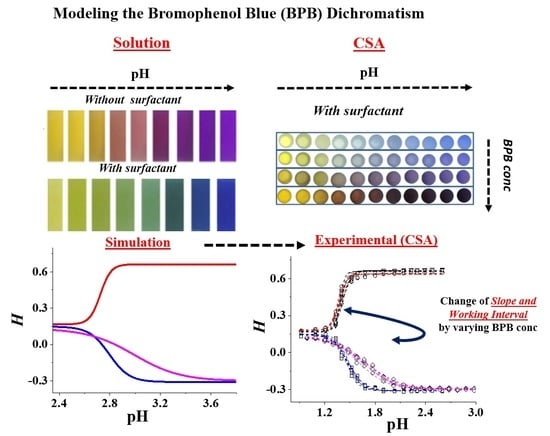
Modeling the Dichromatic Behavior of Bromophenol Blue to Enhance the Analytical Performance of pH Colorimetric Sensor Arrays
Abstract
Share and Cite
Pastore, A.; Badocco, D.; Cappellin, L.; Pastore, P. Modeling the Dichromatic Behavior of Bromophenol Blue to Enhance the Analytical Performance of pH Colorimetric Sensor Arrays. Chemosensors 2022, 10, 87. https://doi.org/10.3390/chemosensors10020087
Pastore A, Badocco D, Cappellin L, Pastore P. Modeling the Dichromatic Behavior of Bromophenol Blue to Enhance the Analytical Performance of pH Colorimetric Sensor Arrays. Chemosensors. 2022; 10(2):87. https://doi.org/10.3390/chemosensors10020087
Chicago/Turabian StylePastore, Andrea, Denis Badocco, Luca Cappellin, and Paolo Pastore. 2022. "Modeling the Dichromatic Behavior of Bromophenol Blue to Enhance the Analytical Performance of pH Colorimetric Sensor Arrays" Chemosensors 10, no. 2: 87. https://doi.org/10.3390/chemosensors10020087
APA StylePastore, A., Badocco, D., Cappellin, L., & Pastore, P. (2022). Modeling the Dichromatic Behavior of Bromophenol Blue to Enhance the Analytical Performance of pH Colorimetric Sensor Arrays. Chemosensors, 10(2), 87. https://doi.org/10.3390/chemosensors10020087