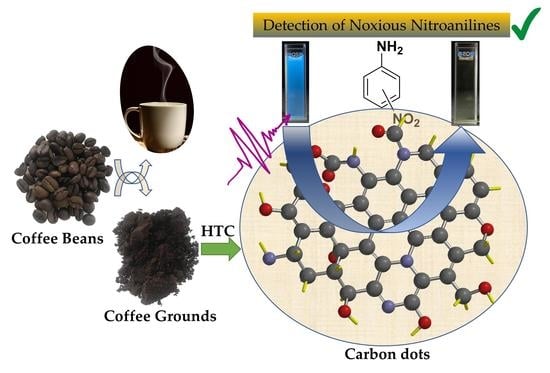
Carbon Dots from Coffee Grounds: Synthesis, Characterization, and Detection of Noxious Nitroanilines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of CGs and CBs
2.3. Instruments and Methods
2.4. General Procedures for the Synthesis of C-Dots from Coffee Grounds
2.4.1. Hydrothermal Carbonization (HTC) Method
2.4.2. Microwave-Assisted Hydrothermal Carbonization (Mw-HTC) Method
2.4.3. Structural Characterization
3. Results
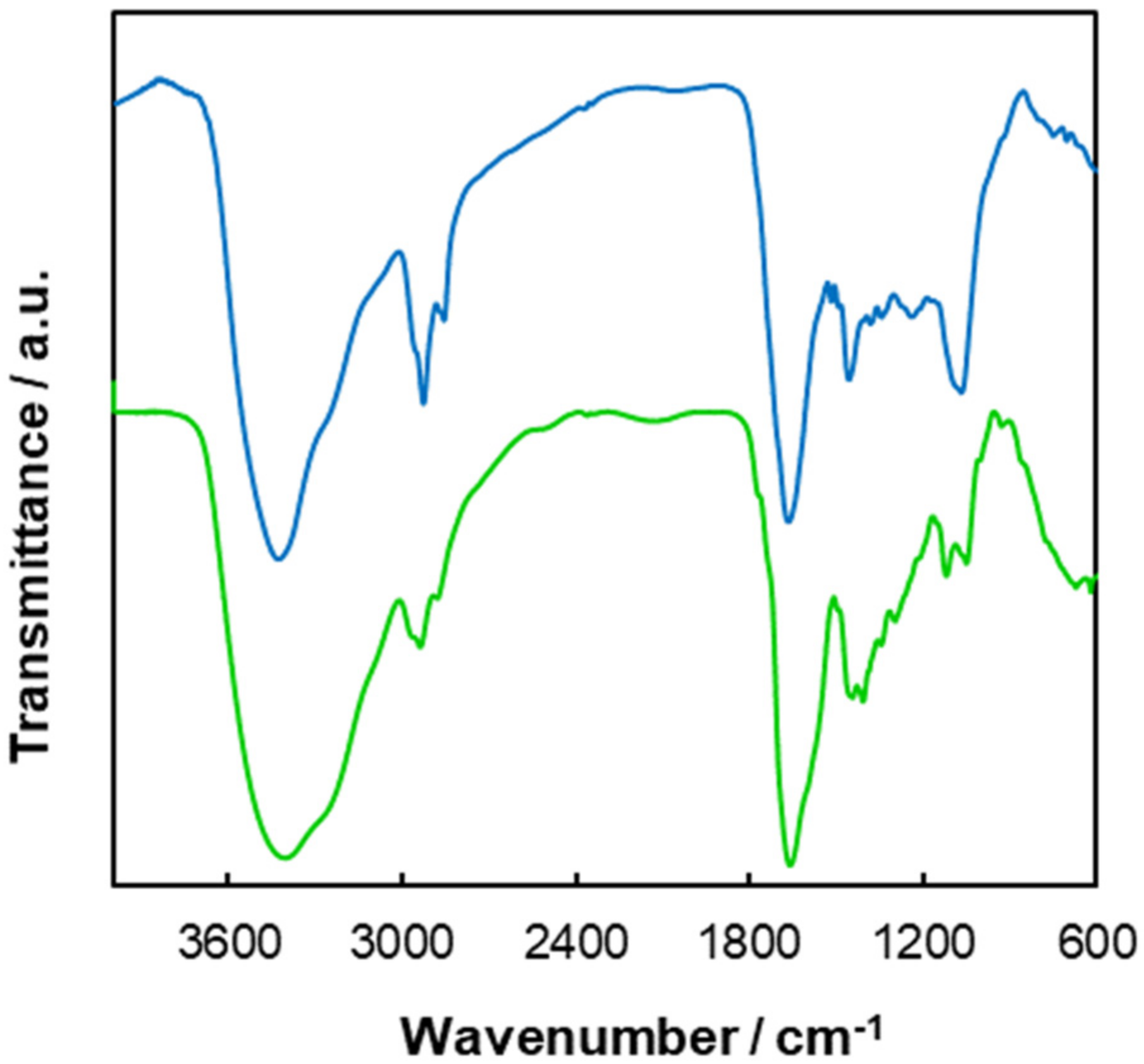
3.1. Coffee Grounds and Coffee Beans Characterization
3.2. Synthesis and Structural Characterization of C-Dots from Coffee Grounds
3.2.1. C-Dots Synthesis by the HTC Method
3.2.2. C-Dots Synthesis by the Mw-HTC Method
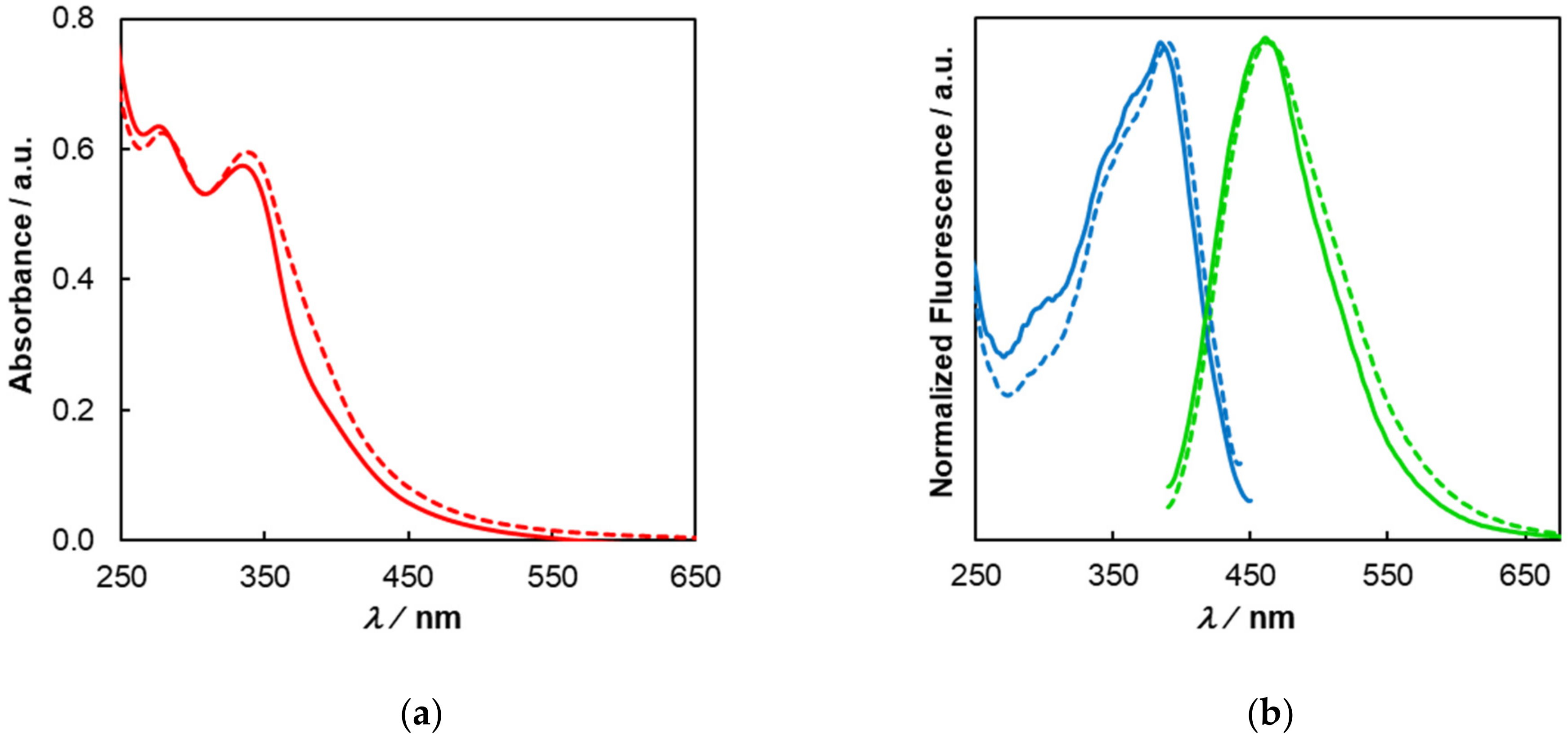
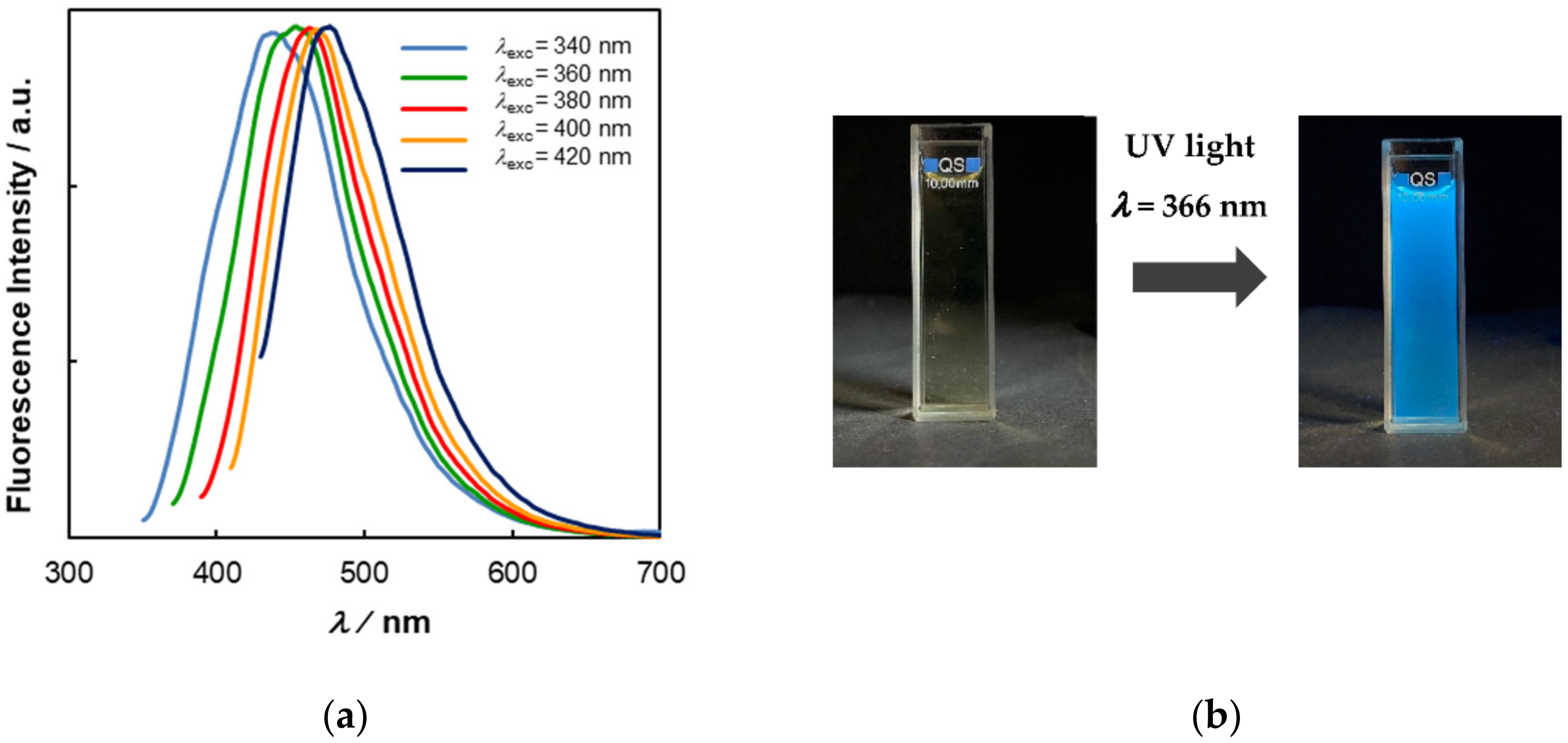
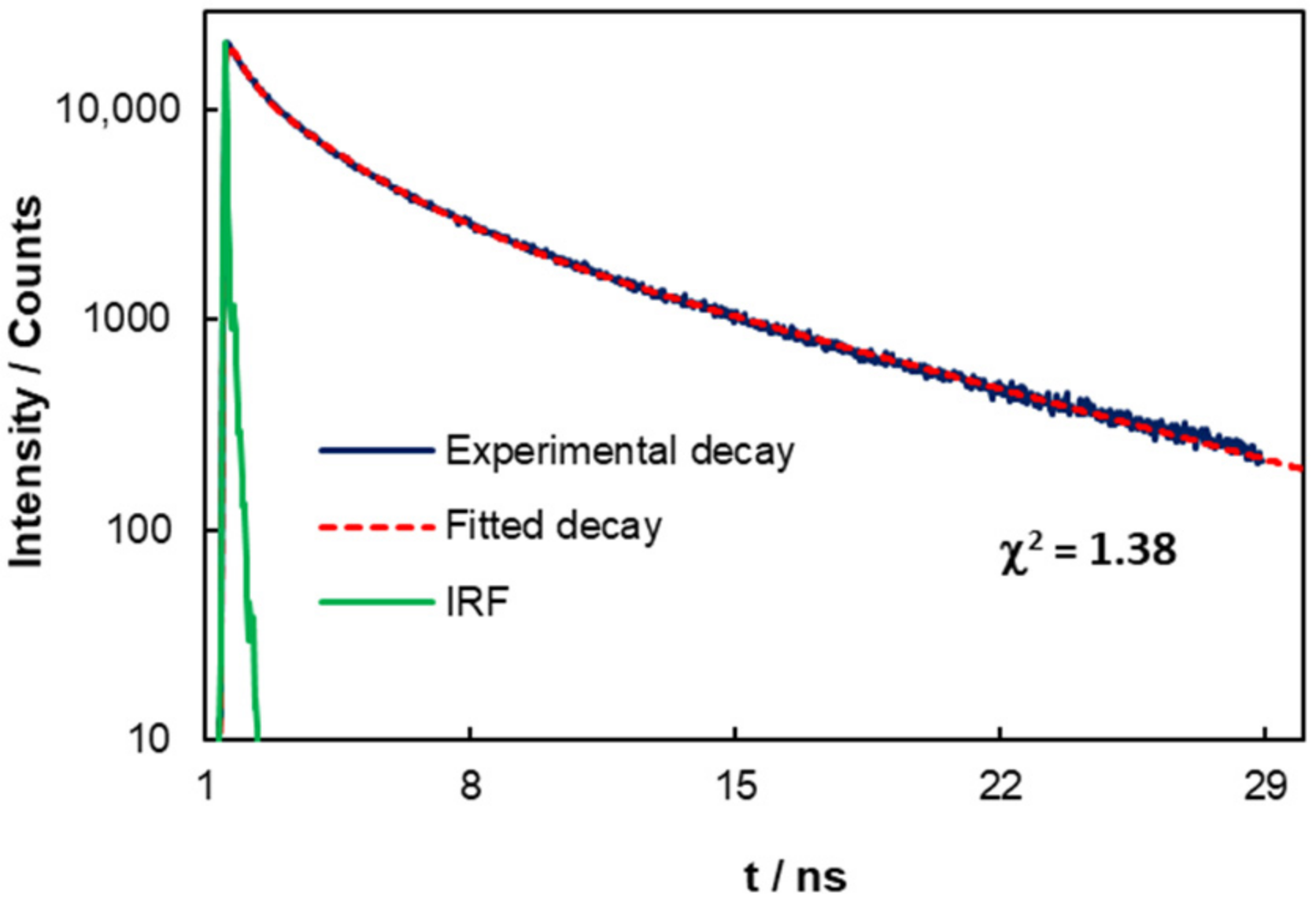
3.3. Photophysical Properties of C-Dots from Coffee Grounds
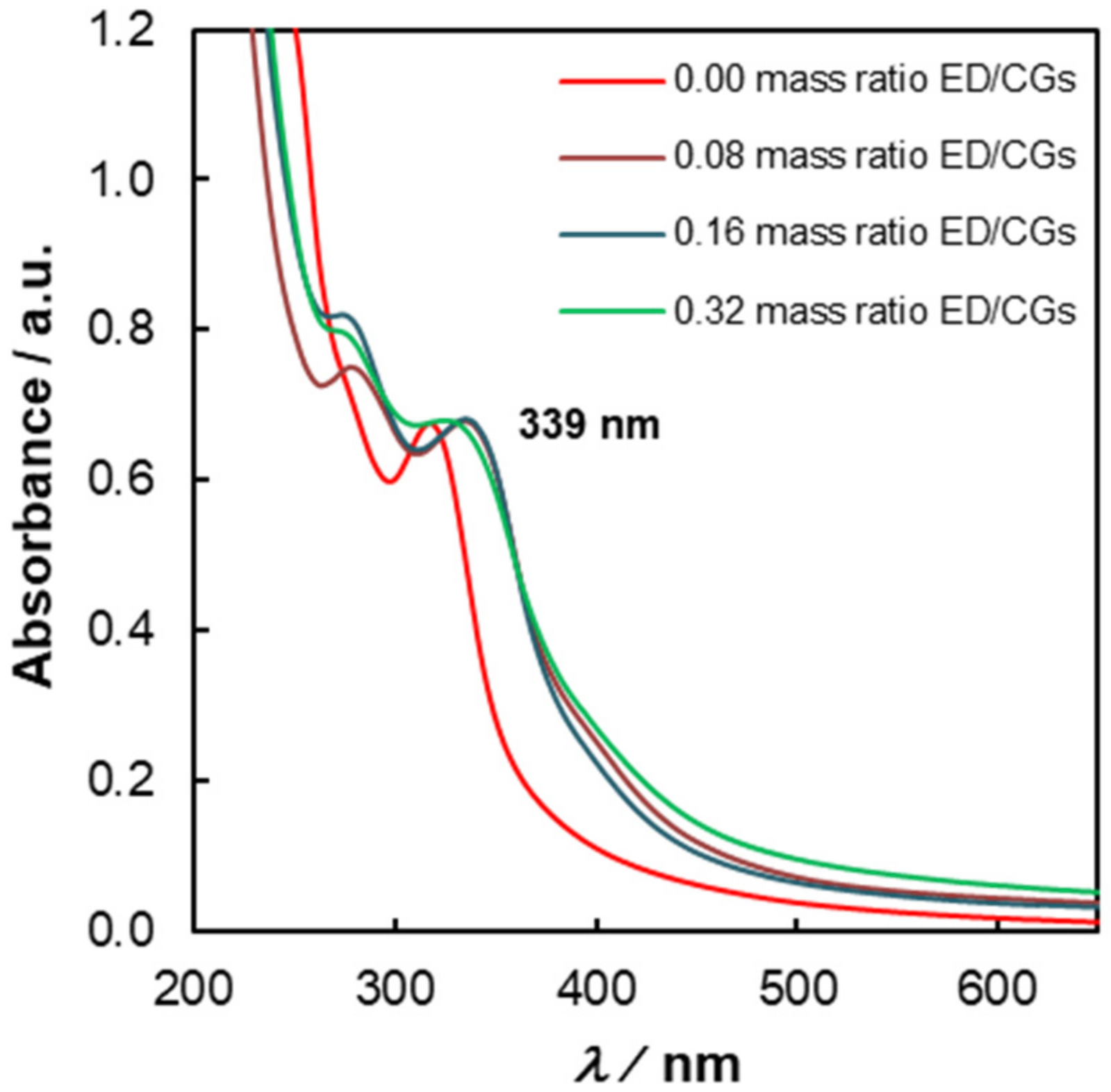
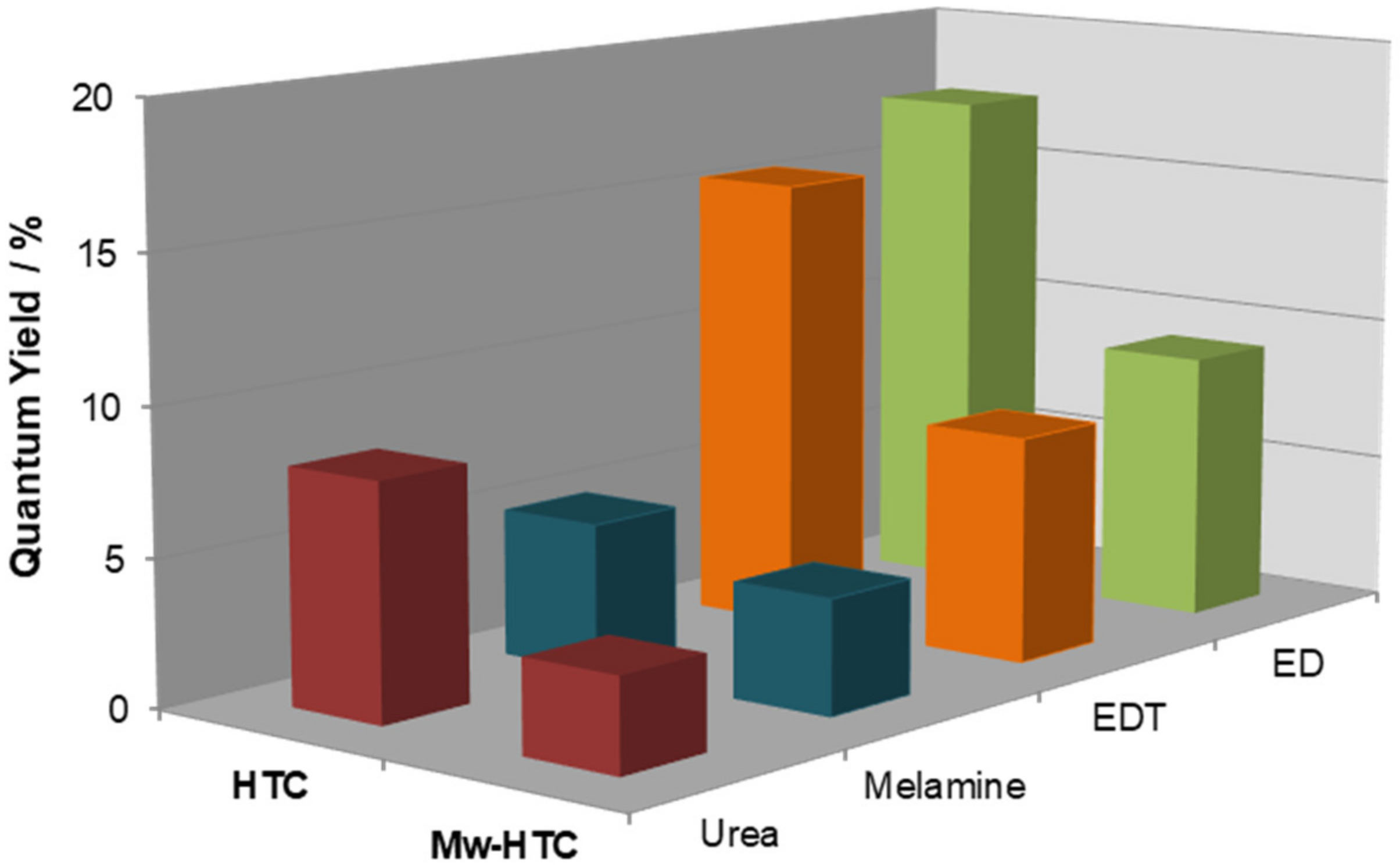
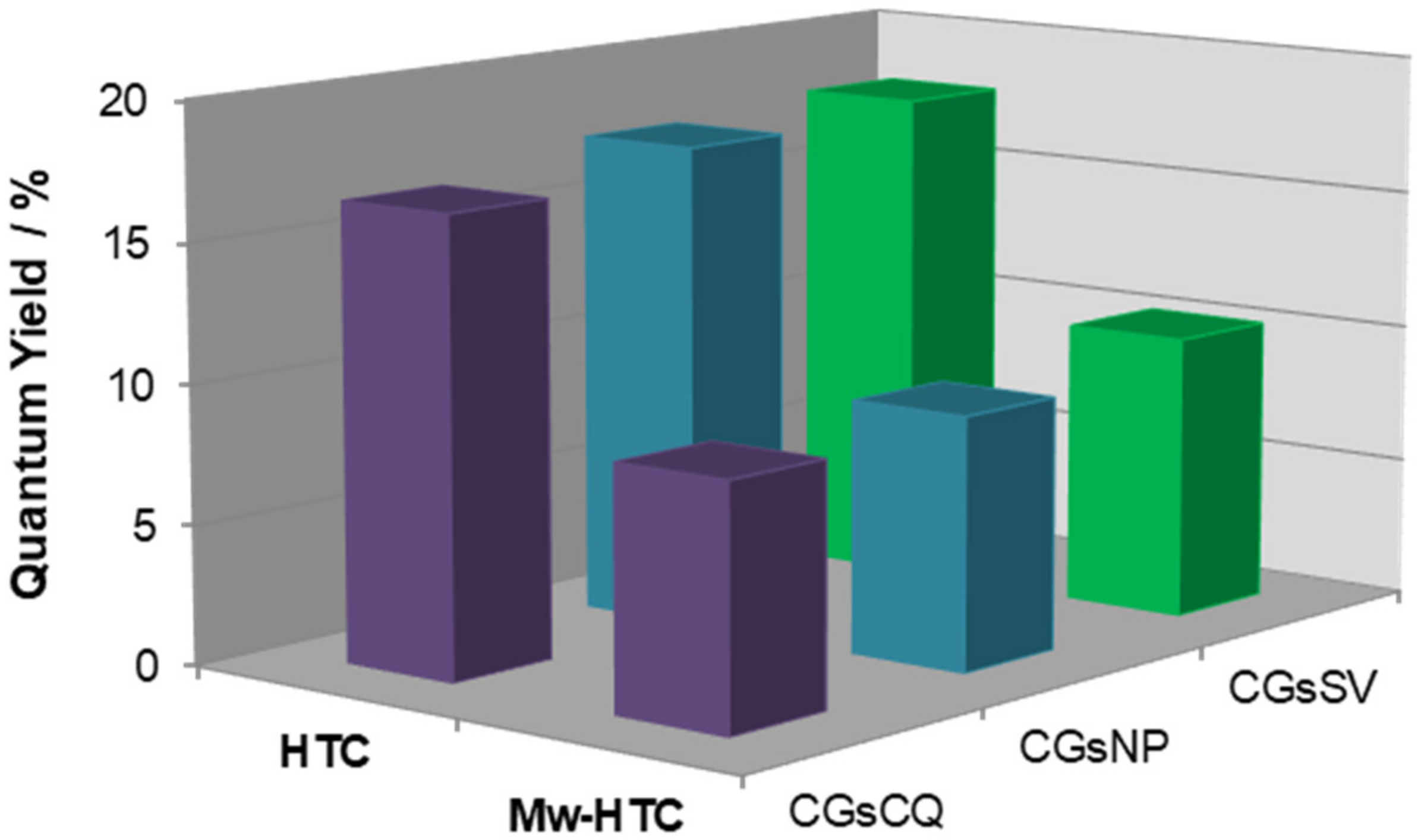
3.4. Influence of Reaction Parameters on the Optical Properties of C-Dots from Coffee Grounds
3.5. Detection of Nitroanilines
