1. Introduction
Hydrogen chloride (HCl) gas is generated by incinerating chloride-containing materials, such as plastics (e.g., polyvinyl chloride), papers, and coals [
1,
2]. It has been recognized as one of the acid gas air pollutants due to its toxic effect on the environment and human health. Exposure to HCl gas can have adverse effects on the human body, including the eyes, mucous membranes, and skin. In addition, exposure to highly concentrated HCl gas (>100 ppm) can lead to acute laryngeal cramps or pulmonary edema [
3]. Therefore, various types of gas sensors, including optochemical [
4,
5,
6,
7,
8], colorimetric [
9,
10], amperometric [
11], conductometric [
12], and electrochemical [
13] sensors, have been proposed to control and monitor HCl gas discharged into the atmosphere. In particular, quartz crystal microbalance (QCM) has been used as a sensing instrument to detect HCl gas because of the ease of quantitative analysis observed by converting a measured frequency variation into a mass change. In a QCM-based sensing system, various functional materials can be used on the QCM electrode in order to amplify the signal resulting from the binding reaction of analytes. In this context, Matsuguchi et al. reported three kinds of poly(acrylamide) derivative-coated quartz resonators for accurately detecting HCl gas in the air [
14]. They found that the sensitivity, response time, and reversibility of resonators depend on the chemical structure of the amide group, and they concluded that poly(
N,
N-dimethylacrylamide) (PDMAA) among the used polymers was the best suited for HCl sensors when considering the relevant data measured using a QCM instrument. Furthermore, they have used various functional polymer-based materials, such as electrodeposited polymer [
15], fluorescent polymer [
16], poly(
N-isopropylacrylamide) (PNIPAM) nanoparticles (NPs) [
17,
18,
19], and PNIPAM brushes [
20,
21] to detect HCl gas. Despite extensive efforts to develop polymer-based gas sensors, a fabrication process which includes greater simplicity and lower cost should be required to produce efficient gas-sensing platforms. In this regard, our group has previously reported aminated polystyrene (a-PS) colloids, which could be prepared through a simple substitution process to detect HCl gas [
22]. Secondary amine salts were formed on the QCM sensors with the a-PS colloids through the reaction between a secondary amine group and HCl gas, resulting in a high binding capacity, of 102 μg/mg, and excellent sensitivity and selectivity. Here, we concluded that the structure of the amine group can influence HCl adsorption properties. However, it was difficult to immobilize spin-coated colloids on quartz crystal (QC) electrodes for the development of QCM-based HCl gas sensors. Moreover, the harsh treatment, with a toxic acid, was essentially required to attach amine groups on the PS colloids.
As an extension of our previous study, we used 2-dimethylaminoethyl methacrylate (DMAEMA) to develop a QCM-based HCl gas-sensing crosslinked copolymer film. The tertiary amine salt is formed by a chemical reaction between the tertiary amine groups of DMAEMA and HCl. As a crosslinking monomer, ethylene glycol dimethacrylate (EGDMA) was used for sufficient polymer film stability; thus, poly(DMAEMA-co-EGDMA) films, denoted by C2-DMA, were prepared on quartz crystal (QC) substrates through ultraviolet (UV) radical polymerization. The mass dependence of the copolymer film was confirmed by comparing HCl-binding capacities on the films fabricated in various masses. Using N,N-dimethylacrylamide (DMAA), proven to be a suitable functional monomer of HCl sensors, poly(DMAA-co-EGDMA) films, i.e., C0-DMA, were also produced as control samples. This copolymer film can interact with HCl gas on the carbonyl group of the tertiary-amide group. Therefore, the HCl adsorption behavior of the two copolymer film types, containing different functional groups (i.e., tertiary amine and amide), was comparatively analyzed. Furthermore, adsorption characteristics, e.g., the binding capacity, oxygen/moisture dependence, sensitivity, and selectivity of the two film types, were investigated using a QCM instrument under flowing HCl gas.
3. Results
The mass of the copolymer film coated on the QCM electrode is an important factor affecting the adsorption capacity and diffusion coefficient in the HCl adsorption process. An ultrathin copolymer film can cause an insufficient signal for HCl adsorption, whereas, in the case of extremely thick film, the diffusion rate may be very slow, even if the detection signal is amplified. Thus, the mass dependence of the C2-DMA film coated on the QCM electrode should be optimized to maximize the adsorption signal. In this regard, various copolymer films, with a mass range of 1.77–11.83 μg, were investigated, monitoring Δ
f values under a 70-ppm HCl gas flow. As shown in
Figure 1b, the Δ
f values of copolymer films with a mass up to 7.5 μg were linearly increased as a function of adsorption time, and this occured because of an increase in adsorption sites for the HCl gas. However, the Δ
f value of the C2-DMA film with a mass of 9.5 μg was slightly reduced because of the relatively lower diffusion rate at the thick film.
