How Surface-Enhanced Raman Spectroscopy Could Contribute to Medical Diagnoses
Abstract
:1. Introduction
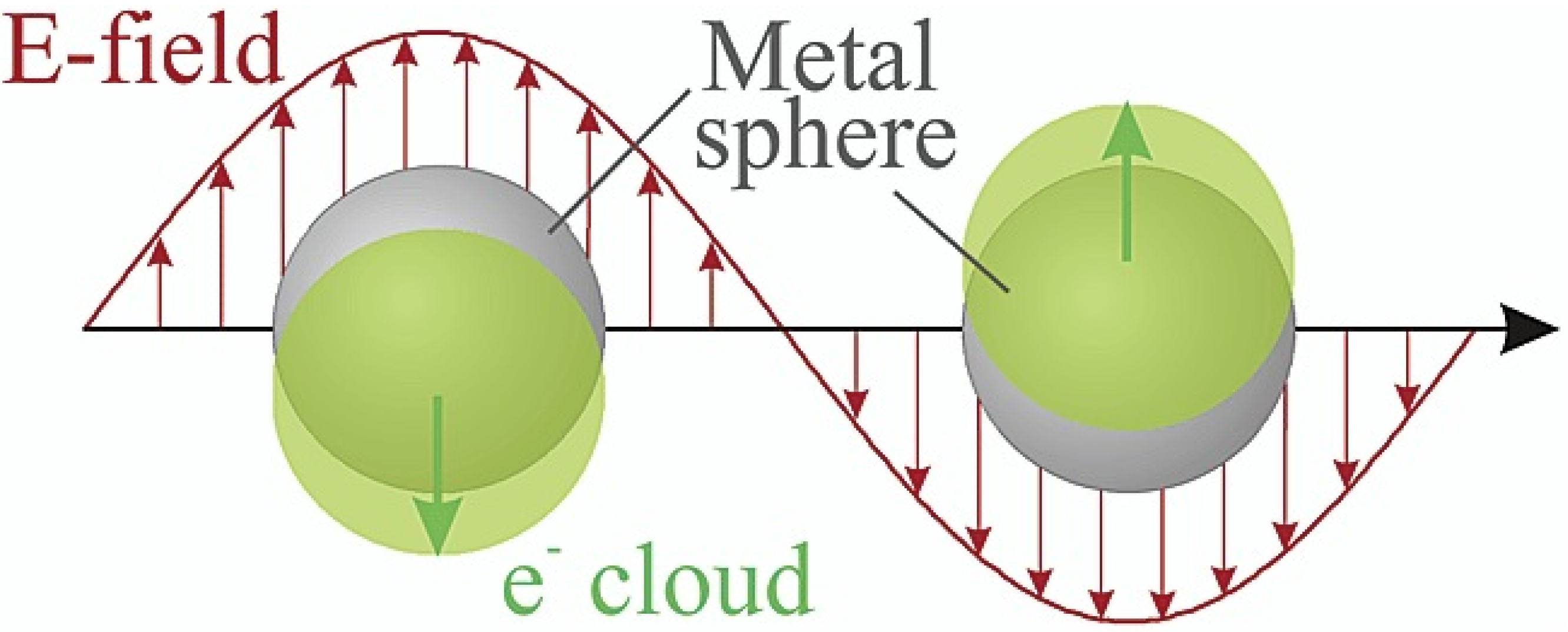
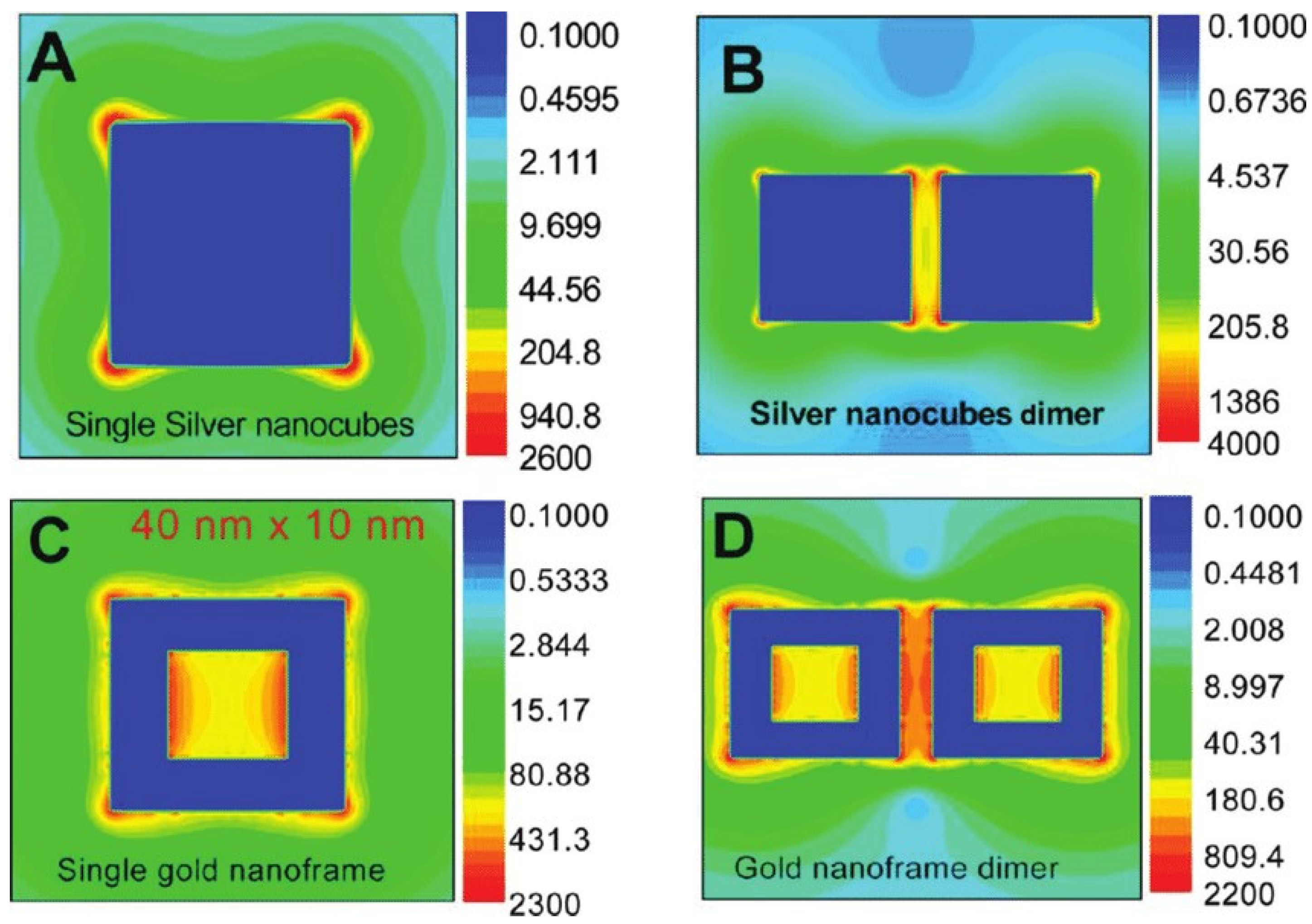
2. Surface-Enhanced Raman Spectroscopy (SERS)
3. Biomedical Applications of SERS—General Principles of the Measurements
3.1. Basics of Label-Free SERS
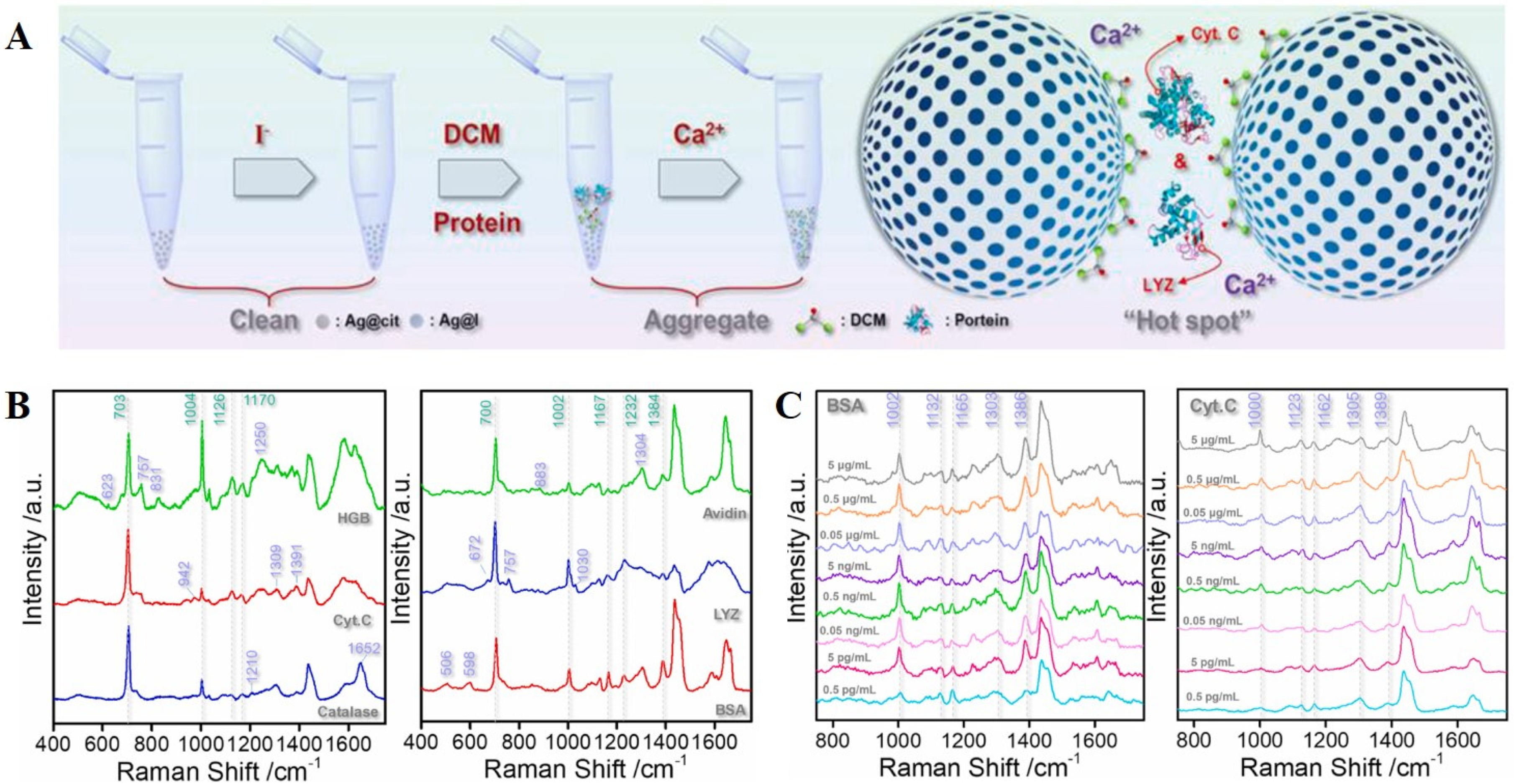
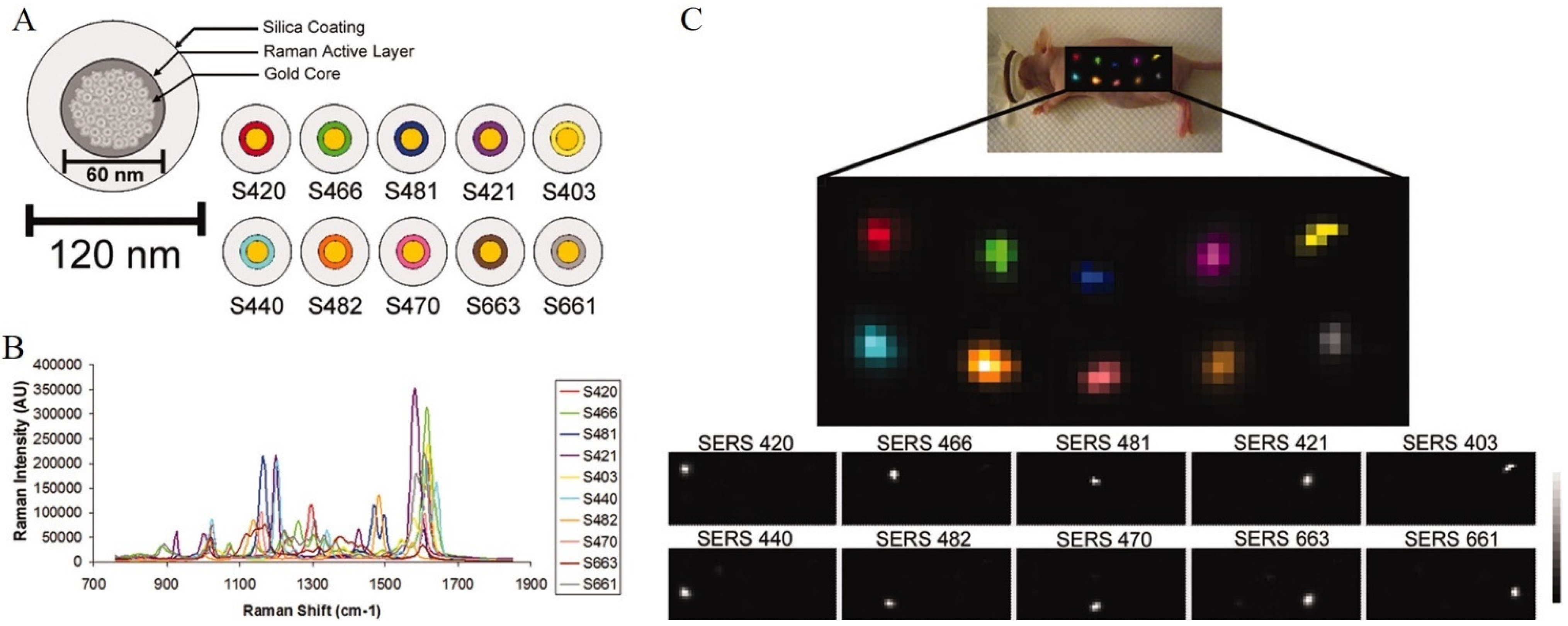
3.2. Basics of SERS Detection Utilizing Raman Reporters—Multiplexing
4. Biomedical Applications of SERS—Sample Implementations
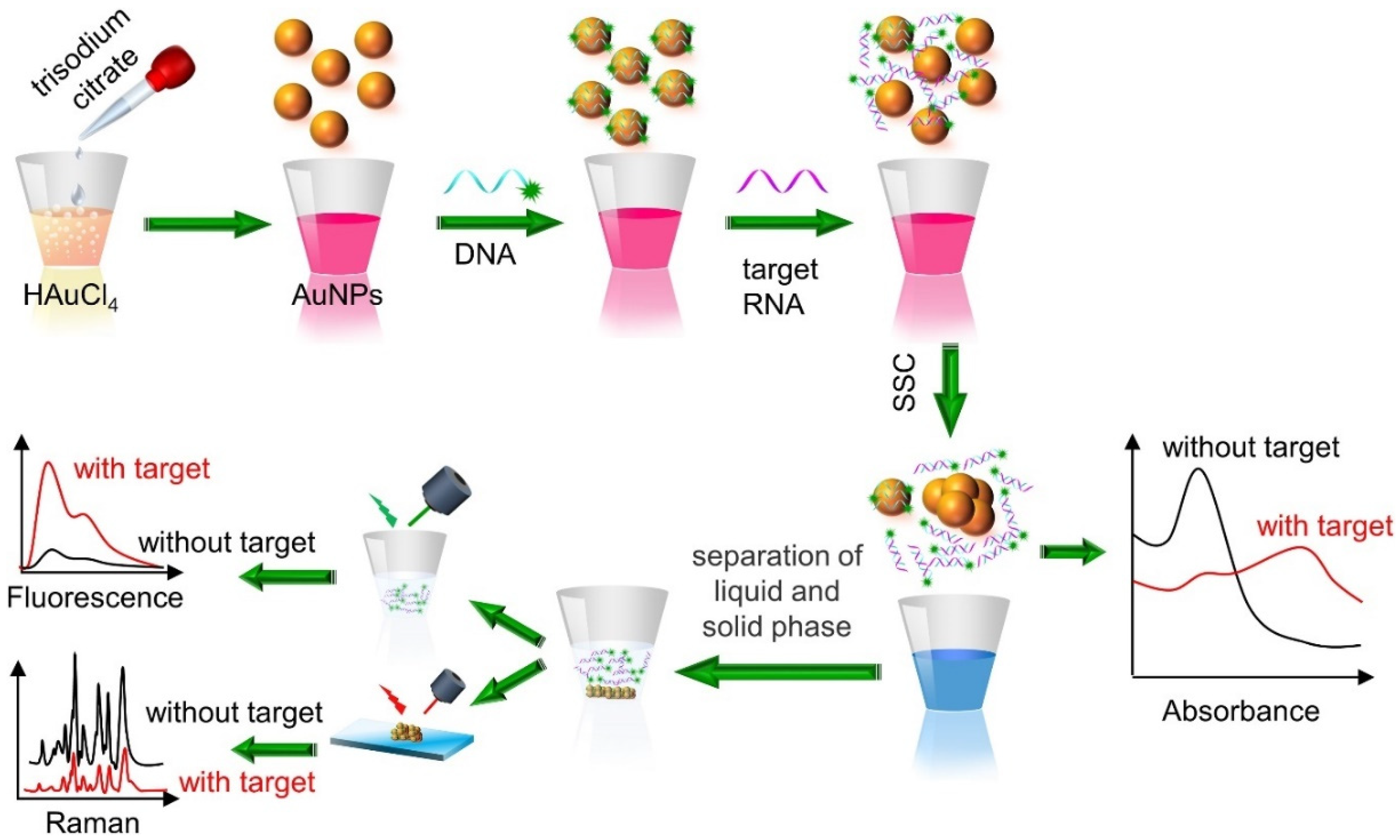
4.1. DNA and RNA Detection
4.2. Drugs Monitoring
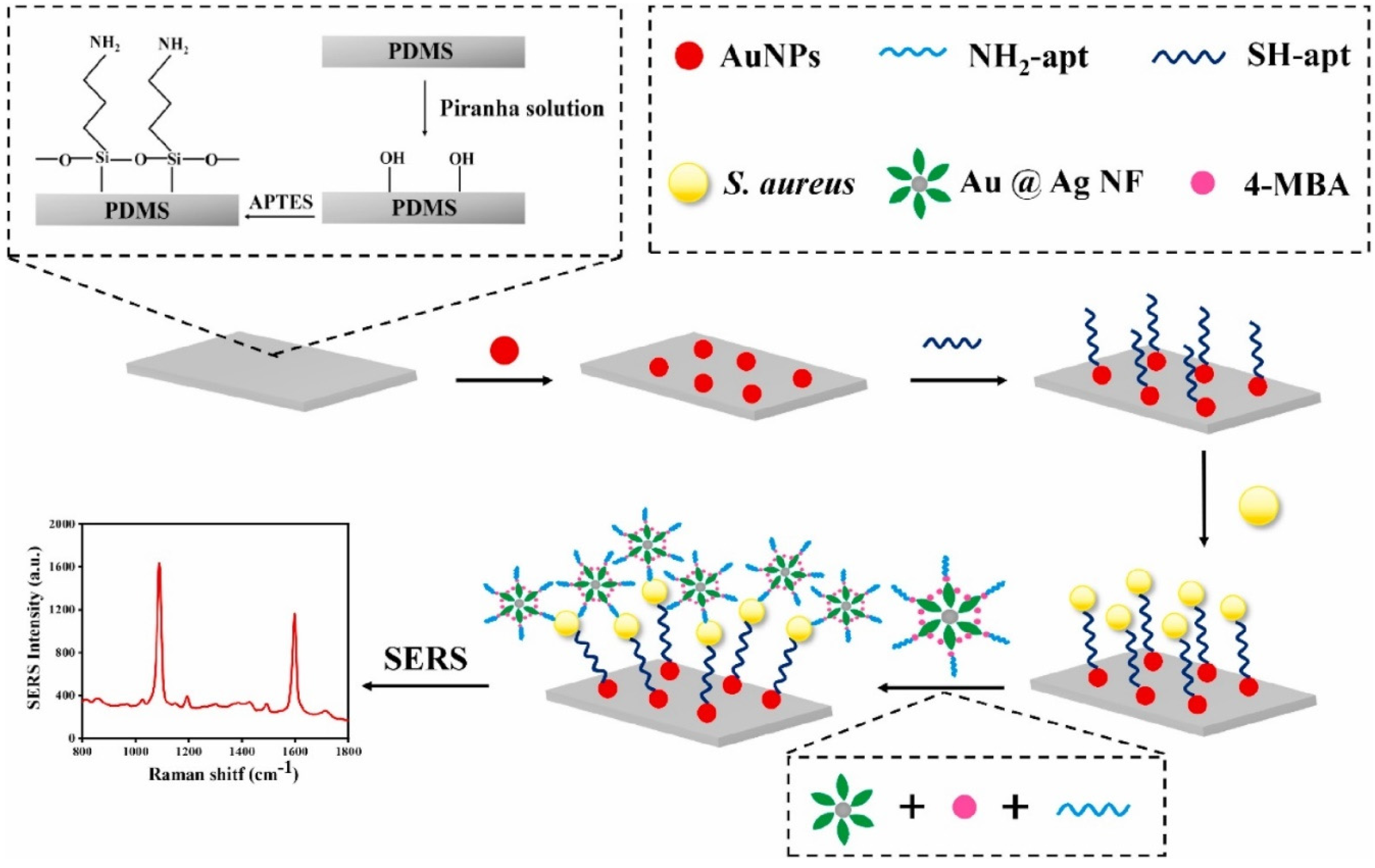
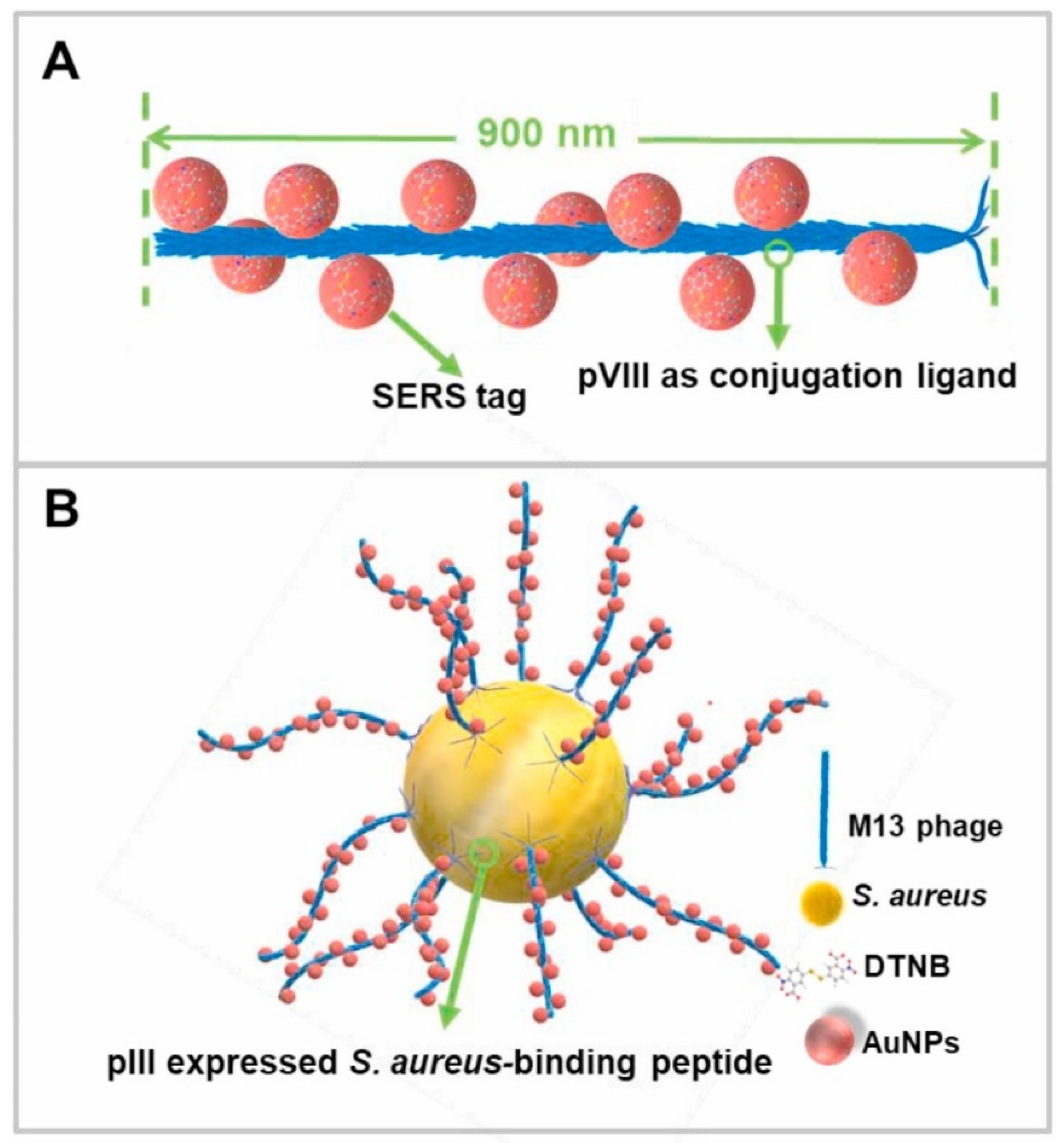
4.3. Detection of Bacteria
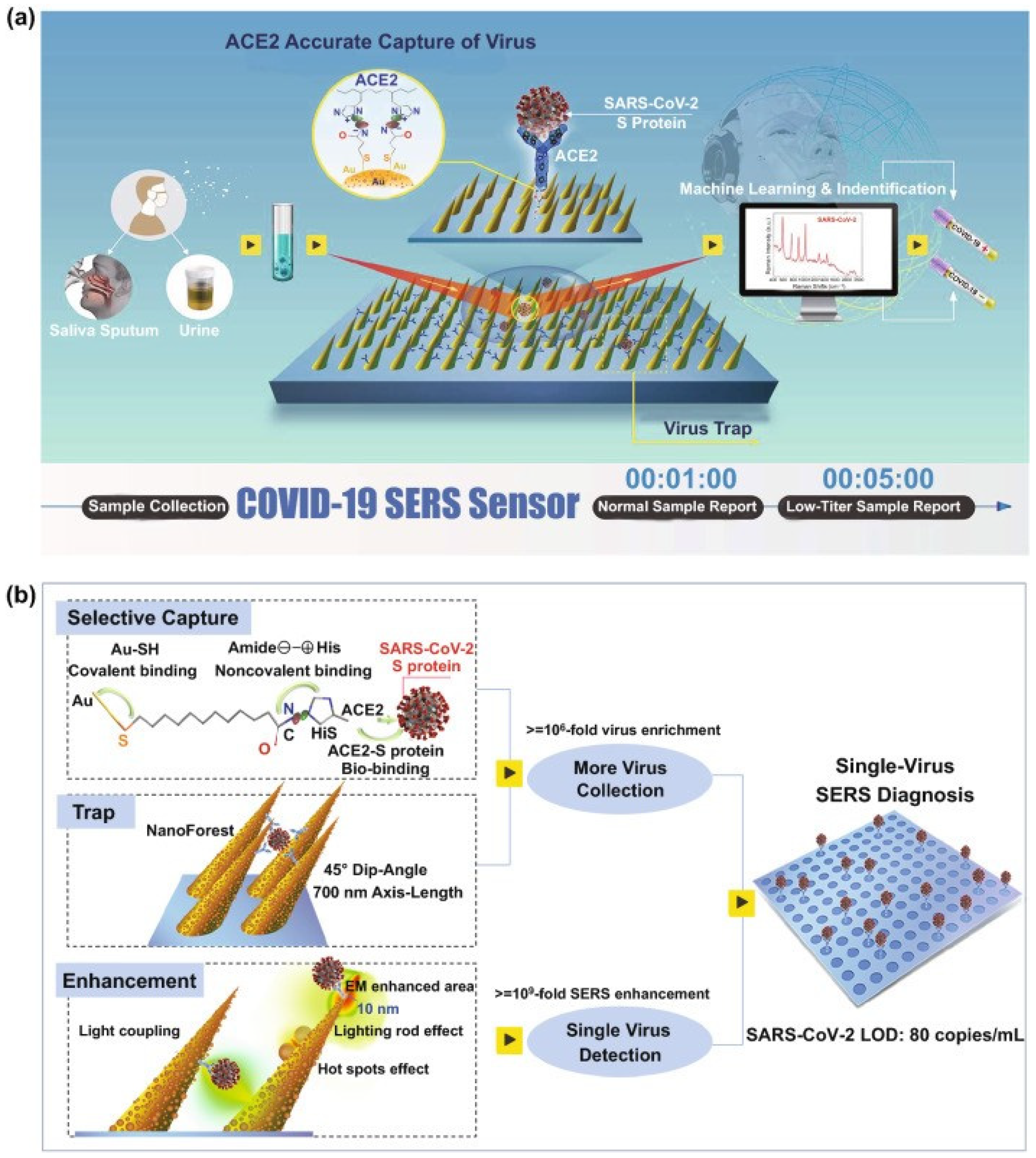
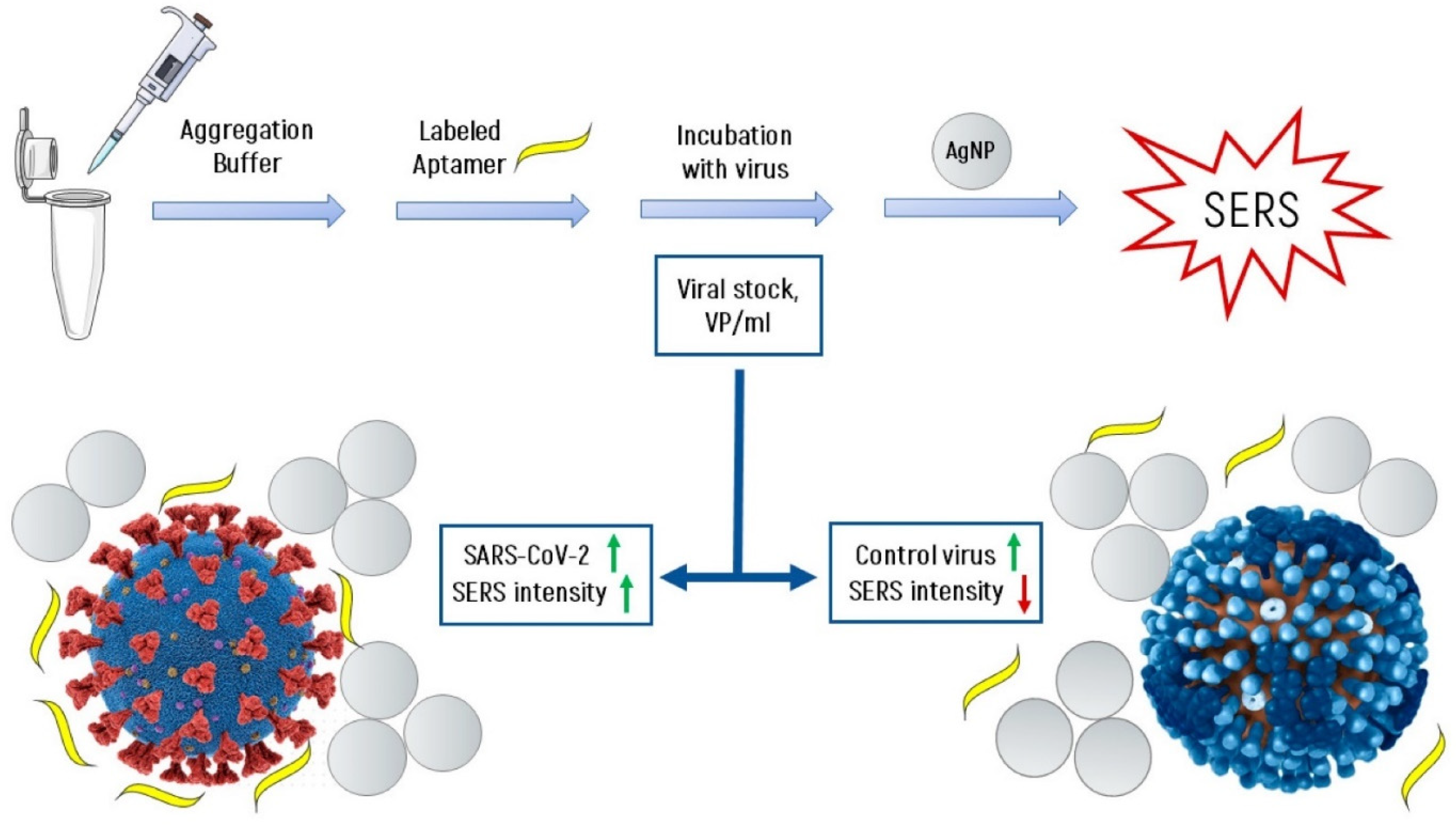
4.4. Detection of Viruses on an Example of SARS-CoV-2
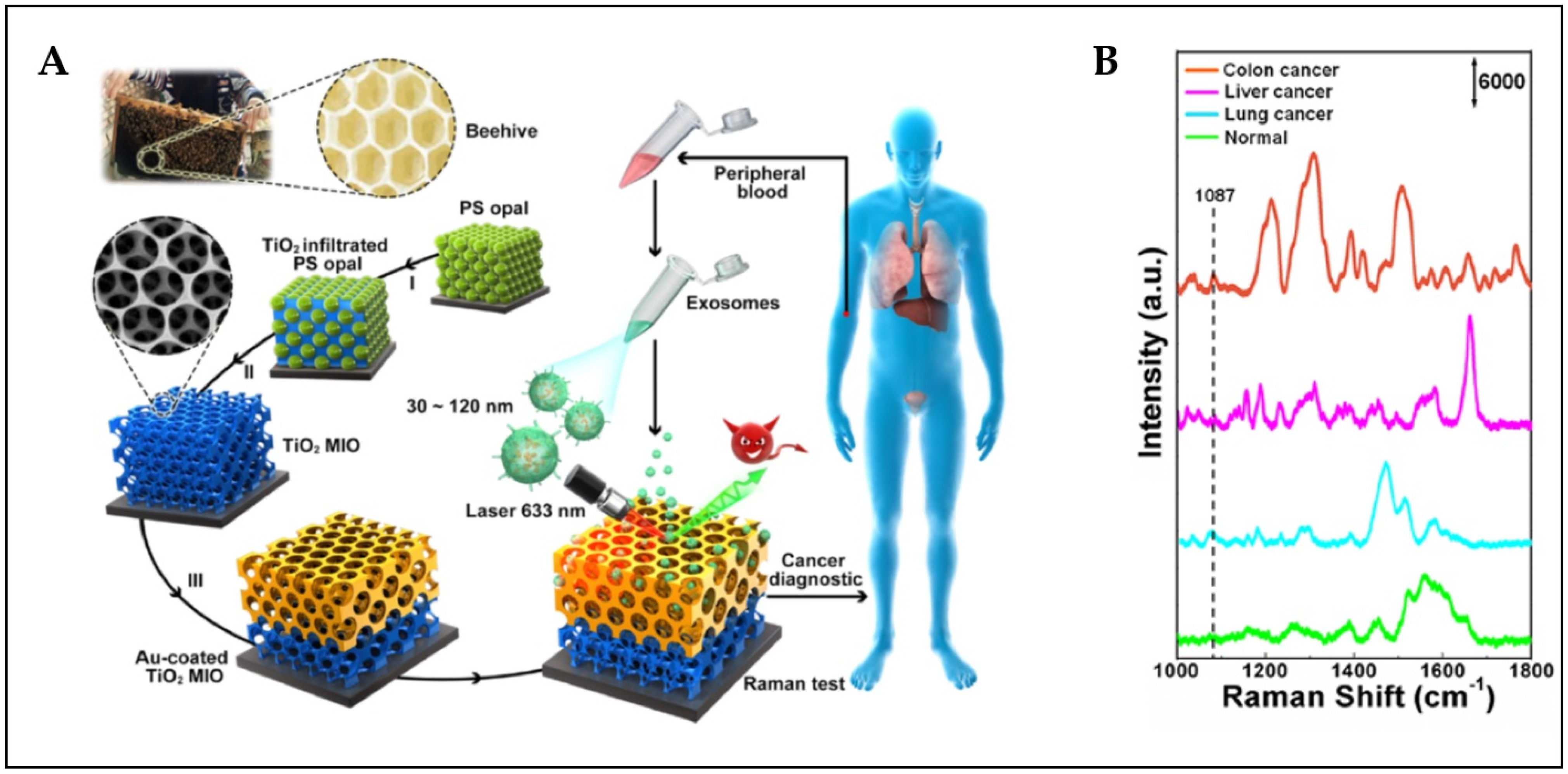
4.5. Liquid Biopsy
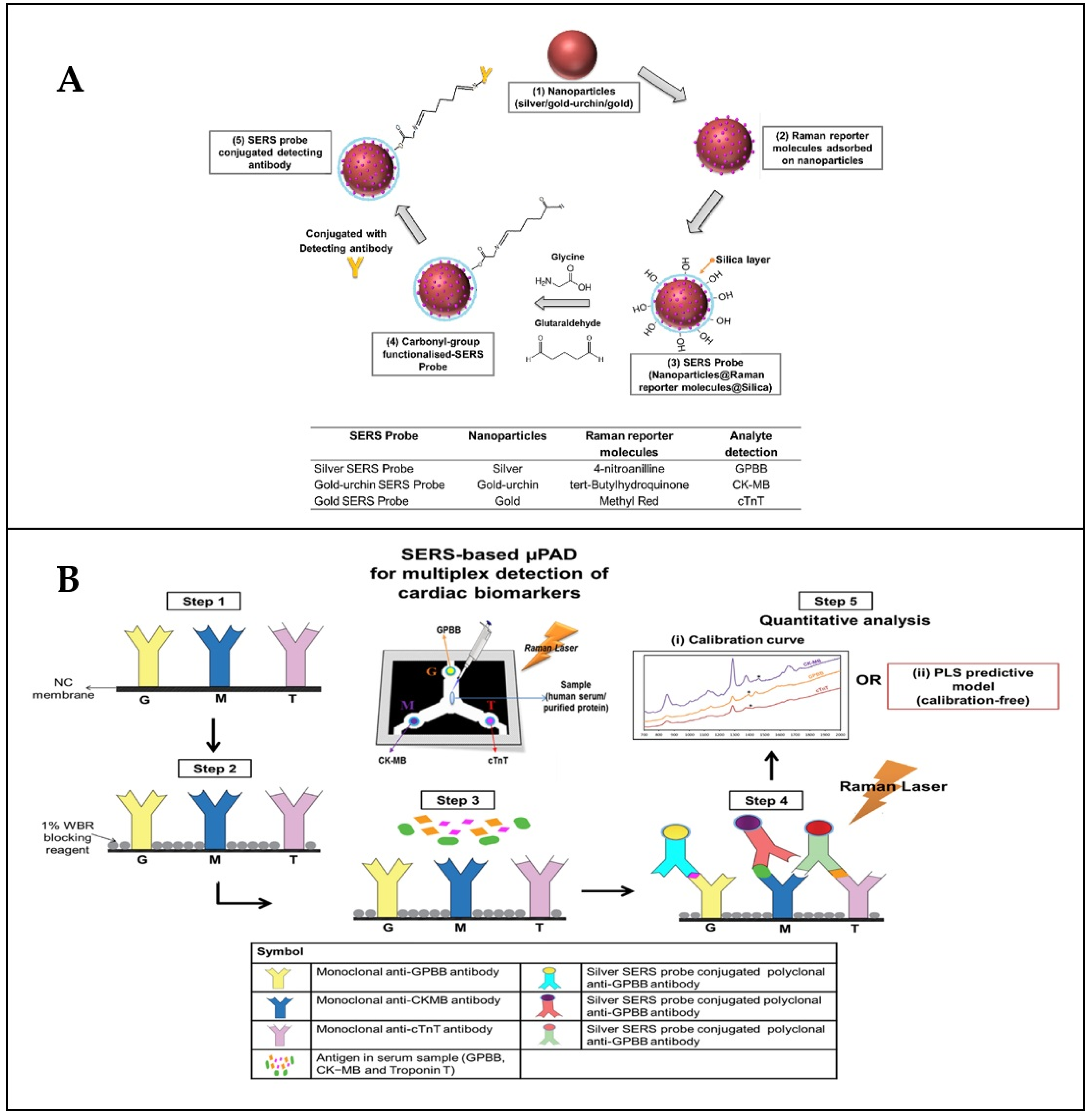
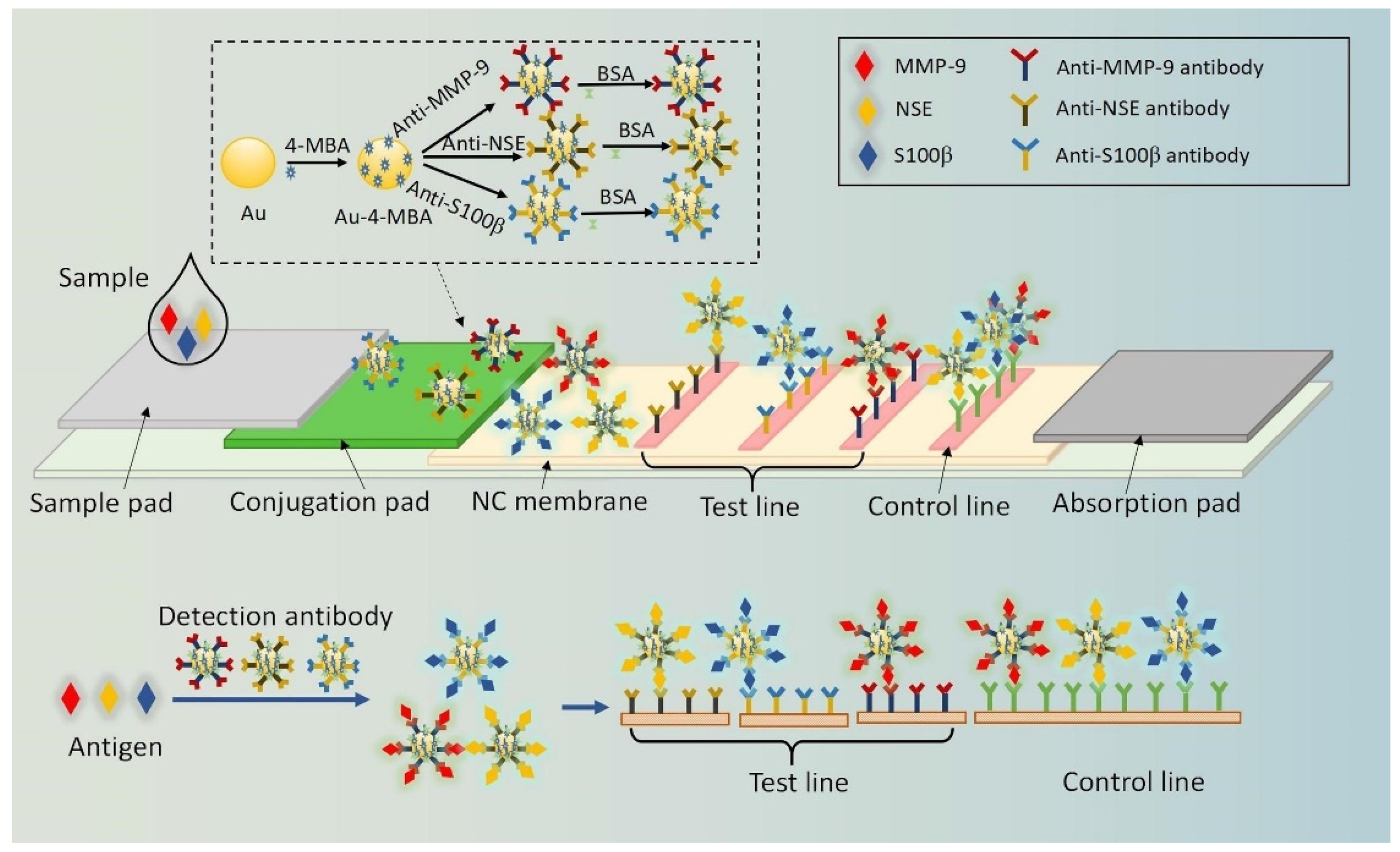
4.6. Detection of Disease Markers on an Example of Lab-On-Chip Procedure
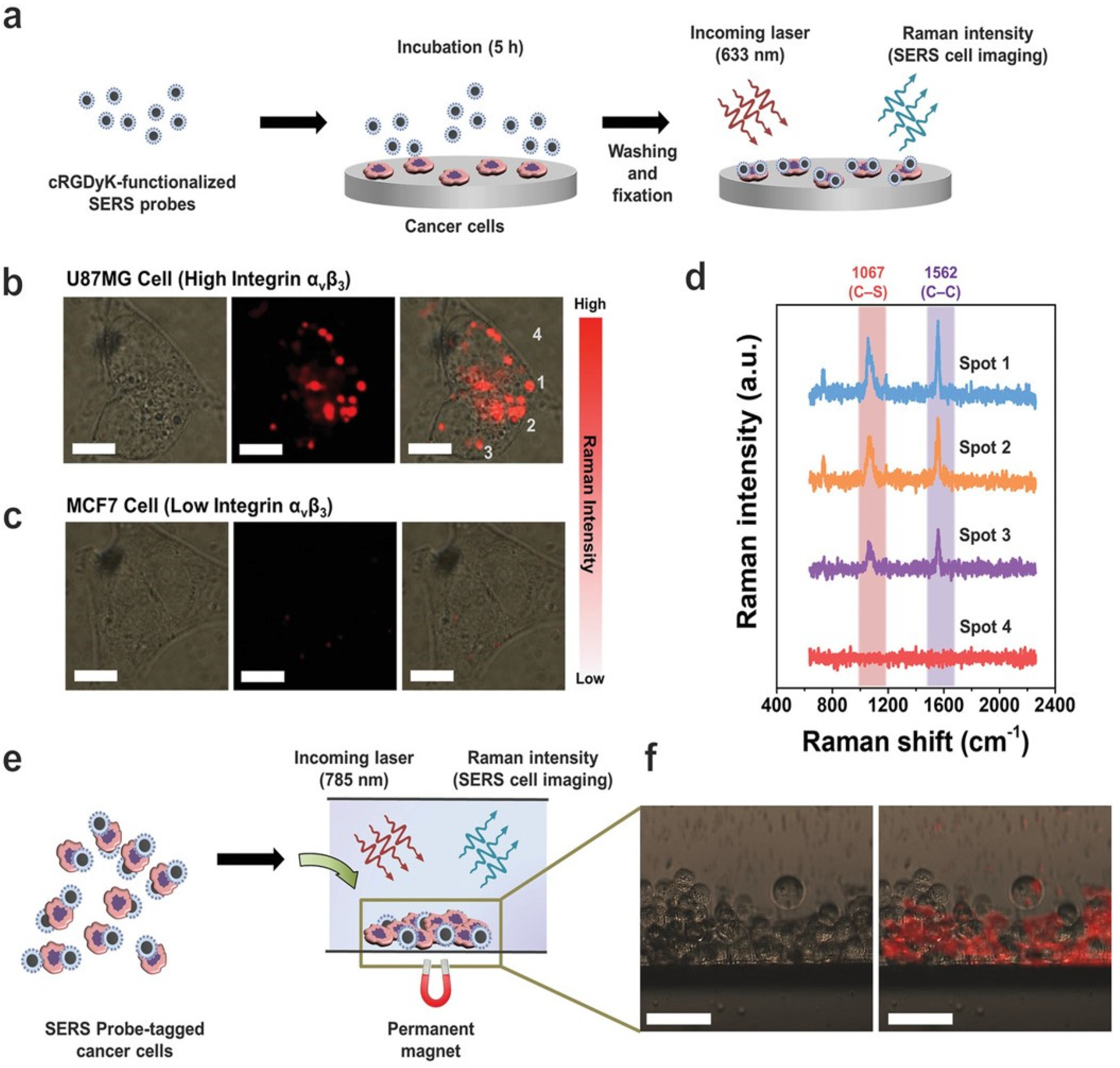
4.7. Expression of Disease Markers from Cells to In Vivo
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Aroca, R. Surface-Enhanced Vibrational Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2006; ISBN 978-0-470-03565-8. [Google Scholar]
- Michaels, A.M.; Nirmal, M.; Brus, L.E. Surface enhanced Raman spectroscopy of individual rhodamine 6G molecules on large Ag nanocrystals. J. Am. Chem. Soc. 1999, 121, 9932–9939. [Google Scholar] [CrossRef]
- Kneipp, K.; Wang, Y.; Kneipp, H.; Perelman, L.T.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Single molecule detection using surface-enhanced Raman scattering (SERS). Phys. Rev. Lett. 1997, 78, 1667–1670. [Google Scholar] [CrossRef] [Green Version]
- Nie, S.; Emory, S.R. Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Jeanmaire, D.L.; Van Duyne, R.P. Surface Raman spectroelectrochemistry: Part I heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J. Electroanal. Chem. Interfacial Electrochem. 1977, 84, 1–20. [Google Scholar] [CrossRef]
- Albrecht, M.G.; Creighton, J.A. Anomalously intense Raman spectra of pyridine at a silver electrode. J. Am. Chem. Soc. 1977, 99, 5215–5217. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Etchegoin, P.G.; Le Ru, E.C. Basic electromagnetic theory of SERS. In Surface Enhanced Raman Spectroscopy; Schlücker, S., Ed.; Wiley-VCH Verlag GmbH & Co., KGaA: Weinheim, Germany, 2010; pp. 1–37. ISBN 978-3-527-63275-6. [Google Scholar]
- Hao, E.; Schatz, G.C. Electromagnetic fields around silver nanoparticles and dimers. J. Chem. Phys. 2004, 120, 357–366. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, M.A.; O’Neil, D.; El-Sayed, M.A. Hollow and solid metallic nanoparticles in sensing and in nanocatalysis. Chem. Mater. 2014, 26, 44–58. [Google Scholar] [CrossRef]
- Kudelski, A. Raman spectroscopy of surfaces. Surf. Sci. 2009, 603, 1328–1334. [Google Scholar] [CrossRef]
- Sur, U.K. Surface-enhanced Raman spectroscopy. Reson 2010, 15, 154–164. [Google Scholar] [CrossRef]
- Kudelski, A.; Bukowska, J. The chemical effect in surface enhanced Raman scattering (SERS) for piperidine adsorbed on a silver electrode. Surf. Sci. 1996, 368, 396–400. [Google Scholar] [CrossRef]
- Jiang, X.; Campion, A. Chemical effects in surface-enhanced Raman scattering: Pyridine chemisorbed on silver adatoms on Rh (100). Chem. Phys. Lett. 1987, 140, 95–100. [Google Scholar] [CrossRef]
- Zheng, X.-S.; Jahn, I.J.; Weber, K.; Cialla-May, D.; Popp, J. Label-free SERS in biological and biomedical applications: Recent progress, current challenges and opportunities. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 197, 56–77. [Google Scholar] [CrossRef] [PubMed]
- Szaniawska, A.; Kudelski, A. Applications of surface-enhanced Raman scattering in biochemical and medical analysis. Front. Chem. 2021, 9, 664134. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Z.; Wang, X.; Wang, Y.; Gao, X.; Li, Y. A direct method for detecting proteins in body fluids by surface-enhanced Raman spectroscopy under native conditions. Biosens. Bioelectron. 2022, 200, 113907. [Google Scholar] [CrossRef] [PubMed]
- Prakash, O.; Sil, S.; Verma, T.; Umapathy, S. Direct detection of bacteria using positively charged Ag/Au bimetallic nanoparticles: A Label-free surface-enhanced Raman scattering study coupled with multivariate analysis. J. Phys. Chem. C 2020, 124, 861–869. [Google Scholar] [CrossRef] [Green Version]