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gayen, B.; Palchoudhury, S.; Chowdhury, J. Carbon Dots: A Mystic Star in the World of Nanoscience. J. Nanomater. 2019, 2019, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Jagdale, P.; Ziegler, D.; Rovere, M.; Tulliani, J.M.; Tagliaferro, A. Waste Coffee Ground Biochar: A Material for Humidity Sensors. Sensors 2019, 19, 801–816. [Google Scholar] [CrossRef] [Green Version]
- Du, F.; Zhang, M.; Li, X.; Li, J.; Jiang, X.; Li, Z.; Hua, Y.; Shao, G.; Jin, J.; Shao, Q.; et al. Economical and green synthesis of bagasse derived fluorescent carbon dots for biomedical applications. Nanotechnology 2014, 25, 315702–315711. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tian, J.; Wang, L.; Zhang, Y.; Qin, X.; Luo, Y.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Hydrothermal Treatment of Grass: A Low-Cost, Green Route to Nitrogen-Doped, Carbon-Rich, Photoluminescent Polymer Nanodots as an Effective Fluorescent Sensing Platform for Label-Free Detection of Cu(II) Ions. Adv. Mat. 2012, 24, 2037–2041. [Google Scholar] [CrossRef] [PubMed]
- Baig, N.; Kammakakam, I.; Falatha, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Kurian, M.; Pau, A. Recent trends in the use of green sources for carbon dot synthesis—A short review. Carbon Trends 2021, 3, 100032. [Google Scholar] [CrossRef]