Figure 1c depicts HCl mass and adsorption capacity (
Qt) value, in 2-h adsorption, pertaining to various C2-DMA masses on the films. The film with a mass of 4.6 μg had the highest
Qt value (162 mg/g). As a comparison sample, a C0-DMA film was also prepared with a mass range of 5.1–5.5 μg. The thicknesses of the C2-DMA (
mp = 5.54 μg) and C0-DMA (
mp = 5.11 μg) films were 227 ± 8.9 and 195 ± 16.6 nm, respectively (
Figure 1d).
To investigate the adsorption response for each crosslinked film, Δ
f values were recorded while flowing dry HCl of 70 ppm in a chamber until the frequency reached an equilibrium state in the process of adsorption (2-h for C2-DMA and 10-min for C0-DMA) and desorption (10-min for both films).
Figure 2 and
Figure S2 show
Qt and Δ
f values as a function of time for two different films. In both crosslinked films, the
Qt values gradually increased with time, owing to HCl adsorption toward functional groups on each film. The C2-DMA film exhibited an equilibrium adsorption capacity (
Qe) value of 161.7 ± 2.8 ng/μg in the equilibrium state, and a 13.9-times-higher adsorption signal than C0-DMA film (11.6 ± 0.3 ng/μg). The significantly increased adsorption response depended highly on the functional group of the copolymer film. The tertiary amine groups of the DMAEMA could interact with HCl molecules through strong chemical bonds, forming tertiary amine salts, whereas the carbonyl groups of the DMAA may form relatively weak hydrogen bonds with HCl gas. The origin of different interactions resulted in vastly different desorption behaviors.
In order to confirm the desorption behavior of the two copolymer films, the recovery value (
Re) was calculated using the following equation:
where
Qe(HCl) is the equilibrium binding capacity after the binding process of HCl gas and
Qe(air) is the equilibrium binding capacity after flowing synthetic air. The adsorption signal of the C0-DMA film was almost restored to its original state, with a
Re value of 98.8%, during the air flowing process due to the easy desorption of HCl gas caused by a weak hydrogen-bonding interaction between carbonyl groups and HCl molecules (
Figure 2b). However, the
Re value of the C2-DMA film was extremely low, approximately 14.3%, because of an irreversible reaction between the tertiary amine groups of the copolymer film and HCl gas; in other words, this irreversible adsorption–desorption behavior was derived from strong ionic bonding between protonated tertiary amine groups of DMAEMA and chloride ions (
Figure 2a). The film might be recovered through post-treatment by immersing it in NaOH aqueous solution to eliminate the bound Cl
− ions [
22]. However, the C2-DMA film could be used only as disposable HCl gas; this is because of low film stability on the electrode in the recovery process. Similar adsorption–desorption behaviors were exhibited on both the films while flowing 100 ppm HCl gas for three-cycles (
Figure S3). The Δ
f value of C0-DMA was almost recovered as initial value according to the elapsed cycle. However, the Δ
f value of C2-DMA was accumulated by binding HCl gas without restoration during the desorption process. The
Re values for C2-DMA and C0-DMA were 95.3% and 12.4%, respectively.
In order to gain further understanding of the interaction mechanisms between polymer films and gas analytes, XPS wide-scan spectra were performed, with two different films, before and after HCl adsorption (
Figure S4). Three sharp peaks were observed in the C0-DMA film at 532.08 eV for O 1s, 400.08 eV for N 1s, and 285.08 eV for C 1s before the HCl adsorption process. After HCl adsorption, no signals appeared, and this was related to HCl adsorption, because of the detachment of unstable adsorbed HCl molecules in ambient conditions. However, a new peak corresponding to the Cl 2p signal was observed at 198.08 eV in the C2-DMA film after the adsorption process, confirming that the HCl analyte irreversibly interacted with DMAEMA. More specifically,
Figure 3a illustrates the high-resolution XPS N 1s spectra ranging between 394 and 406 eV for the two films. In the C0-DMA film, only one peak corresponding to the tertiary-amide nitrogen was observed at 399.78 eV for preadsorption and 399.48 eV for postadsorption. In contrast, the C2-DMA film exhibited one peak at 398.78 eV due to neutral nitrogen atoms in tertiary amine groups, whereas a new peak appeared at 401.58 eV, implying positively charged amine (N
+) in tertiary ammonium salt after the HCl adsorption process [
24]. Furthermore, the XPS Cl 2p scan spectra of the two films are presented in
Figure 3b. No noticeable peaks were observed on both the films at HCl preadsorption. However, after HCl adsorption occured on the C2-DMA film, two clear peaks including 2p
3/2 and 2p
1/2 chloride ion (Cl
−) were observed at 197.08 and 198.38, respectively [
25]. These results were reconfirmed, via FT-IR spectra, before and after HCl adsorption. As shown in
Figure 3c, a strong peak was observed at 1618 cm
−1, and this was associated with CO–N stretching on the IR spectrum of the C0-DMA film before HCl adsorption. The absorption bands at 1496, 1354, and 1138 cm
−1 are attributed to the C–H bending, C–N stretching, and C–O stretching, respectively. The two weak peaks can be assigned to the O–H stretching and C–H stretching at 3464 and 2927 cm
−1, respectively. Interestingly, two IR spectra of the C0-DMA film before and after HCl adsorption were almost overlapped, on the basis of the CO–N stretching peak at 1628 cm
−1, because there was no chemical interaction between HCl gas and the amide-based film. In the case of C2-DMA film, the absorption peaks were observed at 1723, 1455, and 1146 cm
−1 for C–O stretching, C–H bending, and C–O stretching vibration, respectively. The peaks in ranges of 2944–2769 cm
−1 were ascribed to the C–H stretching of tertiary amine. Remarkably, for the C2-DMA film, N–H and C–H stretching peaks appeared in ranges of 3410 to 2463 cm
−1, owing to protonation on tertiary amine groups, which is similar to the FT-IR peaks of pure triethylamine hydrochloride [
26].