- Hunter, R.; Sohi, A.N.; Khatoon, Z.; Berthiaume, V.R.; Alarcon, E.I.; Godin, M.; Anis, H. Optofluidic label-free SERS platform for rapid bacteria detection in serum. Sens. Actuators B Chem. 2019, 300, 126907. [Google Scholar] [CrossRef]
- Hao, N.; Wang, Z.; Liu, P.; Becker, R.; Yang, S.; Yang, K.; Pei, Z.; Zhang, P.; Xia, J.; Shen, L.; et al. Acoustofluidic multimodal diagnostic system for alzheimer’s disease. Biosens. Bioelectron. 2022, 196, 113730. [Google Scholar] [CrossRef]
- Gahlaut, S.K.; Savargaonkar, D.; Sharan, C.; Yadav, S.; Mishra, P.; Singh, J.P. SERS platform for dengue diagnosis from clinical samples employing a hand held Raman spectrometer. Anal. Chem. 2020, 92, 2527–2534. [Google Scholar] [CrossRef]
- Ma, Y.; Chi, J.; Zheng, Z.; Attygalle, A.; Kim, I.Y.; Du, H. Therapeutic prognosis of prostate cancer using surface-enhanced Raman scattering of patient urine and multivariate statistical analysis. J. Biophotonics 2021, 14, e202000275. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, X.; Yang, H.; Xie, J.; Liu, A. Diagnosis and staging of diffuse large B-cell lymphoma using label-free surface-enhanced Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 267, 120571. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Huang, Z.; Lin, X.; Wu, Q.; Quan, K.; Cheng, Y.; Zheng, M.; Xu, J.; Dai, Y.; Qiu, H.; et al. Rapid and label-free urine test based on surface-enhanced Raman spectroscopy for the non-invasive detection of colorectal cancer at different stages. Biomed. Opt. Express BOE 2020, 11, 7109–7119. [Google Scholar] [CrossRef] [PubMed]
- Moisoiu, T.; Dragomir, M.P.; Iancu, S.D.; Schallenberg, S.; Birolo, G.; Ferrero, G.; Burghelea, D.; Stefancu, A.; Cozan, R.G.; Licarete, E.; et al. Combined MiRNA and SERS urine liquid biopsy for the point-of-care diagnosis and molecular stratification of bladder cancer. Mol. Med. 2022, 28, 39. [Google Scholar] [CrossRef]
- Ye, M.; Chen, Y.; Wang, Y.; Xiao, L.; Lin, Q.; Lin, H.; Duan, Z.; Feng, S.; Cao, Y.; Zhang, J.; et al. Subtype discrimination of acute myeloid leukemia based on plasma SERS technique. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 271, 120865. [Google Scholar] [CrossRef]
- Kim, S.; Kim, T.G.; Lee, S.H.; Kim, W.; Bang, A.; Moon, S.W.; Song, J.; Shin, J.-H.; Yu, J.S.; Choi, S. Label-free surface-enhanced Raman spectroscopy biosensor for on-site breast cancer detection using human tears. ACS Appl. Mater. Interfaces 2020, 12, 7897–7904. [Google Scholar] [CrossRef]
- Shin, H.; Oh, S.; Hong, S.; Kang, M.; Kang, D.; Ji, Y.; Choi, B.H.; Kang, K.-W.; Jeong, H.; Park, Y.; et al. Early-stage lung cancer diagnosis by deep learning-based spectroscopic analysis of circulating exosomes. ACS Nano 2020, 14, 5435–5444. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Yin, P.; Hu, Y.; Szydzik, C.; Khan, M.W.; Xu, K.; Thurgood, P.; Mahmood, N.; Dekiwadia, C.; Afrin, S.; et al. Highly accurate and label-free discrimination of single cancer cell using a plasmonic oxide-based nanoprobe. Biosens. Bioelectron. 2022, 198, 113814. [Google Scholar] [CrossRef]
- Dey, P.; Vaideanu, A.; Mosca, S.; Salimi, M.; Gardner, B.; Palombo, F.; Uchegbu, I.; Baumberg, J.; Schatzlein, A.; Matousek, P.; et al. Surface enhanced deep Raman detection of cancer tumour through 71 mm of heterogeneous tissue. Nanotheranostics 2022, 6, 337–349. [Google Scholar] [CrossRef]
- Nikelshparg, E.I.; Baizhumanov, A.A.; Bochkova, Z.V.; Novikov, S.M.; Yakubovsky, D.I.; Arsenin, A.V.; Volkov, V.S.; Goodilin, E.A.; Semenova, A.A.; Sosnovtseva, O.; et al. Detection of hypertension-induced changes in erythrocytes by SERS nanosensors. Biosensors 2022, 12, 32. [Google Scholar] [CrossRef]
- Zyubin, A.; Rafalskiy, V.; Tcibulnikova, A.; Moiseeva, E.; Matveeva, K.; Tsapkova, A.; Lyatun, I.; Medvedskaya, P.; Samusev, I.; Demin, M. Surface-enhanced raman spectroscopy for antiplatelet therapy effectiveness assessment. Laser Phys. Lett. 2020, 17, 045601. [Google Scholar] [CrossRef]
- Li, X.; Yang, T.; Li, C.S.; Song, Y.; Wang, D.; Jin, L.; Lou, H.; Li, W. Polymerase chain reaction—Surface-enhanced Raman spectroscopy (PCR-SERS) method for gene methylation level detection in plasma. Theranostics 2020, 10, 898–909. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Purrà, M.; Roig-Solvas, B.; Rodriguez-Quijada, C.; Leonardo, B.M.; Hamad-Schifferli, K. Reporter selection for nanotags in multiplexed surface enhanced raman spectroscopy assays. ACS Omega 2018, 3, 10733–10742. [Google Scholar] [CrossRef] [PubMed]
