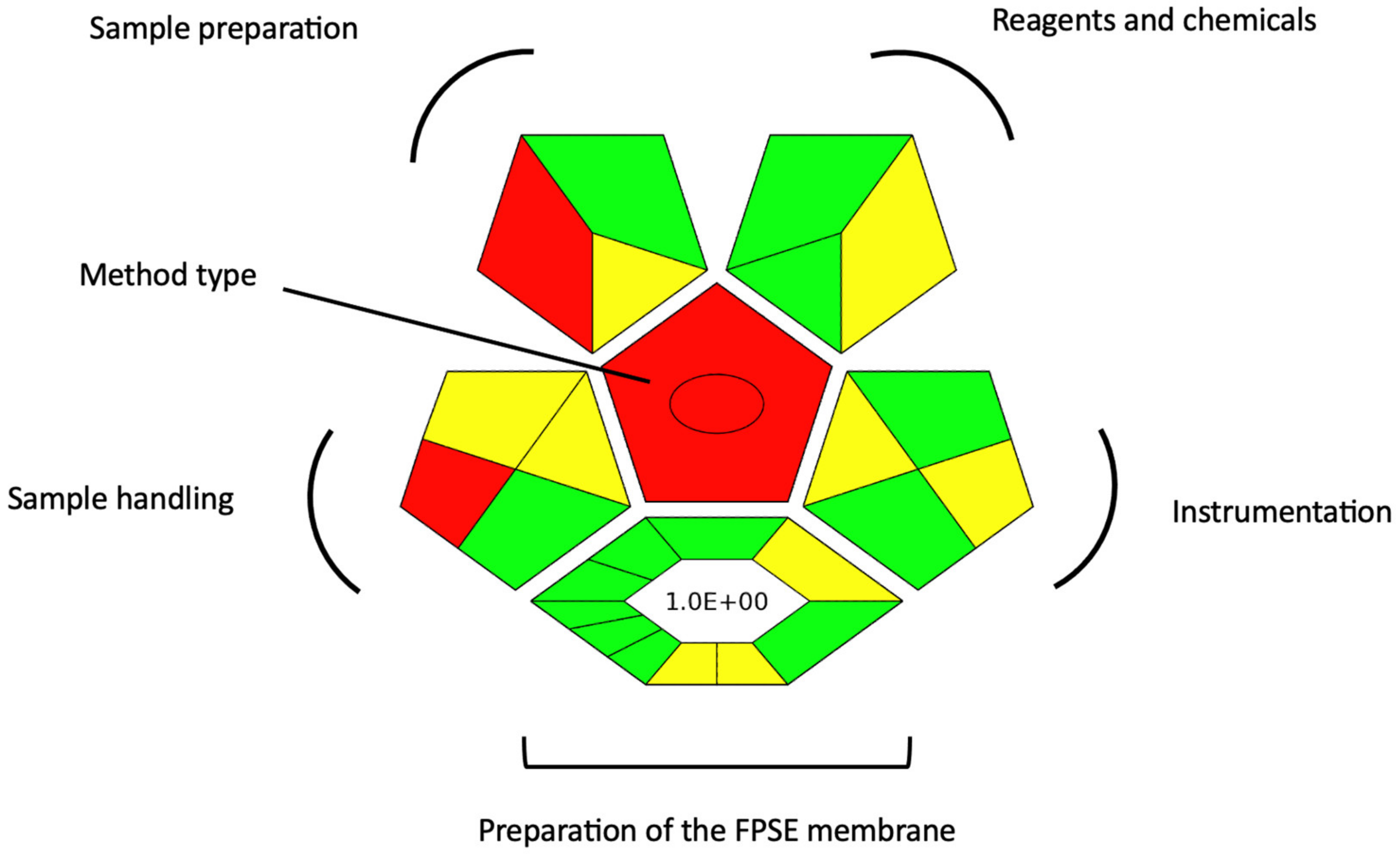
3.8. Evaluation of the Green Character of the FPSE-HPLC-UV Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patel, P.A.; Patravale, V.B. AmbiOnp: Solid Lipid Nanoparticles of Amphotericin B for Oral Administration. J. Biomed. Nanotechnol. 2011, 7, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Davi Marena, G.; Aparecido dos Santos Ramos, M.; Maria Bauab, T.; Chorilli, M.; Davi Marena, Ã.G.; Aparecido dos Santos Ramos, Ã.M.; ıs Maria Bauab, T. A Critical Review of Analytical Methods for Quantification of Amphotericin B in Biological Samples and Pharmaceutical Formulations. Crit. Rev. Anal. Chem. 2020, 52, 555–576. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Petersen, M.E.; Lin, P.; Dressler, D.; Bekersky, I. Quantitation of Free and Total Amphotericin B in Human Biologic Matrices by a Liquid Chromatography Tandem Mass Spectrometric Method. Ther. Drug Monit. 2001, 23, 268–276. [Google Scholar] [CrossRef]
- Bekersky, I.; Fielding, R.M.; Dressler, D.E.; Lee, J.W.; Buell, D.N.; Walsh, T.J. Pharmacokinetics, Excretion, and Mass Balance of Liposomal Amphotericin B (AmBisome) and Amphotericin B Deoxycholate in Humans. Antimicrob. Agents Chemother. 2002, 46, 828–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.H.; Smith, P.C.; Anderson, K.L.; Fielding, R.M. High-Performance Liquid Chromatographic Analysis of Amphotericin B in Plasma, Blood, Urine and Tissues for Pharmacokinetic and Tissue Distribution Studies. J. Chromatogr. B Biomed. Sci. Appl. 1992, 579, 259–268. [Google Scholar] [CrossRef]