- Pan, M.; Xie, X.; Liu, K.; Yang, J.; Hong, L.; Wang, S. Fluorescent Carbon Quantum Dots—Synthesis, Functionalization and Sensing Application in Food Analysis. Nanomaterials 2020, 10, 930. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef]
- Tuerhong, M.; Yang, X.; Xue-Bo, Y. Review on Carbon Dots and Their Applications. Chin. J. Anal. Chem. 2017, 45, 139–150. [Google Scholar] [CrossRef]
- Liu, M.L.; Chen, B.B.; Li, C.M.; Huang, C.Z. Carbon dots: Synthesis, formation mechanism, fluorescence origin and sensing applications. Green Chem. 2019, 21, 449–471. [Google Scholar] [CrossRef]
- Lin, F.; Li, C.; Chen, Z. Bacteria-Derived Carbon Dots Inhibit Biofilm Formation of Escherichia coli without Affecting Cell Growth. Front. Microbiol. 2015, 9, 259. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Das, J. Small molecules derived carbon dots: Synthesis and applications in sensing, catalysis, imaging, and biomedicine. J. Nanobiotechnol. 2019, 17, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasouni, A.; Chatzimitakos, T.; Stalikas, C. Bioimaging Applications of Carbon Nanodots: A Review. C 2019, 5, 19. [Google Scholar] [CrossRef] [Green Version]