- Laing, S.; Gracie, K.; Faulds, K. Multiplex in vitro detection using SERS. Chem. Soc. Rev. 2016, 45, 1901–1918. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.H.; Steinberg, I.; Davis, R.M.; Malkovskiy, A.V.; Zlitni, A.; Radzyminski, R.K.; Jung, K.O.; Chung, D.T.; Curet, L.D.; D’Souza, A.L.; et al. Noninvasive and highly multiplexed five-color tumor imaging of multicore near-infrared resonant surface-enhanced Raman nanoparticles in vivo. ACS Nano 2021, 15, 19956–19969. [Google Scholar] [CrossRef]
- Zavaleta, C.L.; Smith, B.R.; Walton, I.; Doering, W.; Davis, G.; Shojaei, B.; Natan, M.J.; Gambhir, S.S. Multiplexed imaging of surface enhanced raman scattering nanotags in living mice using noninvasive Raman spectroscopy. Proc. Natl. Acad. Sci. USA 2009, 106, 13511–13516. [Google Scholar] [CrossRef] [Green Version]
- Bronkhorst, A.J.; Ungerer, V.; Holdenrieder, S. The emerging role of cell-free DNA as a molecular marker for cancer management. Biomol. Detect. Quantif. 2019, 17, 100087. [Google Scholar] [CrossRef]
- Pyrak, E.; Krajczewski, J.; Kowalik, A.; Kudelski, A.; Jaworska, A. Surface enhanced Raman spectroscopy for DNA biosensors—How far are we? Molecules 2019, 24, 4423. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Dong, Y.; Zhu, W.; Xie, D.; Zhao, Y.; Yang, D.; Li, M. Ultrasensitive detection of circulating tumor DNA of lung cancer via an enzymatically amplified SERS-based frequency shift assay. ACS Appl. Mater. Interfaces 2019, 11, 18145–18152. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, H.; Xu, Y.; Laššáková, S.; Korabečná, M.; Neužil, P. PCR past, present and future. BioTechniques 2020, 69, 317–325. [Google Scholar] [CrossRef]
- Garcia-Rico, E.; Alvarez-Puebla, R.A.; Guerrini, L. Direct surface-enhanced Raman scattering (SERS) spectroscopy of nucleic acids: From fundamental studies to real-life applications. Chem. Soc. Rev. 2018, 47, 4909–4923. [Google Scholar] [CrossRef] [PubMed]
- Iancu, S.D.; Stefancu, A.; Moisoiu, V.; Leopold, L.F.; Leopold, N. The role of Ag+, Ca2+, Pb2+ and Al3+ adions in the SERS Turn-on effect of anionic analytes. Beilstein J. Nanotechnol. 2019, 10, 2338–2345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Grasso, M.F.; Cui, X.; Silva, R.N.; Zhang, P. Sensitive and label-free SERS detection of single-stranded DNA assisted by silver nanoparticles and gold-coated magnetic nanoparticles. ACS Appl. Bio Mater. 2020, 3, 2626–2632. [Google Scholar] [CrossRef] [PubMed]
- Safaee Ardekani, G.; Jafarnejad, S.M.; Tan, L.; Saeedi, A.; Li, G. The Prognostic Value of BRAF Mutation in Colorectal Cancer and Melanoma: A Systematic Review and Meta-Analysis. PLoS ONE 2012, 7, e47054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, M. BRAF mutation in thyroid cancer. Endocr. Relat. Cancer 2005, 12, 245–262. [Google Scholar] [CrossRef] [Green Version]
- Roberts, P.J.; Stinchcombe, T.E. KRAS mutation: Should We test for it, and does it matter. J. Clin. Oncol. 2013, 31, 1112–1121. [Google Scholar] [CrossRef]
- Tan, C.; Du, X. KRAS mutation testing in metastatic colorectal cancer. World J. Gastroenterol. WJG 2012, 18, 5171. [Google Scholar] [CrossRef]
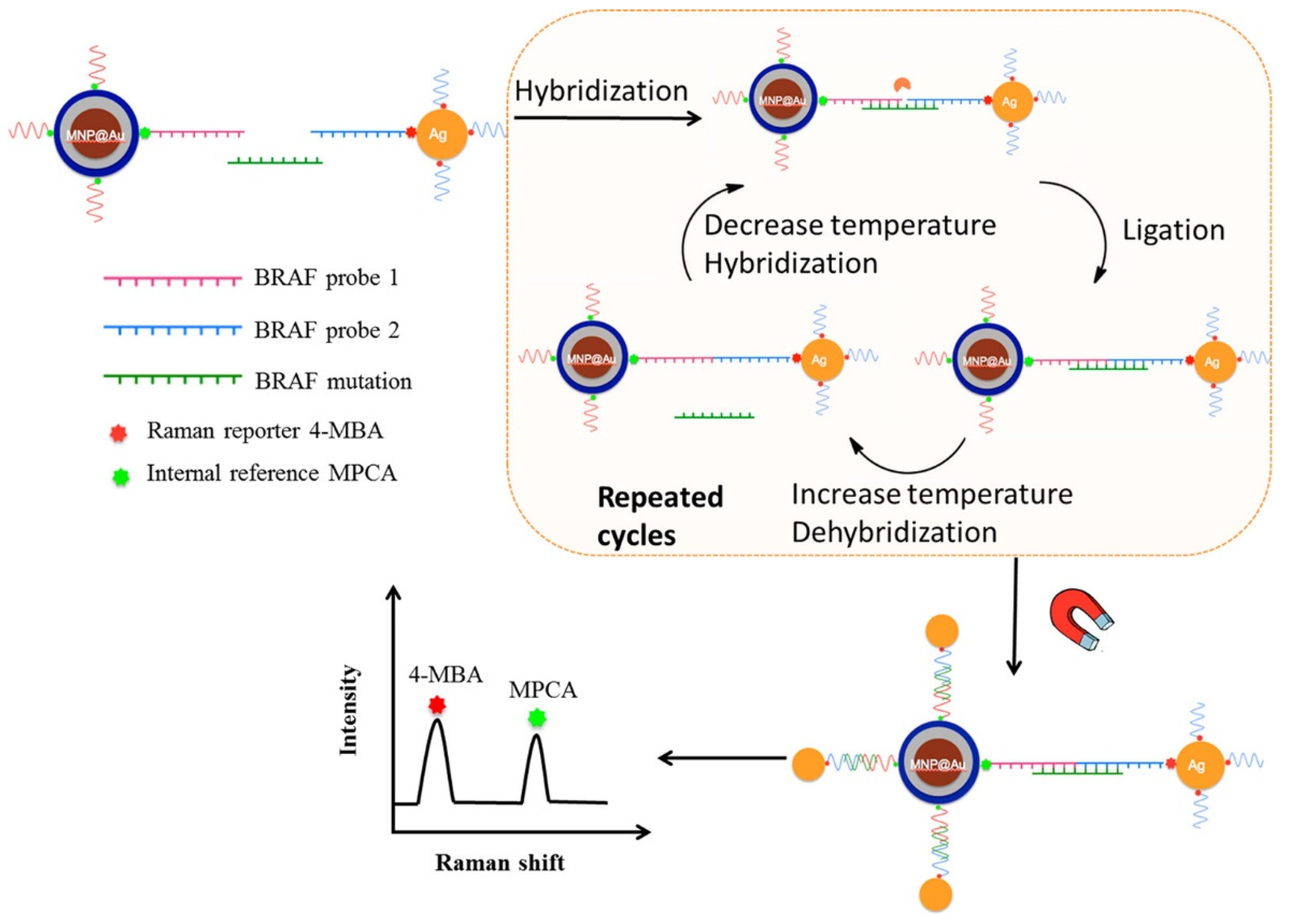
- Lyu, N.; Rajendran, V.K.; Diefenbach, R.J.; Charles, K.; Clarke, S.J.; Engel, A.; Rizos, H.; Molloy, M.P.; Wang, Y. Multiplex detection of CtDNA mutations in plasma of colorectal cancer patients by PCR/SERS assay. Nanotheranostics 2020, 4, 224. [Google Scholar] [CrossRef]
- Lee, H.G.; Choi, W.; Yang, S.Y.; Kim, D.H.; Park, S.G.; Lee, M.Y.; Jung, H.S. PCR-coupled paper-based surface-enhanced Raman scattering (SERS) sensor for rapid and sensitive detection of respiratory bacterial DNA. Sens. Actuators B Chem. 2021, 326, 128802. [Google Scholar] [CrossRef]
- Kim, M.; Costello, J. DNA methylation: An epigenetic mark of cellular memory. Exp. Mol. Med. 2017, 49, e322. [Google Scholar] [CrossRef] [Green Version]
- Luczak, M.W.; Jagodziński, P.P. The role of DNA methylation in cancer development. Folia Histochem. Cytobiol. 2006, 44, 143–154. [Google Scholar] [PubMed]
- Kurdyukov, S.; Bullock, M. DNA methylation analysis: Choosing the right method. Biology 2016, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, S.; Venkatakrishnan, K.; Tan, B. Quantum scale organic semiconductors for SERS detection of DNA methylation and gene expression. Nat. Commun. 2020, 11, 1135. [Google Scholar] [CrossRef] [Green Version]