- Ingle, R.G.; Zeng, S.; Jiang, H.; Fang, W.J. Current Developments of Bioanalytical Sample Preparation Techniques in Pharmaceuticals. J. Pharm. Anal. 2022, 12, 517–529. [Google Scholar] [CrossRef]
- Queiroz, M.E.C.; de Souza, I.D.; de Oliveira, I.G.; Grecco, C.F. In Vivo Solid Phase Microextraction for Bioanalysis. TrAC–Trends Anal. Chem. 2022, 153, 116656. [Google Scholar] [CrossRef]
- Li, F.; Ceballos, M.R.; Balavandy, S.K.; Fan, J.; Khataei, M.M.; Yamini, Y.; Maya, F. 3D Printing in Analytical Sample Preparation. J. Sep. Sci. 2020, 43, 1854–1866. [Google Scholar] [CrossRef]
- Hansen, F.; Øiestad, E.L.; Pedersen-Bjergaard, S. Bioanalysis of Pharmaceuticals Using Liquid-Phase Microextraction Combined with Liquid Chromatography–Mass Spectrometry. J. Pharm. Biomed Anal. 2020, 189, 113446. [Google Scholar] [CrossRef]
- Turoňová, D.; Kujovská Krčmová, L.; Švec, F. Application of Microextraction in Pipette Tips in Clinical and Forensic Toxicology. TrAC–Trends Anal. Chem. 2021, 143, 116404. [Google Scholar] [CrossRef]
- Paiva, A.C.; Crucello, J.; de Aguiar Porto, N.; Hantao, L.W. Fundamentals of and Recent Advances in Sorbent-Based Headspace Extractions. TrAC–Trends Anal. Chem. 2021, 139, 116252. [Google Scholar] [CrossRef]
- Mamounas, G.; Manousi, N.; Kabir, A.; Furton, K.G.; Mystridis, G.A.; Vizirianakis, I.S.; Zacharis, C.K. Designing an “All-in-One” Microextraction Capsule Device for the Liquid Chromatographic-Fluorescence Determination of Doxorubicin and Its Metabolites in Rat Plasma. J. Chromatogr. A 2022, 1680, 463432. [Google Scholar] [CrossRef]
- US20140274660A1–Fabric Phase Sorptive Extractors (Fpse)–Google Patents. Available online: https://patents.google.com/patent/US20140274660A1/en (accessed on 16 October 2022).