- Rosso, C.; Filippini, G.; Prato, M. Carbon Dots as Nano-Organocatalysts for Synthetic Applications. ACS Catal. 2020, 10, 8090–8105. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, T.; Liang, R.; Wei, M. Application of Zero-Dimensional Nanomaterials in Biosensing. Front. Chem. 2020, 8, 320. [Google Scholar] [CrossRef]
- Stepanidenko, E.A.; Ushakova, E.V.; Fedorov, A.V.; Rogach, A.L. Applications of Carbon Dots in Optoelectronics. Nanomaterials 2021, 11, 364. [Google Scholar] [CrossRef]
- Jelinek, R. Materials Science Applications of Carbon-Dots. In Carbon Quantum Dots, Carbon Nanostructures; Springer International Publishing: Cham, Switzerland, 2017; pp. 93–114. [Google Scholar] [CrossRef]
- El-Shabasy, R.M.; Elsadek, M.F.; Ahmed, B.M.; Farahat, M.F.; Mosleh, K.N.; Taher, M.M. Recent Developments in Carbon Quantum Dots: Properties, Fabrication Techniques, and Bio-Applications. Processes 2021, 9, 388. [Google Scholar] [CrossRef]
- Das, R.; Bandyopadhyay, R.; Pramanik, P. Carbon quantum dots from natural resource: A review. Mater. Today Chem. 2018, 8, 96–109. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Vinodh, R.; Sundramoorthy, A.K.; Babu, R.S.; Lee, Y.R. Leftover Kiwi Fruit Peel-Derived Carbon Dots as a Highly Selective Fluorescent Sensor for Detection of Ferric Ion. Chemosensors 2021, 9, 166. [Google Scholar] [CrossRef]