- Stefancu, A.; Moisoiu, V.; Desmirean, M.; Iancu, S.D.; Tigu, A.B.; Petrushev, B.; Jurj, A.; Cozan, R.G.; Budisan, L.; Fetica, B.; et al. SERS-based DNA methylation profiling allows the differential diagnosis of malignant lymphadenopathy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 264, 120216. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.X.; Rothenberg, M.E. MicroRNA. J. Allergy Clin. Immunol. 2018, 141, 1202–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.; Qiang, L.; Gao, Y.; Gao, J.; He, Q.; Liu, H.; Han, L.; Zhang, Y. Large-area surface-enhanced Raman spectroscopy substrate by hybrid porous GaN with Au/Ag for breast cancer MiRNA detection. Appl. Surf. Sci. 2021, 541, 148456. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, L.; Mi, L.; Guo, R.; Li, T. Recent progress of SERS optical nanosensors for MiRNA analysis. J. Mater. Chem. B 2020, 8, 5178–5183. [Google Scholar] [CrossRef]
- Schwarzenbach, H.; Hoon, D.S.B.; Pantel, K. Cell-free nucleic acids as biomarkers in cancer patients. Nature Rev. Cancer 2011, 11, 426–437. [Google Scholar] [CrossRef]
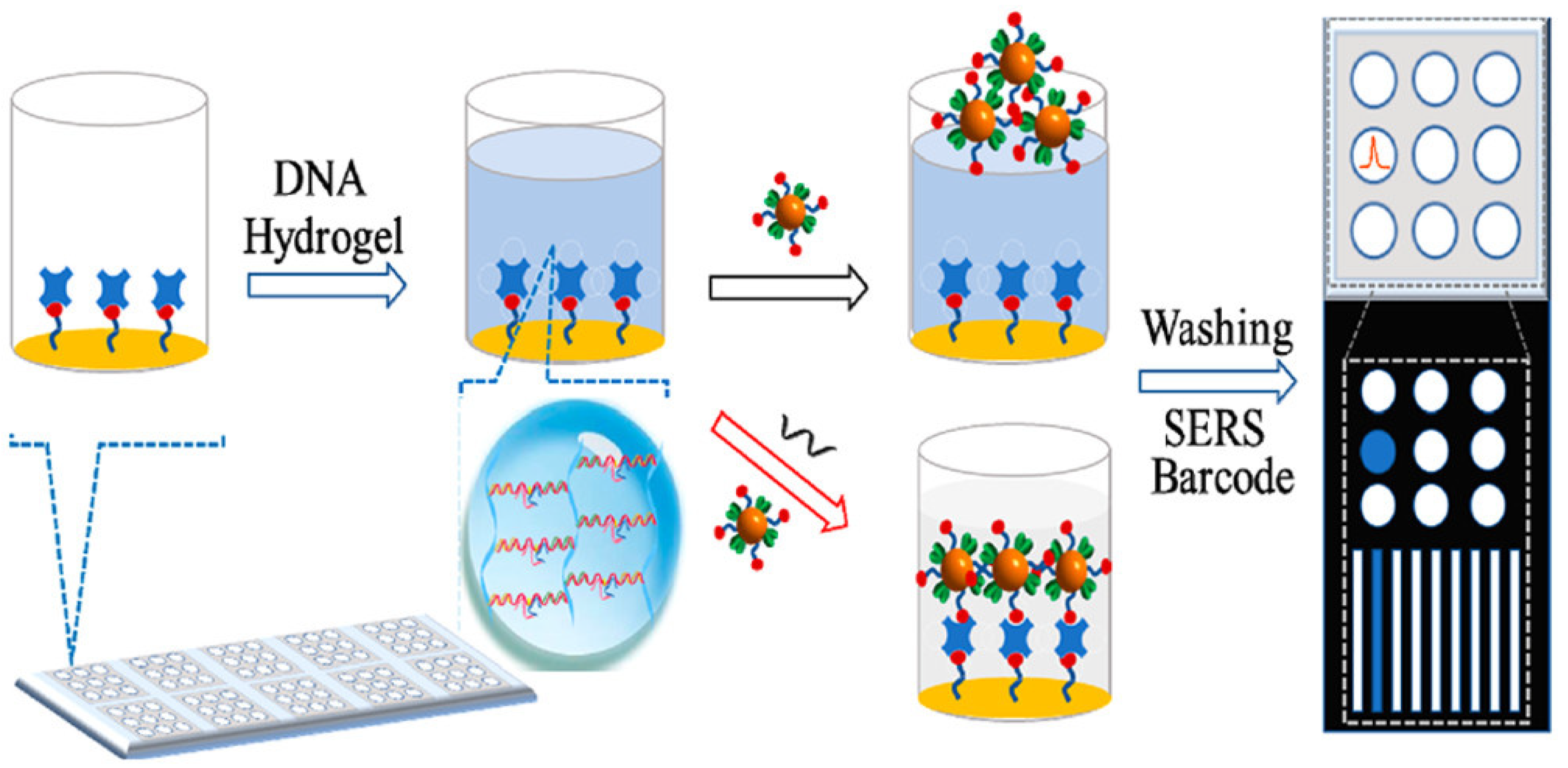
- Si, Y.; Xu, L.; Wang, N.; Zheng, J.; Yang, R.; Li, J. Target MicroRNA-responsive DNA hydrogel-based surface-enhanced Raman scattering sensor arrays for MicroRNA-marked cancer screening. Anal. Chem. 2020, 92, 2649–2655. [Google Scholar] [CrossRef]
- Lee, W.J.; Kim, K.J.; Hossain, M.K.; Cho, H.Y.; Choi, J.W. DNA–gold nanoparticle conjugates for intracellular MiRNA detection using surface-enhanced Raman spectroscopy. Biochip J. 2022, 16, 33–40. [Google Scholar] [CrossRef]
- Jiang, S.; Li, Q.; Wang, C.; Pang, Y.; Sun, Z.; Xiao, R. In situ exosomal MicroRNA determination by target-triggered SERS and Fe3O4@TiO2-based exosome accumulation. ACS Sens. 2021, 6, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Saviñon-Flores, F.; Méndez, E.; López-Castaños, M.; Carabarin-Lima, A.; López-Castaños, K.A.; González-Fuentes, M.A.; Méndez-Albores, A. A review on SERS-based detection of human virus infections: Influenza and coronavirus. Biosensors 2021, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Nasir, S.; Majeed, M.I.; Nawaz, H.; Rashid, N.; Ali, S.; Farooq, S.; Kashif, M.; Rafiq, S.; Bano, S.; Ashraf, M.N.; et al. Surface enhanced Raman spectroscopy of RNA samples extracted from blood of hepatitis C patients for quantification of viral loads. Photodiagnosis Photodyn. Ther. 2021, 33, 102152. [Google Scholar] [CrossRef] [PubMed]
- Dardir, K.; Wang, H.; Martin, B.E.; Atzampou, M.; Brooke, C.B.; Fabris, L. SERS nanoprobe for intracellular monitoring of viral mutations. J. Phys. Chem. C 2020, 124, 3211–3217. [Google Scholar] [CrossRef]
- Jaworska, A.; Fornasaro, S.; Sergo, V.; Bonifacio, A. Potential of surface enhanced raman spectroscopy (SERS) in therapeutic drug monitoring (TDM). A critical review. Biosensors 2016, 6, 47. [Google Scholar] [CrossRef] [Green Version]
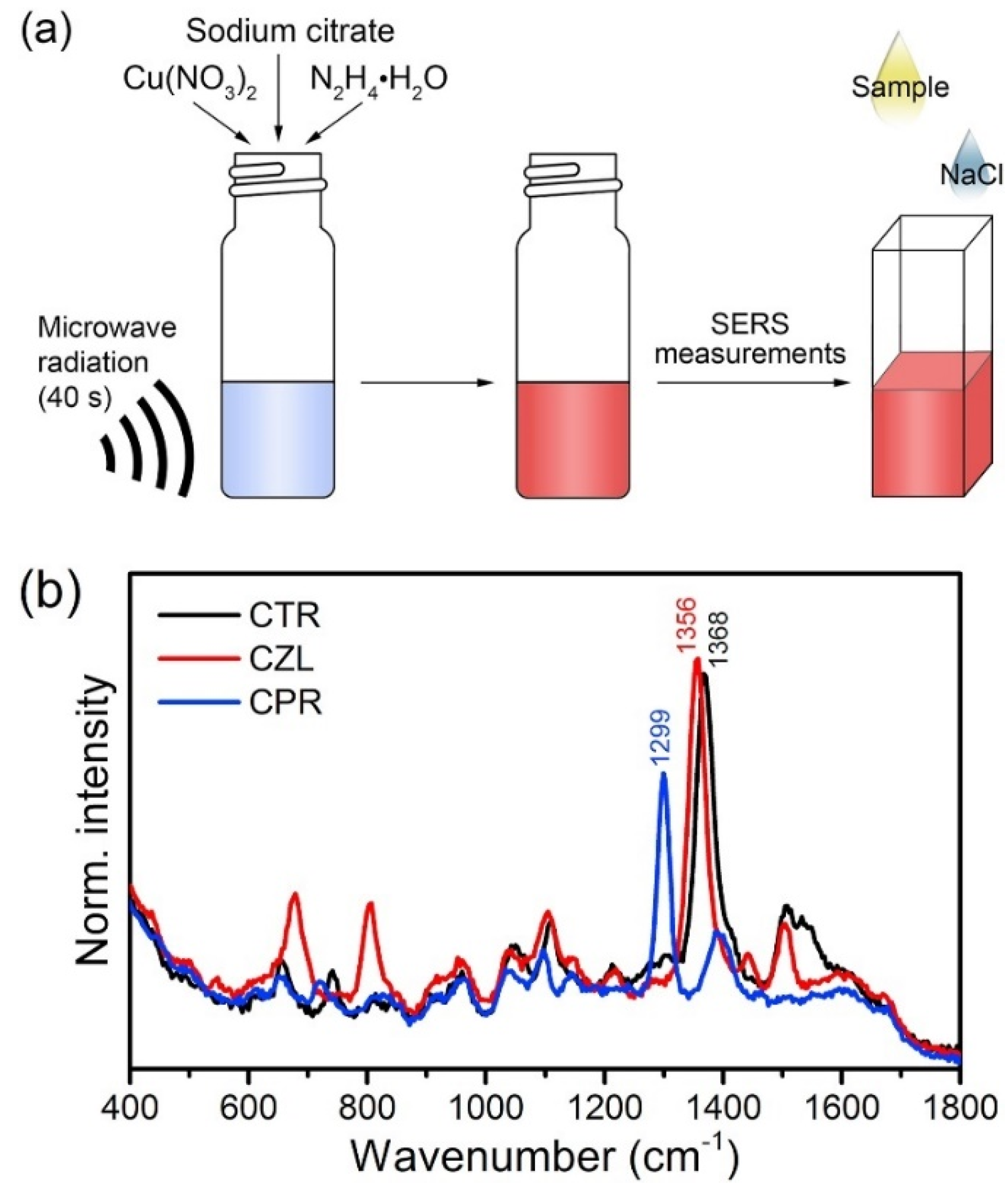
- Markina, N.E.; Ustinov, S.N.; Zakharevich, A.M.; Markin, A.V. Copper nanoparticles for SERS-based determination of some cephalosporin antibiotics in spiked human urine. Anal. Chim. Acta 2020, 1138, 9–17. [Google Scholar] [CrossRef]
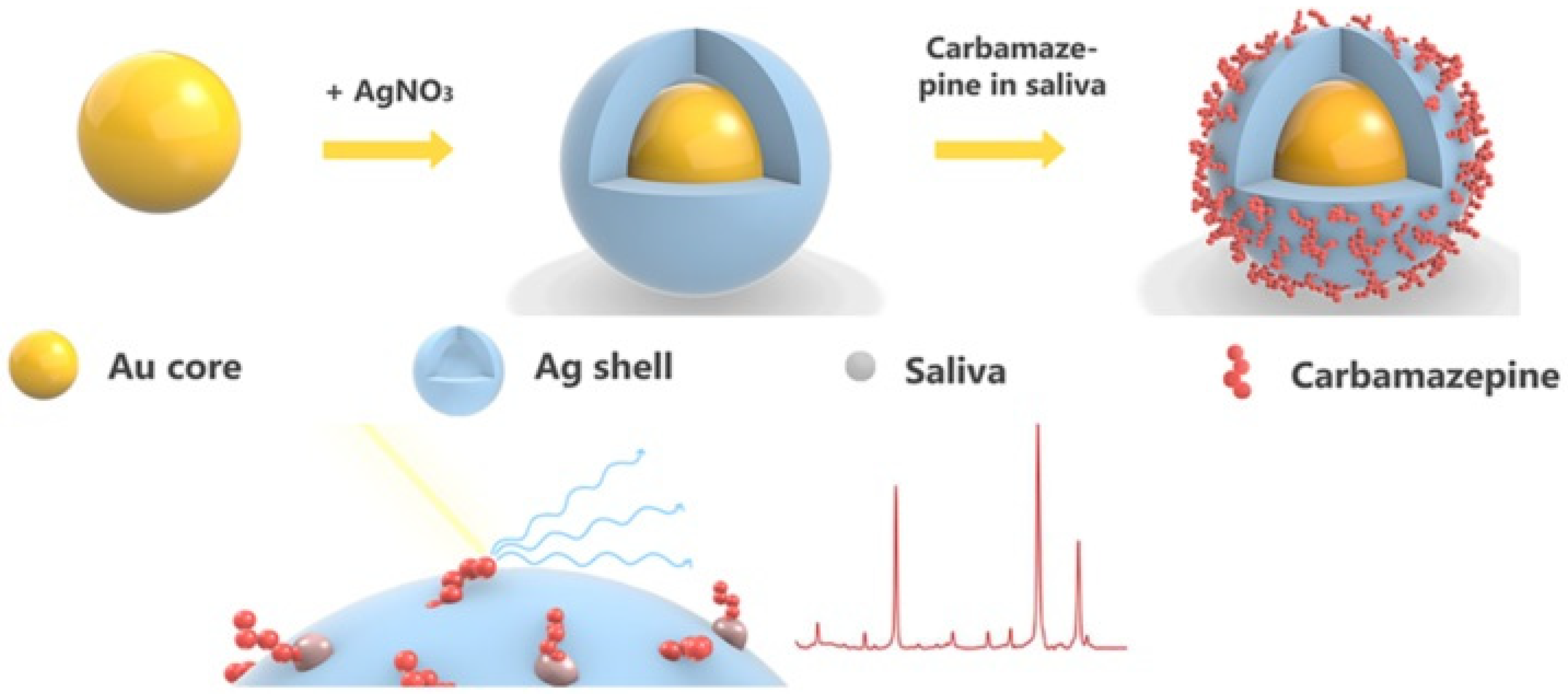
- Chen, N.; Yuan, Y.; Lu, P.; Wang, L.; Zhang, X.; Zhang, X.; Chen, H.; Ma, P. Detection of carbamazepine in saliva based on surface-enhanced Raman spectroscopy. Biomed. Opt. Express BOE 2021, 12, 7673–7688. [Google Scholar] [CrossRef]
- Barveen, N.R.; Wang, T.-J.; Chang, Y.-H. A Photochemical approach to anchor Au NPs on MXene as a prominent SERS substrate for ultrasensitive detection of chlorpromazine. Mikrochim. Acta 2021, 189, 16. [Google Scholar] [CrossRef]
- Martin, J.H.; Dimmitt, S. The rationale of dose-response curves in selecting cancer drug dosing. Br. J. Clin. Pharmacol. 2019, 85, 2198–2204. [Google Scholar] [CrossRef]
- Clarke, W.A.; Chatelut, E.; Fotoohi, A.K.; Larson, R.A.; Martin, J.H.; Mathijssen, R.H.J.; Salamone, S.J. Therapeutic drug monitoring in oncology: International association of therapeutic drug monitoring and clinical toxicology consensus guidelines for imatinib therapy. Eur. J. Cancer 2021, 157, 428–440. [Google Scholar] [CrossRef]
- Evans, W.E.; Pratt, C.B.; Taylor, R.H.; Barker, L.F.; Crom, W.R. Pharmacokinetic monitoring of high-dose methotrexate. Early recognition of high-risk patients. Cancer Chemother. Pharm. 1979, 3, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Markina, N.E.; Zakharevich, A.M.; Markin, A.V. Determination of methotrexate in spiked human urine using SERS-active sorbent. Anal. Bioanal. Chem. 2020, 412, 7757–7766. [Google Scholar] [CrossRef] [PubMed]