- Parla, A.; Zormpa, E.; Paloumpis, N.; Kabir, A.; Furton, K.G.; Roje, Ž.; Samanidou, V.; Vrček, I.V.; Panderi, I. Determination of Intact Parabens in the Human Plasma of Cancer and Non-Cancer Patients Using a Validated Fabric Phase Sorptive Extraction Reversed-Phase Liquid Chromatography Method with Uv Detection. Molecules 2021, 26, 1526. [Google Scholar] [CrossRef] [PubMed]
- Agadellis, E.; Tartaglia, A.; Locatelli, M.; Kabir, A.; Furton, K.G.; Samanidou, V. Mixed-Mode Fabric Phase Sorptive Extraction of Multiple Tetracycline Residues from Milk Samples Prior to High Performance Liquid Chromatography-Ultraviolet Analysis. Microchem. J. 2020, 159, 105437. [Google Scholar] [CrossRef]
- Alampanos, V.; Kabir, A.; Furton, K.G.; Samanidou, V.; Papadoyannis, I. Fabric Phase Sorptive Extraction for Simultaneous Observation of Four Penicillin Antibiotics from Human Blood Serum Prior to High Performance Liquid Chromatography and Photo-Diode Array Detection. Microchem. J. 2019, 149, 103964. [Google Scholar] [CrossRef]
- Locatelli, M.; Tinari, N.; Grassadonia, A.; Tartaglia, A.; Macerola, D.; Piccolantonio, S.; Sperandio, E.; D’Ovidio, C.; Carradori, S.; Ulusoy, H.I.; et al. FPSE-HPLC-DAD Method for the Quantification of Anticancer Drugs in Human Whole Blood, Plasma, and Urine. J. Chromatogr. B 2018, 1095, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, A.; Kabir, A.; D’Ambrosio, F.; Ramundo, P.; Ulusoy, S.; Ulusoy, H.I.; Merone, G.M.; Savini, F.; D’Ovidio, C.; de Grazia, U.; et al. Fast Off-Line FPSE-HPLC-PDA Determination of Six NSAIDs in Saliva Samples. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2020, 1144, 122082. [Google Scholar] [CrossRef]
- FDA. Bioanalytical Method Validation Guidance. 2018. Available online: https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf (accessed on 10 December 2022).
- Brooks, T.; Keevil, C.W. A Simple Artificial Urine for the Growth of Urinary Pathogens. Lett. Appl. Microbiol. 1997, 24, 203–206. [Google Scholar] [CrossRef]
- Kumar, R.; Gaurav; Heena; Malik, A.K.; Kabir, A.; Furton, K.G. Efficient Analysis of Selected Estrogens Using Fabric Phase Sorptive Extraction and High Performance Liquid Chromatography-Fluorescence Detection. J. Chromatogr. A 2014, 1359, 16–25. [Google Scholar] [CrossRef]
- Aznar, M.; Úbeda, S.; Nerin, C.; Kabir, A.; Furton, K.G. Fabric Phase Sorptive Extraction as a Reliable Tool for Rapid Screening and Detection of Freshness Markers in Oranges. J. Chromatogr. A 2017, 1500, 32–42. [Google Scholar] [CrossRef]
- Alcudia-León, M.C.; Lucena, R.; Cárdenas, S.; Valcárcel, M.; Kabir, A.; Furton, K.G. Integrated Sampling and Analysis Unit for the Determination of Sexual Pheromones in Environmental Air Using Fabric Phase Sorptive Extraction and Headspace-Gas Chromatography–Mass Spectrometry. J. Chromatogr. A 2017, 1488, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Mazaraki, K.; Kabir, A.; Furton, K.G.; Fytianos, K.; Samanidou, V.F.; Zacharis, C.K. Fast Fabric Phase Sorptive Extraction of Selected β-Blockers from Human Serum and Urine Followed by UHPLC-ESI-MS/MS Analysis. J. Pharm. Biomed Anal. 2021, 199, 114053. [Google Scholar] [CrossRef] [PubMed]