- Kang, C.; Huang, Y.; Yang, H.; Yan, X.F.; Chen, Z.P. A Review of Carbon Dots Produced from Biomass Wastes. Nanomaterials 2020, 10, 2316. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Zeng, L.; Cao, X.; Wang, Q.; Wang, X.; Sun, R. Sustainable carbon quantum dots from forestry and agricultural biomass with amplified photoluminescence by simple NH4OH passivation. J. Mater. Chem. C 2014, 2, 9760–9766. [Google Scholar] [CrossRef]
- Oliveira, B.P.; Abreu, F.O.M.S. Carbon Quantum Dots Synthesis from Waste and by-products: Perspectives and challenges. Mater. Lett. 2020, 282, 128764. [Google Scholar] [CrossRef]
- Sharma, V.; Tiwari, P.; Mobin, S.M. Sustainable Carbon-dots: Recent advances in green carbon dots for sensing and bioimaging. J. Mater. Chem. B 2017, 5, 8904–8924. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Li, D.; Liu, Y.; Xu, X.; Xiong, C. Nitrogen-Doped Carbon Dots from Plant Cytoplasm as Selective and Sensitive Fluorescent Probes for Detecting p-Nitroaniline in Both Aqueous and Soil Systems. Analyst 2015, 140, 1428–1431. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, M.R.; Costa, A.I.; Berberan-Santos, M.N.; Prata, J.V. Finding Value in Wastewaters from the Cork Industry: Carbon Dots Synthesis and Fluorescence for Hemeprotein Detection. Molecules 2020, 25, 2320–2337. [Google Scholar] [CrossRef]
- Prata, J.V.; Alexandre, M.R.; Costa, A.I. Produção de Nanomateriais de Carbono Luminescentes a partir de Águas de Cozedura Industriais da Cortiça. (Production of Luminescent Carbon Nanomaterials from Cork Industry Wastewaters). Portuguese Patent NO. 109379, 10 May 2016. [Google Scholar]
- Sousa, D.A.; Costa, A.I.; Alexandre, M.R.; Prata, J.V. How an Environmental Issue could Turn into Useful High-valued Products: The Olive Mill Wastewater Case. Sci. Total Environ. 2019, 647, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.V.; Costa, A.I. Nanomateriais de Carbono Luminescentes obtidos por Conversão Química das Águas Residuais do Processamento Industrial do Azeite (Luminescent Carbon Nanomaterials by Chemical Conversion of Olive Mill Wastewaters). Portuguese Patent NO. 110131, 8 June 2017. [Google Scholar]
- Coffee Market Report, December 2021, International Coffee Organization. Available online: https://www.ico.org/Market-Report-21-22-e.asp (accessed on 5 January 2022).