As shown in
Figure 4a,b, HCl adsorption (2-h for C2-DMA and 10-min for C0-DMA) and desorption (10-min for both films) behaviors of crosslinked films were investigated while flowing three individual carrier gases, i.e., dried N
2 (RH 10%) or synthetic air (RH 10% or 50%), including 50-ppm dried HCl, to identify the effects of oxygen and moisture on the adsorption response. For the C2-DMA film, the
Qe values for 2-h HCl adsorption were 150.7 ± 7.1 ng/μg in N
2 and 148.0 ± 9.1 ng/μg in the air, respectively. Considering that the protonated tertiary ammonium salt ionically bonded with the HCl analyte during the adsorption process, the adsorption response did not exhibit any significant difference, and this finding occurred, notably, in the presence of oxygen in the HCl/air–gas mixture. After the desorption process through air gas flow, the
Qe value was not recovered, and this was because of the strong bonding of the HCl analyte to the film. At high RH (50%), the
Qe value was approximately 100-ng/μg higher than that at the other conditions (RH 10%), owing to the effect of a signal increase which was caused by water moisture adsorbed on the film [
27]. However, the
Qe value decreased to 168.8 ± 1.4 ng/μg after 10-min desorption process, nearly reaching the value reflected by adsorbed HCl mass itself, with no moisture effect, because the adsorbed moisture on the film was removed. In contrast, the
Qe value of the C0-DMA film in the dried air (7.26 ± 0.7 ng/μg) showed a 0.45-fold decrease compared with that in the N
2 gas (15.9 ± 3.5 ng/μg), and this was due to the interference effect of oxygen on the hydrogen bond between the carbonyl group and HCl. In addition, the increase in
Qe value caused by the adsorption of water molecules also occurred on the C0-DMA film at high RH (50%). After the desorption process,
Qe values became zero, indicating that few HCl molecules, weakly bonded on the film, were easily removed; thus, this film exhibited a worse adsorption response than the C2-DMA film.
As shown in
Figure 4c,d and
Figure S5, the 2-h adsorption responses, i.e.,
Qe and Δ
f values, were studied with respect to an HCl gas concentration ranging from 10 to 70 ppm in order to determine the concentration dependence of the two crosslinked films. As shown in
Figure 4c and
Figure S5a, the
Qe and Δ
f values of the C2-DMA film were significantly increased within the HCl concentration range of 10–50 ppm, and they were saturated at higher concentrations (>50 ppm). On the other hand, for the C0-DMA film, the adsorption responses gradually increased with an increase in the concentration of HCl gas (
Figure 4d and
Figure S5b). A linear regression curve of the C2-DMA film, for adsorption at a concentration range of less than 50 ppm, is shown in the inset of
Figure 4c. Its sensitivity was calculated to be 2.51 (ng/μg)(1/ppm) with a coefficient of determination (
R2) of 0.977, indicating that it was 14.8-fold higher than the C0-DMA film with 0.17 (ng/μg)(1/ppm) and
R2 = 0.984. The sensitivity of the C2-DMA film could also be obtained from the linear regression curve in the plot of Δ
f value as a function of HCl concentration in ranges of 10–50 ppm (
Figure S6). The sensitivity (13 Hz/ppm) of the C2-DMA film was much higher than other sensors in previously reported studies (
Table S1). Furthermore, the limit of quantitation (LOQ), and the limit of detection (LOD), for the two copolymer films were determined using equations such as LOQ = 10 S/m and LOD = 3.3 S/m, where S represents the standard deviation of the y-intercept, and m represents the slope of the linear calibration curve [
28,
29]. LOQ and LOD values of 1.67 and 0.55 ppm were obtained for the C2-DMA film, and 4.84 and 1.59 ppm for the C0-DMA film, respectively.