- Panikar, S.S.; Banu, N.; Escobar, E.-R.; García, G.-R.; Cervantes-Martínez, J.; Villegas, T.-C.; Salas, P.; De la Rosa, E. Stealth modified bottom up SERS substrates for label-free therapeutic drug monitoring of doxorubicin in blood serum. Talanta 2020, 218, 121138. [Google Scholar] [CrossRef] [PubMed]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharm. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, S.; Liu, J.; Zhao, X.; Feng, Z.; Wang, D.; Gong, Z.; Fan, M. Quantitative detection of 6-thioguanine in body fluids based on a free-standing liquid membrane SERS substrate. Anal. Bioanal. Chem. 2022, 414, 1663–1670. [Google Scholar] [CrossRef]
- Larsen, R.H.; Rank, C.U.; Grell, K.; Møller, L.N.; Overgaard, U.M.; Kampmann, P.; Nersting, J.; Degn, M.; Nielsen, S.N.; Holst, H.; et al. Increments in DNA-thioguanine level during thiopurine-enhanced maintenance therapy of acute lymphoblastic leukemia. Haematologica 2021, 106, 2824–2833. [Google Scholar] [CrossRef]
- Koch, B.C.P.; Muller, A.E.; Hunfeld, N.G.M.; de Winter, B.C.M.; Ewoldt, T.M.J.; Abdulla, A.; Endeman, H. Therapeutic drug monitoring of antibiotics in critically III patients: Current practice and future perspectives with a focus on clinical outcome. Ther. Drug Monit. 2022, 44, 11–18. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Wang, X.; Wang, Y.; Xiao, Y.; Zhuo, Z.; Li, Y. A versatile technique based on surface-enhanced Raman spectroscopy for label-free detection of amino acids and peptide formation in body fluids. Mikrochim. Acta 2022, 189, 82. [Google Scholar] [CrossRef]
- Markina, N.E.; Markin, A.V.; Weber, K.; Popp, J.; Cialla-May, D. Liquid-liquid extraction-assisted SERS-based determination of sulfamethoxazole in spiked human urine. Anal. Chim. Acta 2020, 1109, 61–68. [Google Scholar] [CrossRef]
- Dina, N.E.; Gherman, A.M.R.; Colniță, A.; Marconi, D.; Sârbu, C. Fuzzy characterization and classification of bacteria species detected at single-cell level by surface-enhanced Raman scattering. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 247, 119149. [Google Scholar] [CrossRef]
- Ding, J.; Lin, Q.; Zhang, J.; Young, G.M.; Jiang, C.; Zhong, Y.; Zhang, J. Rapid identification of pathogens by using surface-enhanced Raman spectroscopy and multi-scale convolutional neural network. Anal. Bioanal. Chem. 2021, 413, 3801–3811. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, A.B.; Czaplicka, M.; Szymborski, T.; Kamińska, A. Combined negative dielectrophoresis with a flexible SERS plat-form as a novel strategy for rapid detection and identification of bacteria. Anal. Bioanal. Chem. 2021, 413, 2007–2020. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Wang, S.; Shao, Q.; Cheng, Y. A Rapid and facile analytical approach to detecting salmonella enteritidis with ap-tamer-based surface-enhanced Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 267, 120625. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Ali, S.; Xu, Y.; Ouyang, Q.; Chen, Q. A SERS aptasensor based on AuNPs functionalized PDMS film for selective and sensitive detection of staphylococcus aureus. Biosens. Bioelectron. 2021, 172, 112806. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Li, D.; Yin, P.; Xie, Q.; Li, Y.; Lin, Q.; Duan, Y. A novel surface-enhanced Raman scattering (SERS) strategy for ultrasensitive detection of bacteria based on three-dimensional (3D) DNA walker. Biosens. Bioelectron. 2021, 172, 112758. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Amaya, S.; Lin, L.-K.; Deering, A.J.; Stanciu, L.A. Aptamer-based SERS biosensor for whole cell analytical detection of E. coli O157:H7. Anal. Chim. Acta 2019, 1081, 146–156. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Yang, J.-Y.; Wang, Y.-T.; Zhang, H.-C.; Chen, M.-L.; Yang, T.; Wang, J.-H. M13 phage-based nanoprobe for SERS detection and inactivation of staphylococcus aureus. Talanta 2021, 221, 121668. [Google Scholar] [CrossRef]
- Pang, Y.; Wan, N.; Shi, L.; Wang, C.; Sun, Z.; Xiao, R.; Wang, S. Dual-recognition surface-enhanced Raman scattering (SERS) biosensor for pathogenic bacteria detection by using vancomycin-SERS tags and aptamer-Fe3O4@Au. Anal. Chim. Acta 2019, 1077, 288–296. [Google Scholar] [CrossRef]
- Li, A.; Zuo, P.; Ye, B.-C. An aptamer biosensor based dual signal amplification system for the detection of salmonella typhimurium. Anal. Biochem. 2021, 615, 114050. [Google Scholar] [CrossRef]
- Xie, B.; Wang, Z.-P.; Zhang, R.; Zhang, Z.; He, Y. A SERS aptasensor based on porous Au-NC nanoballoons for staphylococcus aureus detection. Anal. Chim. Acta 2022, 1190, 339175. [Google Scholar] [CrossRef]