- Figueroa, G.A.; Homann, T.; Rawel, H.M. Coffee Production Wastes: Potentials and Perspectives. Austin Food Sci. 2016, 1, 1014–1018. [Google Scholar]
- Mussatto, S.I.; Machado, E.M.S.; Martins, S.; Teixeira, J.A. Production, Composition, and Application of Coffee. Food Bioproc. Tech. 2011, 4, 661–672. [Google Scholar] [CrossRef] [Green Version]
- Tokimoto, T.; Kawasaki, N.; Nakamura, T.; Akutagawa, J.; Tanada, S.J. Removal of lead ions in drinking water by coffee grounds as vegetable biomass. J. Colloid Interface Sci. 2005, 281, 56–61. [Google Scholar] [CrossRef]
- Echeverria, M.C.; Nuti, M. Valorisation of the Residues of Coffee Agro-industry: Perspectives and Limitations. Open Waste Manag. J. 2017, 10, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Unni, M.S.; George, L.; Bhange, S.N.; Devi, N.; Kurungot, S. Valorization of coffee bean waste: A coffee bean waste derived multifunctional catalyst for photocatalytic hydrogen production and electrocatalytic oxygen reduction reactions. RSC Adv. 2016, 6, 82103–82111. [Google Scholar] [CrossRef]
- Hsu, P.C.; Shih, Z.Y.; Lee, C.H.; Chang, H.T. Synthesis and analytical applications of photoluminescent carbon nanodots. Green Chem. 2012, 14, 917–920. [Google Scholar] [CrossRef]
- Wang, L.; Li, W.; Wu, B.; Li, Z.; Wang, S.; Liu, Y.; Pan, D.; Wu, M. Facile synthesis of fluorescent graphene quantum dots from coffee grounds for bioimaging and sensing. Chem. Eng. J. 2016, 300, 75–82. [Google Scholar] [CrossRef]
- Ge, L.; Yu, H.; Ren, H.; Shi, B.; Guo, Q.; Gao, W.; Li, Z.; Li, J. Photoluminescence of carbon dots and their applications in Hela cell imaging and Fe3+ ion detection. J. Mater. Sci. 2017, 52, 9979–9989. [Google Scholar] [CrossRef]
- Xu, H.; Xie, L.; Hakkarainen, M. Coffee-Ground-Derived Quantum Dots for Aqueous Processable Nanoporous Graphene Membranes. ACS Sustain. Chem. Eng. 2017, 5, 5360–5367. [Google Scholar] [CrossRef]
- Chen, J.; Du, H.; Xu, Y.; Ma, B.; Zheng, Z.; Li, P.; Jiang, Y. A turn-on fluorescent sensor based on coffee-ground carbon dots for the detection of sodium cyclamate. J. Mater. Sci.: Mater. Electron. 2021, 32, 13581–13587. [Google Scholar] [CrossRef]
- Honga, W.T.; Yang, H.K. Anti-counterfeiting application of fluorescent carbon dots derived from wasted coffee grounds. Optik 2021, 241, 16644. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Hajimahmoodi, M.; Moghaddam, G.; Ranjbar, A.M.; Khazani, H.; Sadeghi, N.; Oveisi, M.R.; Jannat, B. Total phenolic, flavonoids, tannin content and antioxidant power of some iranian pomegranate flower cultivars (Punica granatum L.). Am. J. Plant Sci. 2013, 4, 1815–1820. [Google Scholar] [CrossRef] [Green Version]
- Pekal, A.; Pyrynska, K. Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef] [Green Version]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Sedmak, J.J.; Grossberg, S.E. A rapid, sensitive, and versatile assay for protein using Coomassie Brilliant Blue G250. Anal. Biochem. 1977, 79, 544–552. [Google Scholar] [CrossRef]
- Rabemanolontsoa, H.; Ayada, S.; Saka, S. Quantitative method applicable for various biomass species to determine their chemical composition. Biomass Bioenerg. 2011, 35, 4630–4635. [Google Scholar] [CrossRef] [Green Version]
- Anon. A Guide to Recording Fluorescence Quantum Yields, Horiba Scientific. 2018. Available online: http://www.horiba.com/fileadmin/uploads/Scientific/Documents/Fluorescence/quantumyieldstrad.pdf (accessed on 10 January 2022).
- Borissevitch, I.E. More about the inner filter effect: Corrections of Stern-Volmer fluorescence quenching constants are necessary at very low optical absorption of the quencher. J. Lumin. 1999, 81, 219–224. [Google Scholar] [CrossRef]
- Long, G.L.; Winefordner, J.D. Limit of detection a closer look at the IUPAC definition. Anal. Chem. 1983, 55, 712–724. [Google Scholar] [CrossRef]
- Consonni, R.; Cagliani, L.R.; Cogliati, C. NMR based geographical characterization of roasted coffee. Talanta 2012, 88, 420–426. [Google Scholar] [CrossRef]
- Miller, F.A.; Wilkins, C.H. Infrared spectra and characteristic frequencies of inorganic ions. Anal. Chem. 1952, 24, 1253–1294. [Google Scholar] [CrossRef]
- Hola, K.; Bourlinos, A.B.; Kozak, O.; Berka, K.; Siskova, K.M.; Havrdova, M.; Tucek, J.; Safarova, K.; Otyepka, M.; Giannelis, E.P. Photoluminescence effects of graphitic core size and surface functional groups in carbon dots: COO- induced red-shift emission. Carbon 2014, 70, 279–286. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Feng, X.; Zhang, F.; Yang, Y.; Liu, X. Effect of reaction temperature on structure and fluorescence properties of nitrogen-doped carbon dots. Appl. Surf. Sci. 2016, 387, 1236–1246. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, S.; Zhang, S.; Fu, Y.; Wang, L.; Zhao, X.; Yang, B. Investigation from chemical structure to photoluminescent mechanism: A type of carbon dots from the pyrolysis of citric acid and an amine. J. Mater. Chem. C 2015, 3, 5976–5984. [Google Scholar] [CrossRef]
- Shuker, L.K.; Batt, S.; Rystedt, I.; Berlin, M. The Health Effects of Aromatic Amines—A Review, A Technological Report; Monitoring and Assessment Research Centre: Cambridge, MA, USA, 1986; Available online: https://apps.who.int/iris/handle/10665/40073 (accessed on 26 January 2022).