Furthermore, the Langmuir and Freundlich isotherm models were used to compare the adsorption behavior of the two films. The Langmuir isotherm model assumes that adsorption behavior is based on monolayer adsorption on a solid surface, which has a homogeneous surface for the target molecules. However, the Freundlich isotherm describes multilayer adsorption for a heterogeneous surface. Equations (3) and (4) describe the Langmuir and Freundlich models, respectively [
30,
31]:
where
Qe (mg/g) is the equilibrium adsorption capacity,
Ce (mg/L) is the equilibrium concentration of HCl during adsorption,
Qm (mg/g) is the maximum adsorption capacity, 1/
n is a factor used to evaluate surface heterogeneity, and
kL and
kF represent the Langmuir and Freundlich adsorption equilibrium constants, respectively.
Figure 5 depicts the nonlinear fitted curves of the Langmuir isotherm and Freundlich isotherm models, respectively, pertaining to HCl adsorbed on the surface of the two films during the adsorption process. The parameters of the two isotherm models are presented in
Table S2. Based on
R2 values, the experimental data points of the C2-DMA film were better fitted to the Langmuir model than the Freundlich isotherm model. This result indicates that only one HCl molecule is adsorbed at a tertiary amine group of the copolymer film. For the C0-DMA film, the experimental data points were not fitted to both models due to low
Qe values caused by the small adsorption response for HCl adsorption. According to these results, the experimental data of the C2-DMA film were well fitted to the Langmuir linear model because the copolymer film is based on a stoichiometric chemical reaction with HCl gas.
In order to evaluate the selective adsorption performance of the two crosslinked films, Δ
f values by gas adsorption and/or desorption were monitored, flowing 70 ppm each gas of HCHO and HF, including HCl in a chamber until the sensing signal was stabilized in the process of adsorption (2-h for C2-DMA and 10-min for C0-DMA) and desorption (10-min for both films) (
Figure 6). On the C2-DMA film, the Δ
f value with HCl gas adsorption reached approximately −767 Hz, whereas the value was maintained at −61.6 ± 6.6 Hz for HCHO gas adsorption and −57.4 ± 11.4 Hz for HF gas adsorption in a 2-h gas flow. With regard to the desorption process under air gas flow, the Δ
f value was slightly decreased before it stabilized because of the irreversible binding of HCl molecules. However, the Δ
f value was approximately zero in nonselective adsorption of other gases (
Figure 6a). Furthermore, on the C2-DMA film,
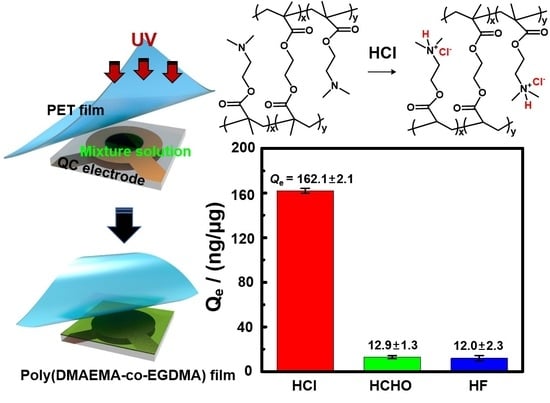
Qe values were 12.9 ± 1.4 and 12.0 ± 2.4 ng/μg for the HCHO and HF gases, respectively, which are extremely low compared with the adsorption response of HCl gas (162.1 ± 2.2 ng/μg) (
Figure 6a). The selectivity coefficient (
k*), as the ratio of the
Qe,HCl/
Qe,(HCHO or HF), was especially high, with numbers of 12.6 and 13.5 for the HCHO and HF gases, respectively, because HCl gas reacts by protonation and ionic bonding toward the tertiary amine groups. In addition, the
Re values for HCHO and HF were 103.5% and 99.9% after the desorption process of the adsorbed toxic gas, and this was due to the breakdown of hydrogen-bonding interactions. In contrast, the adsorption response on the C0-DMA film for 10-min was extremely low, with a Δ
f value between −39 and −61 Hz, regardless of the type of toxic gas.
Qe values were 11.6 ± 0.3, 9.8 ± 0.4, and 8.0 ± 0.3 ng/μg for the HCl, HCHO, and HF gases, respectively; the
k* values of 1.2 for HCHO and 1.5 for HF were significantly lower than those of the C2-DMA film. Thus, the use of an amine-based crosslinked film could lead to a highly sensitive response to, and an improved selectivity for, HCl gas.