- Tian, T.; Yi, J.; Liu, Y.; Li, B.; Liu, Y.; Qiao, L.; Zhang, K.; Liu, B. Self-assembled plasmonic nanoarrays for enhanced bacterial identification and discrimination. Biosens. Bioelectron. 2022, 197, 113778. [Google Scholar] [CrossRef] [PubMed]
- Gukowsky, J.C.; Yang, T.; He, L. Assessment of three SERS approaches for studying E. coli O157:H7 susceptibility to ampicillin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 264, 120239. [Google Scholar] [CrossRef] [PubMed]
- Andrei, C.-C.; Moraillon, A.; Lau, S.; Félidj, N.; Yamakawa, N.; Bouckaert, J.; Larquet, E.; Boukherroub, R.; Ozanam, F.; Szunerits, S.; et al. Rapid and sensitive identification of uropathogenic escherichia coli using a surface-enhanced-Raman-scattering-based biochip. Talanta 2020, 219, 121174. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Dang, H.; Park, S.-G.; Chen, L.; Choo, J. SERS-PCR assays of SARS-CoV-2 target genes using au nanoparti-cles-internalized Au nanodimple substrates. Biosens. Bioelectron. 2022, 197, 113736. [Google Scholar] [CrossRef]
- Cha, H.; Kim, H.; Joung, Y.; Kang, H.; Moon, J.; Jang, H.; Park, S.; Kwon, H.-J.; Lee, I.-C.; Kim, S.; et al. Surface-enhanced Raman scattering-based immunoassay for severe acute respiratory syndrome coronavirus 2. Biosens. Bioelectron. 2022, 202, 114008. [Google Scholar] [CrossRef]
- Sanchez, J.E.; Jaramillo, S.A.; Settles, E.; Salazar, J.J.V.; Lehr, A.; Gonzalez, J.; Aranda, C.R.; Navarro-Contreras, H.R.; Rani-ere, M.O.; Harvey, M.; et al. Detection of SARS-CoV-2 and its S and N proteins using surface enhanced Raman spectroscopy. RSC Adv. 2021, 11, 25788–25794. [Google Scholar] [CrossRef]
- Peng, Y.; Lin, C.; Long, L.; Masaki, T.; Tang, M.; Yang, L.; Liu, J.; Huang, Z.; Li, Z.; Luo, X.; et al. Charge-transfer resonance and electromagnetic enhancement synergistically enabling MXenes with excellent sers sensitivity for SARS-CoV-2 S protein detection. Nanomicro. Lett. 2021, 13, 52. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, Y.; Lin, C.; Long, L.; Hu, J.; He, J.; Zeng, H.; Huang, Z.; Li, Z.-Y.; Tanemura, M.; et al. Human ACE2-functionalized gold “Virus-Trap” nanostructures for accurate capture of SARS-CoV-2 and single-virus SERS detection. Nano-Micro Lett. 2021, 13, 109. [Google Scholar] [CrossRef]
- Zavyalova, E.; Ambartsumyan, O.; Zhdanov, G.; Gribanyov, D.; Gushchin, V.; Tkachuk, A.; Rudakova, E.; Nikiforova, M.; Kuznetsova, N.; Popova, L.; et al. SERS-based aptasensor for rapid quantitative detection of SARS-CoV-2. Nanomaterials 2021, 11, 1394. [Google Scholar] [CrossRef]
- Chen, H.; Park, S.-G.; Choi, N.; Kwon, H.-J.; Kang, T.; Lee, M.-K.; Choo, J. Sensitive detection of SARS-CoV-2 using a SERS-based aptasensor. ACS Sens. 2021, 6, 2378–2385. [Google Scholar] [CrossRef]
- Gao, Y.; Han, Y.; Wang, C.; Qiang, L.; Gao, J.; Wang, Y.; Liu, H.; Han, L.; Zhang, Y. Rapid and sensitive triple-mode detection of causative SARS-CoV-2 virus specific genes through interaction between genes and nanoparticles. Anal. Chim. Acta 2021, 1154, 338330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, X.; Pan, J.; Zhang, Y.; Zhang, L.; Wang, C.; Yan, X.; Liu, X.; Lu, G. Ultrasensitive detection of SARS-CoV-2 spike protein in untreated saliva using SERS-based biosensor. Biosens. Bioelectron. 2021, 190, 113421. [Google Scholar] [CrossRef] [PubMed]
- Mochalov, K.; Samokhvalov, P.; Nifontova, G.; Tsoi, T.; Sukhanova, A.; Nabiev, I. Surface-enhanced Raman scattering of CoV-SARS-2 viral proteins in a strong coupling regime. J. Phys. Conf. Ser. 2021, 2058, 012020. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, X.; Ma, R.; Deng, S.; Wang, X.; Wang, X.; Zhang, X.; Huang, X.; Liu, Y.; Li, G.; et al. Ultra-fast and onsite interrogation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in waters via surface enhanced Raman scattering (SERS). Water Res. 2021, 200, 117243. [Google Scholar] [CrossRef]
- Pramanik, A.; Gao, Y.; Patibandla, S.; Mitra, D.; McCandless, M.G.; Fassero, L.A.; Gates, K.; Tandon, R.; Ray, P.C. The rapid diagnosis and effective inhibition of coronavirus using spike antibody attached gold nanoparticles. Nanoscale Adv. 2021, 3, 1588–1596. [Google Scholar] [CrossRef]
- Dell’Olio, F. Multiplexed liquid biopsy and tumor imaging using surface-enhanced Raman scattering. Biosensors 2021, 11, 449. [Google Scholar] [CrossRef]
- Kowalska, A.A.; Nowicka, A.B.; Szymborski, T.; Piecyk, P.; Kamińska, A. SERS-based sensor for direct L-selectin level determination in plasma samples as alternative method of tumor detection. J. Biophotonics 2021, 14, e202000318. [Google Scholar] [CrossRef]