- Strong, C.; Guo, Y.; Liu, W. Simultaneous determination of five nitroaniline and dinitroaniline isomers in wastewaters by solid-phase extraction and high-performance liquid chromatography with ultraviolet detection. Chemosphere 2010, 81, 430–435. [Google Scholar] [CrossRef]
- Lin, X.; Ni, Y.; Kokot, S. Voltammetric analysis with the use of a novel electro-polymerised graphene-nafion film modified glassy carbon electrode: Simultaneous analysis of noxious nitroaniline isomers. J. Hazard. Mater. 2012, 243, 232–241. [Google Scholar] [CrossRef]
- Ji, N.-N.; Shi, Z.-Q.; Hu, H.-L.; Zheng, H.-G. A triphenylamine-functionalized luminescent sensor for efficient p-nitroaniline detection. Dalton Trans. 2018, 47, 7222–7228. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Bu, M.; You, X.; Dai, Z.; Shi, J. High-performance detection of p-nitroaniline on defect-graphene SERS substrate utilizing molecular imprinting technique. Microchem. J. 2021, 168, 106536. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: Berlin, Germany, 2006; ISBN 978-0387-31278-1. [Google Scholar]
- Oudar, J.L.; Chemla, D.S. Hyperpolarizabilities of nitroanilines. J. Chem. Phys. 1977, 66, 2664–2668. [Google Scholar] [CrossRef]
- Wang, J.H.; Li, G.Y.; Liu, X.J.; Feng, R.; Zhang, H.J.; Zhang, S.Y.; Zhang, Y.H. A fluorescent anthracene-based metal-organic framework for highly selective detection of nitroanilines. Inorg. Chim. Acta 2018, 473, 70–74. [Google Scholar] [CrossRef]



| Parameter | Coffee Grounds | Coffee Beans | ||||
|---|---|---|---|---|---|---|
| CGsSV | CGsCQ | CGsNP | CBsSV | CBsCQ | CBsNP | |
| TS-WE (g/100 g) | 6.85 | 7.41 | 12.45 | 21.77 | 22.38 | 28.20 |
| pH (WE) | 5.0 | 5.6 | 5.1 | - | - | - |
| Ashes (%) | 1.21 | 1.83 | 1.50 | 4.55 | 4.19 | 4.39 |
| Microanalysis | C, 45.95%; H, 5.11%; N, 3.53% O (calc.), 44.20%; S < 0.3% | - | C, 45.86%; H, 5.26%; N, 4.16% O (calc.), 43.21%; S < 0.3% | C, 49.68%; H, 5.33%; N, 5.16% O (calc.), 35.28%; S < 0.3% | - | C, 47.44%; H, 5.24%; N, 4.69% O (calc.), 38.24%; S < 0.3% |
| Entry | T (°C) | ΦF (λexc = 380 nm) | λem max (nm) | ηmass (%) |
|---|---|---|---|---|
| 1 | 200 | 0.068 | 464 | 15.5 |
| 2 | 250 | 0.12 | 461 | 16.5 |
| 3 | 300 | 0.18 | 449 | 9.8 |
| Entry | Additive | ΦF (λexc = 380 nm) | ηmass (%) |
|---|---|---|---|
| 1 | - | 0.05 | 10.0 |
| 2 | ED | 0.18 | 16.0 |
| 3 | Melamine | 0.05 | 10.3 |
| 4 | Urea | 0.08 | 10.8 |
| 5 | DET | 0.15 | 20.4 |
| Entry | Mass Ratio ED/CGs | ΦF (λexc = 380 nm) | ηmass (%) |
|---|---|---|---|
| 1 | - | 0.05 | 10.0 |
| 2 | 0.08 | 0.09 | 14.5 |
| 3 | 0.16 | 0.12 | 16.5 |
| 4 | 0.32 | 0.14 | 25.5 |
| Entry | Analyte | Ksv/M−1 | LOD/ppb |
|---|---|---|---|
| 1 | o-NA | 6.00 ± 0.05 × 103 (R2 = 0.994) | 129 |
| 2 | m-NA | 2.21 ± 0.02 × 103 (R2 = 0.969) | 350 |
| 3 | p-NA | 1.13 ± 0.10 × 104 (R2 = 0.992) | 68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, A.I.; Barata, P.D.; Moraes, B.; Prata, J.V. Carbon Dots from Coffee Grounds: Synthesis, Characterization, and Detection of Noxious Nitroanilines. Chemosensors 2022, 10, 113. https://doi.org/10.3390/chemosensors10030113
Costa AI, Barata PD, Moraes B, Prata JV. Carbon Dots from Coffee Grounds: Synthesis, Characterization, and Detection of Noxious Nitroanilines. Chemosensors. 2022; 10(3):113. https://doi.org/10.3390/chemosensors10030113
Chicago/Turabian StyleCosta, Alexandra I., Patrícia D. Barata, Bianca Moraes, and José V. Prata. 2022. "Carbon Dots from Coffee Grounds: Synthesis, Characterization, and Detection of Noxious Nitroanilines" Chemosensors 10, no. 3: 113. https://doi.org/10.3390/chemosensors10030113
APA StyleCosta, A. I., Barata, P. D., Moraes, B., & Prata, J. V. (2022). Carbon Dots from Coffee Grounds: Synthesis, Characterization, and Detection of Noxious Nitroanilines. Chemosensors, 10(3), 113. https://doi.org/10.3390/chemosensors10030113