- Faustino, C.; Pinheiro, L. Lipid Systems for the Delivery of Amphotericin B in Antifungal Therapy. Pharmaceutics 2020, 12, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 Principles of Green Analytical Chemistry and the SIGNIFICANCE Mnemonic of Green Analytical Practices. TrAC–Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Bekersky, I.; Fielding, R.M.; Dressler, D.E.; Lee, J.W.; Buell, D.N.; Walsh, T.J. Plasma Protein Binding of Amphotericin B and Pharmacokinetics of Bound versus Unbound Amphotericin B after Administration of Intravenous Liposomal Amphotericin B (AmBisome) and Amphotericin B Deoxycholate. Antimicrob. Agents Chemother. 2002, 46, 834–840. [Google Scholar] [CrossRef] [Green Version]
- Pippa, L.F.; Marques, M.P.; da Silva, A.C.T.; Vilar, F.C.; de Haes, T.M.; da Fonseca, B.A.L.; Martinez, R.; Coelho, E.B.; Wichert-Ana, L.; Lanchote, V.L. Sensitive LC-MS/MS Methods for Amphotericin B Analysis in Cerebrospinal Fluid, Plasma, Plasma Ultrafiltrate, and Urine: Application to Clinical Pharmacokinetics. Front. Chem. 2021, 9, 998. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Wojnowski, W. Complementary Green Analytical Procedure Index (ComplexGAPI) and Software. Green Chem. 2021, 23, 8657–8665. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J. A New Tool for the Evaluation of the Analytical Procedure: Green Analytical Procedure Index. Talanta 2018, 181, 204–209. [Google Scholar] [CrossRef]

| Intra-Day (n = 3) | Inter-Day (n = 3) | |||
|---|---|---|---|---|
| Added Concentration (μg mL−1) | Precision (%RSD) | Accuracy RR 1 (%) | Precision (%RSD) | Accuracy RR (%) |
| 0.10 (LLOQ) | 12.7 | 88.1 | 9.8 | 96.4 |
| 0.25 (LQC) | 7.0 | 91.6 | 9.3 | 97.7 |
| 1.0 (MQC) | 5.7 | 110.3 | 8.4 | 108.3 |
| 10.0 (HQC) | 3.0 | 107.6 | 7.4 | 103.5 |
| Added Concentration (μg mL−1) | Relative Recoveries (%) (RSD%, n = 3) | ||
|---|---|---|---|
| Sample#1 | Sample#2 | Sample#3 | |
| 0.10 | 89.2 (3.0) | 106.9 (7.5) | 82.6 (12.0) |
| 0.25 | 110.7 (4.0) | 95.4 (9.5) | 98.3 (9.7) |
| 1.0 | 105.8 (6.3) | 106.9 (6.7) | 110.9 (5.0) |
| 10.0 | 98.6 (7.0) | 89.1 (4.8) | 89.7 (4.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asimakopoulou, E.; Manousi, N.; Anaxagorou, N.; Kabir, A.; Furton, K.G.; Zacharis, C.K. Fabric Phase Sorptive Extraction Combined with HPLC-UV for the Quantitation of Amphotericin B in Human Urine. Chemosensors 2022, 10, 537. https://doi.org/10.3390/chemosensors10120537
Asimakopoulou E, Manousi N, Anaxagorou N, Kabir A, Furton KG, Zacharis CK. Fabric Phase Sorptive Extraction Combined with HPLC-UV for the Quantitation of Amphotericin B in Human Urine. Chemosensors. 2022; 10(12):537. https://doi.org/10.3390/chemosensors10120537
Chicago/Turabian StyleAsimakopoulou, Evmorfia, Natalia Manousi, Nikoleta Anaxagorou, Abuzar Kabir, Kenneth G. Furton, and Constantinos K. Zacharis. 2022. "Fabric Phase Sorptive Extraction Combined with HPLC-UV for the Quantitation of Amphotericin B in Human Urine" Chemosensors 10, no. 12: 537. https://doi.org/10.3390/chemosensors10120537
APA StyleAsimakopoulou, E., Manousi, N., Anaxagorou, N., Kabir, A., Furton, K. G., & Zacharis, C. K. (2022). Fabric Phase Sorptive Extraction Combined with HPLC-UV for the Quantitation of Amphotericin B in Human Urine. Chemosensors, 10(12), 537. https://doi.org/10.3390/chemosensors10120537