- Gholami, M.D.; Sonar, P.; Ayoko, G.A.; Izake, E.L. A highly sensitive SERS quenching nanosensor for the determination of tumor necrosis factor alpha in blood. Sens. Actuators B Chem. 2020, 310, 127867. [Google Scholar] [CrossRef]
- Muhammad, M.; Shao, C.; Huang, Q. Aptamer-functionalized Au nanoparticles array as the effective SERS biosensor for label-free detection of interleukin-6 in serum. Sens. Actuators B Chem. 2021, 334, 129607. [Google Scholar] [CrossRef]
- Dong, S.; Wang, Y.; Liu, Z.; Zhang, W.; Yi, K.; Zhang, X.; Zhang, X.; Jiang, C.; Yang, S.; Wang, F.; et al. Beehive-inspired macroporous SERS probe for cancer detection through capturing and analyzing exosomes in plasma. ACS Appl. Mater. Interfaces 2020, 12, 5136–5146. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, H.; Li, X.; Chen, Y.; Gu, C.; Wei, G.; Zhou, J.; Jiang, T. Nonmetallic SERS-based immunosensor byintegrating MoS2 nanoflower and nanosheet towards the direct serum detection of carbohydrate antigen 19-9. Biosens. Bioelectron. 2021, 193, 113481. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.Y.; Goh, C.-H.; Thevarajah, T.M.; Goh, B.T.; Khor, S.M. Using SERS-based microfluidic paper-based device (ΜPAD) for calibration-free quantitative measurement of AMI cardiac biomarkers. Biosens. Bioelectron. 2020, 147, 111792. [Google Scholar] [CrossRef]
- Yang, S.J.; Lee, J.U.; Jeon, M.J.; Sim, S.J. Highly sensitive surface-enhanced Raman scattering-based immunosensor incorporating half antibody-fragment for quantitative detection of alzheimer’s disease biomarker in blood. Anal. Chim. Acta 2022, 1195, 339445. [Google Scholar] [CrossRef] [PubMed]
- Turan, E.; Zengin, A.; Suludere, Z.; Kalkan, N.Ö.; Tamer, U. Construction of a sensitive and selective plasmonic biosensor for prostate specific antigen by combining magnetic molecularly-imprinted polymer and surface-enhanced Raman spectroscopy. Talanta 2022, 237, 122926. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Sun, J.; Li, C.; Lin, L.; Gao, F.; Yang, M.; Sun, B.; Wang, Y. Long-term monitoring of blood biomarkers related to intrauterine growth restriction using AgNPs SERS tags-based lateral flow assay. Talanta 2022, 241, 123128. [Google Scholar] [CrossRef] [PubMed]
- False Positive Result—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/medicine-and-dentistry/false-positive-result (accessed on 25 February 2022).
- Song, Y.; Sun, J.; Zhao, S.; Gao, F.; Yuan, H.; Sun, B.; Wang, B.; Wang, Y. Based lateral flow immunosensor for ultrasensitive and selective surface-enhanced Raman spectroscopy stroke biomarkers detection. Appl. Surf. Sci. 2022, 571, 151153. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, S.; Liu, Q.; Gao, B.; Zhao, X.; Sun, F. SERS-based lateral flow immunoassay strip for ultrasensitive and quan-titative detection of acrosomal protein SP10. Microchem. J. 2022, 175, 107191. [Google Scholar] [CrossRef]
- Yan, S.; Liu, C.; Fang, S.; Ma, J.; Qiu, J.; Xu, D.; Li, L.; Yu, J.; Li, D.; Liu, Q. SERS-based lateral flow assay combined with machine learning for highly sensitive quantitative analysis of escherichia coli O157:H7. Anal. Bioanal. Chem. 2020, 412, 7881–7890. [Google Scholar] [CrossRef]
- Yadav, S.; Sadique, M.A.; Ranjan, P.; Kumar, N.; Singhal, A.; Srivastava, A.K.; Khan, R. SERS based lateral flow immunoassay for point-of-care detection of SARS-CoV-2 in clinical samples. ACS Appl. Bio. Mater. 2021, 4, 2974–2995. [Google Scholar] [CrossRef]
- Fornasaro, S.; Alsamad, F.; Baia, M.; de Carvalho, L.A.E.B.; Beleites, C.; Byrne, H.J.; Chiadò, A.; Chis, M.; Chisanga, M.; Daniel, A.; et al. Surface enhanced Raman spectroscopy for quantitative analysis: Results of a large-scale european multi-instrument interlaboratory study. Anal. Chem. 2020, 92, 4053–4064. [Google Scholar] [CrossRef]
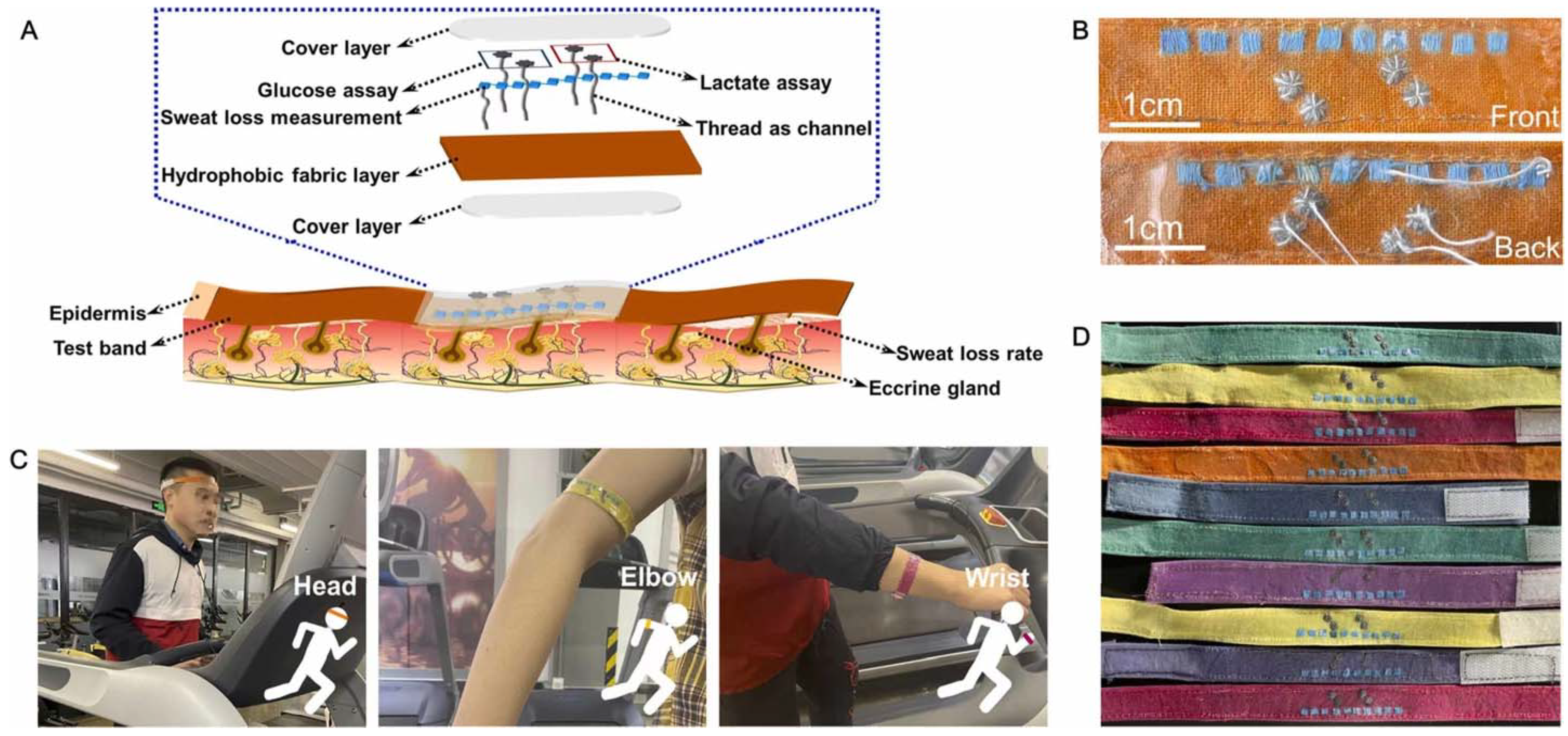
- Zhao, Z.; Li, Q.; Dong, Y.; Gong, J.; Li, Z.; Zhang, J. Core-Shell structured gold nanorods on thread-embroidered fabric-based microfluidic device for ex situ detection of glucose and lactate in sweat. Sens. Actuators B Chem. 2022, 353, 131154. [Google Scholar] [CrossRef]
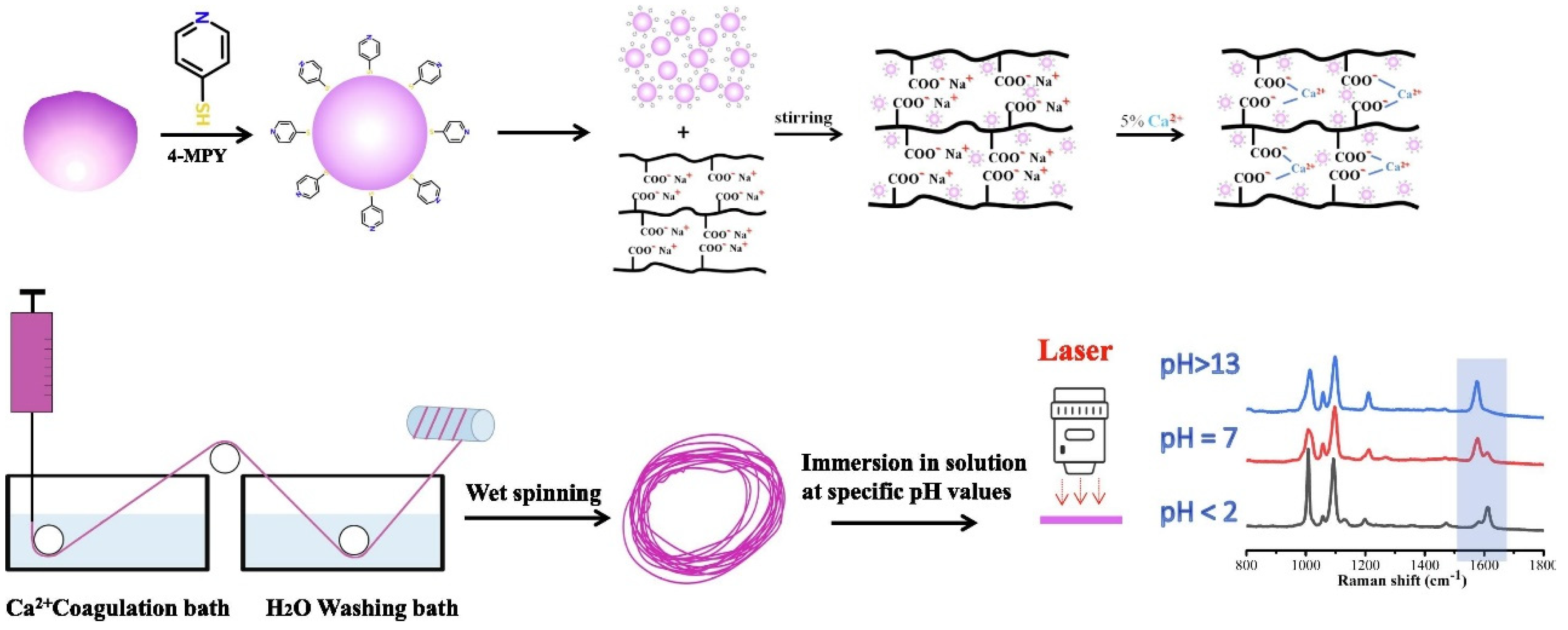
- Zhang, Y.; Zhou, J.; He, Y.; Ye, Y.; An, J. SERS active fibers from wet-spinning of alginate with gold nanoparticles for PH sensing. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 271, 120848. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Tian, Y.; Jiao, A.; Wang, C.; Zhang, M.; Zheng, L.; Li, S.; Chen, M. Silk fibroin-decorated with tunable Au/Ag nanodendrites: A plastic near-infrared SERS substrate with periodic microstructures for ultra-sensitive monitoring of lactic acid in human sweat. Vib. Spectrosc. 2022, 118, 103330. [Google Scholar] [CrossRef]
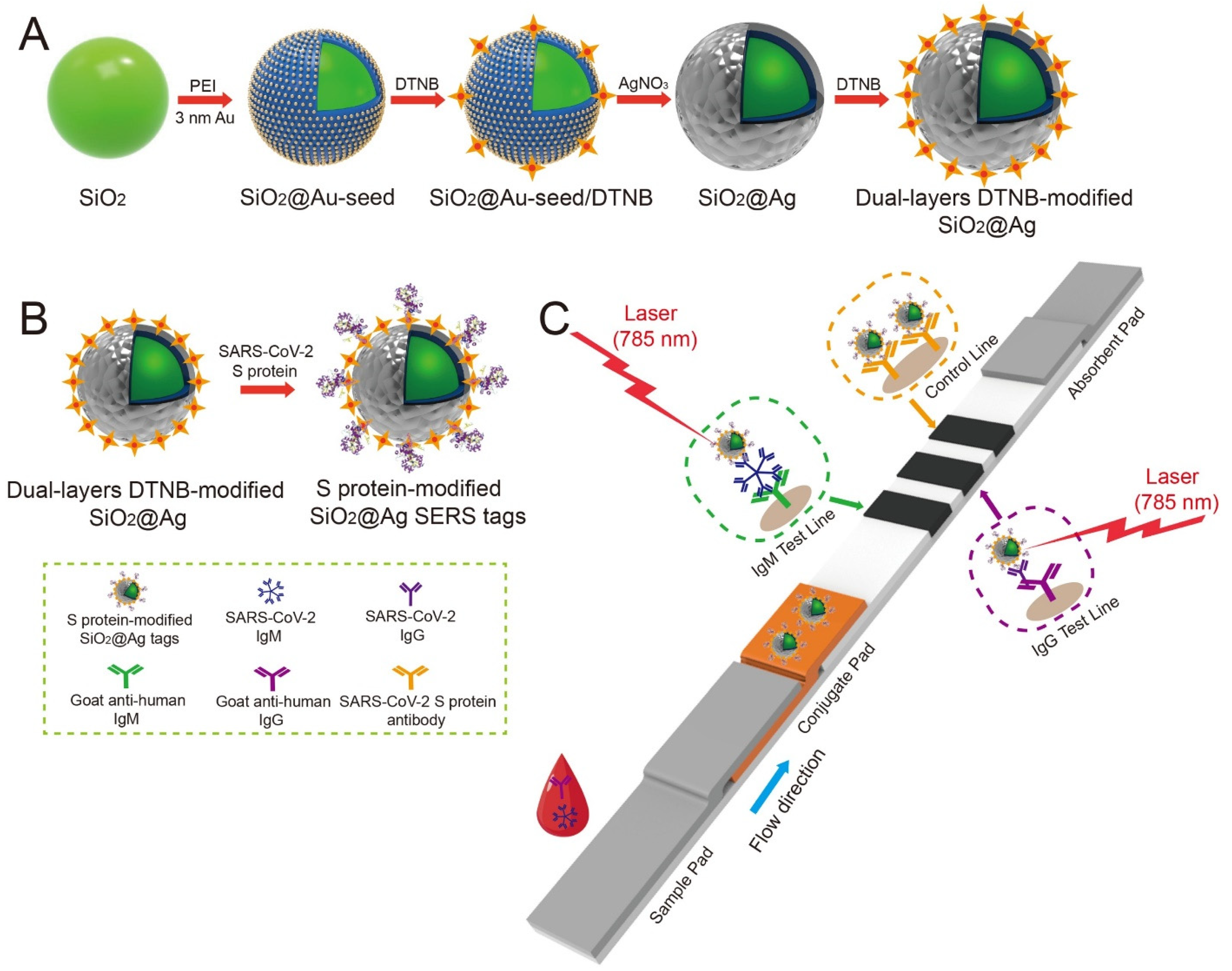
- Liu, H.; Dai, E.; Xiao, R.; Zhou, Z.; Zhang, M.; Bai, Z.; Shao, Y.; Qi, K.; Tu, J.; Wang, C.; et al. Development of a SERS-based lateral flow immunoassay for rapid and ultra-sensitive detection of Anti-SARS-CoV-2 IgM/IgG in clinical samples. Sens. Actuators B Chem. 2021, 329, 129196. [Google Scholar] [CrossRef]
- Chen, S.; Meng, L.; Wang, L.; Huang, X.; Ali, S.; Chen, X.; Yu, M.; Yi, M.; Li, L.; Chen, X.; et al. SERS-based lateral flow immunoassay for sensitive and simultaneous detection of Anti-SARS-CoV-2 IgM and IgG antibodies by using gap-enhanced Raman nanotags. Sens. Actuators B Chem. 2021, 348, 130706. [Google Scholar] [CrossRef]
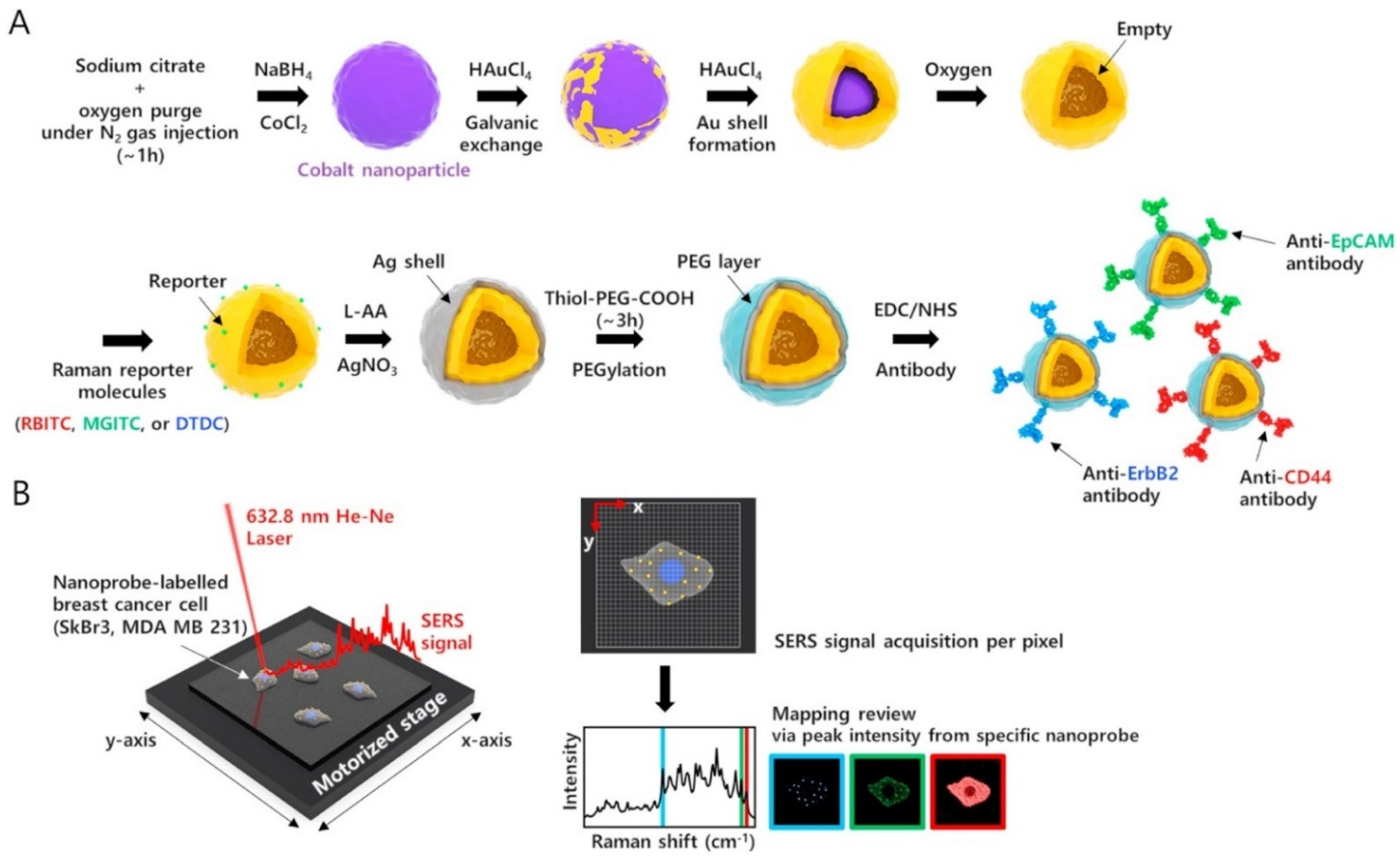
- Choi, N.; Dang, H.; Das, A.; Sim, M.S.; Chung, I.Y.; Choo, J. SERS biosensors for ultrasensitive detection of multiple biomarkers expressed in cancer cells. Biosens. Bioelectron. 2020, 164, 112326. [Google Scholar] [CrossRef]
- Guo, X.; Wu, X.; Sun, M.; Xu, L.; Kuang, H.; Xu, C. Tetrahedron probes for ultrasensitive in situ detection of telomerase and surface glycoprotein activity in living cells. Anal. Chem. 2020, 92, 2310–2315. [Google Scholar] [CrossRef]
- Luo, S.; Ma, L.; Tian, F.; Gu, Y.; Li, J.; Zhang, P.; Yang, G.; Li, H.; Qu, L.-L. Fluorescence and surface-enhanced Raman scattering dual-mode nanoprobe for monitoring telomerase activity in living cells. Microchem. J. 2022, 175, 107171. [Google Scholar] [CrossRef]
- Lv, J.; Chang, S.; Wang, X.; Zhou, Z.; Chen, B.; Qian, R.; Li, D. Live-cell profiling of membrane sialic acids by fluorescence imaging combined with SERS labelling. Sens. Actuators B Chem. 2022, 351, 130877. [Google Scholar] [CrossRef]
- Kapara, A.; Paterson, K.A.F.; Brunton, V.G.; Graham, D.; Zagnoni, M.; Faulds, K. Detection of estrogen receptor alpha and assessment of fulvestrant activity in MCF-7 tumor spheroids using microfluidics and SERS. Anal. Chem. 2021, 93, 5862–5871. [Google Scholar] [CrossRef]
- Zhang, Y.; Tran, V.; Adanalic, M.; Schlücker, S. Chapter 9—ISERS microscopy: Point-of-care diagnosis and tissue imaging. In Principles and Clinical Diagnostic Applications of Surface-Enhanced Raman Spectroscopy; Wang, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 327–372. ISBN 978-0-12-821121-2. [Google Scholar]
- Rotter, L.K.; Berisha, N.; Hsu, H.-T.; Burns, K.H.; Andreou, C.; Kircher, M.F. Visualizing surface marker expression and intratumoral heterogeneity with SERRS-NPs imaging. Nanotheranostics 2022, 6, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Park, B.C.; Kim, Y.J.; Lee, J.H.; Koo, T.M.; Ko, M.J.; Kim, Y.K. Design of magnetic-plasmonic nanoparticle assemblies via interface engineering of plasmonic shells for targeted cancer cell imaging and separation. Small 2020, 16, 2001103. [Google Scholar] [CrossRef] [PubMed]
- Kenry, F.N.; Clark, L.; Panikkanvalappil, S.R.; Andreiuk, B.; Andreou, C. Advances in surface enhanced Raman spectroscopy for in vivo imaging in oncology. Nanotheranostics 2022, 6, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Karabeber, H.; Huang, R.; Iacono, P.; Samii, J.M.; Pitter, K.; Holland, E.C.; Kircher, M.F. Guiding brain tumor resection using surface-enhanced Raman scattering nanoparticles and a hand-held Raman scanner. ACS Nano 2014, 8, 9755–9766. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szaniawska, A.; Mazur, K.; Kwarta, D.; Pyrak, E.; Kudelski, A. How Surface-Enhanced Raman Spectroscopy Could Contribute to Medical Diagnoses. Chemosensors 2022, 10, 190. https://doi.org/10.3390/chemosensors10050190
Szaniawska A, Mazur K, Kwarta D, Pyrak E, Kudelski A. How Surface-Enhanced Raman Spectroscopy Could Contribute to Medical Diagnoses. Chemosensors. 2022; 10(5):190. https://doi.org/10.3390/chemosensors10050190
Chicago/Turabian StyleSzaniawska, Aleksandra, Kinga Mazur, Dominika Kwarta, Edyta Pyrak, and Andrzej Kudelski. 2022. "How Surface-Enhanced Raman Spectroscopy Could Contribute to Medical Diagnoses" Chemosensors 10, no. 5: 190. https://doi.org/10.3390/chemosensors10050190
APA StyleSzaniawska, A., Mazur, K., Kwarta, D., Pyrak, E., & Kudelski, A. (2022). How Surface-Enhanced Raman Spectroscopy Could Contribute to Medical Diagnoses. Chemosensors, 10(5), 190. https://doi.org/10.3390/chemosensors10050190






