Gas-Sensing Properties of Dissolved Gases in Insulating Material Adsorbed on SnO2–GeSe Monolayer
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
- (1)
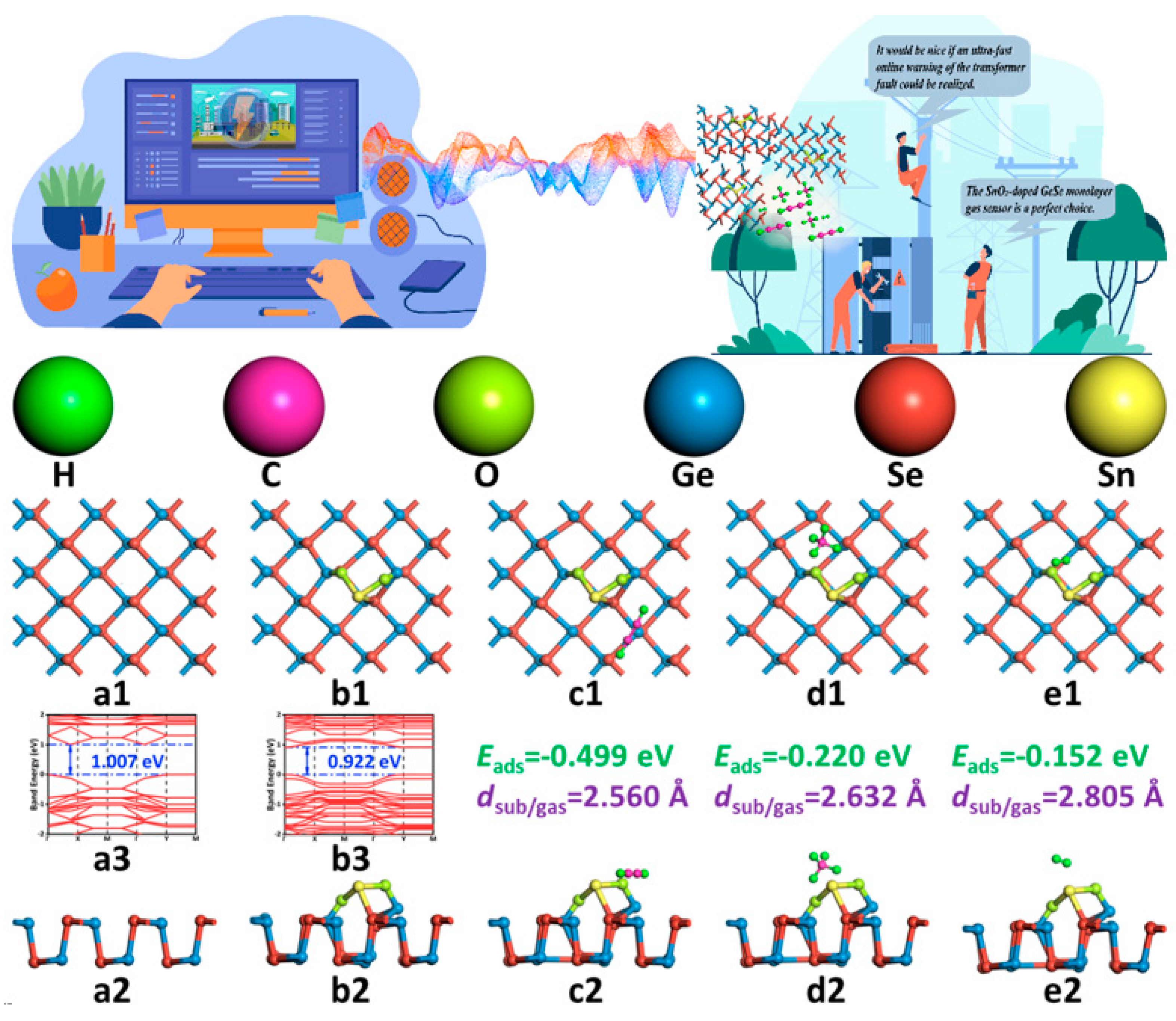
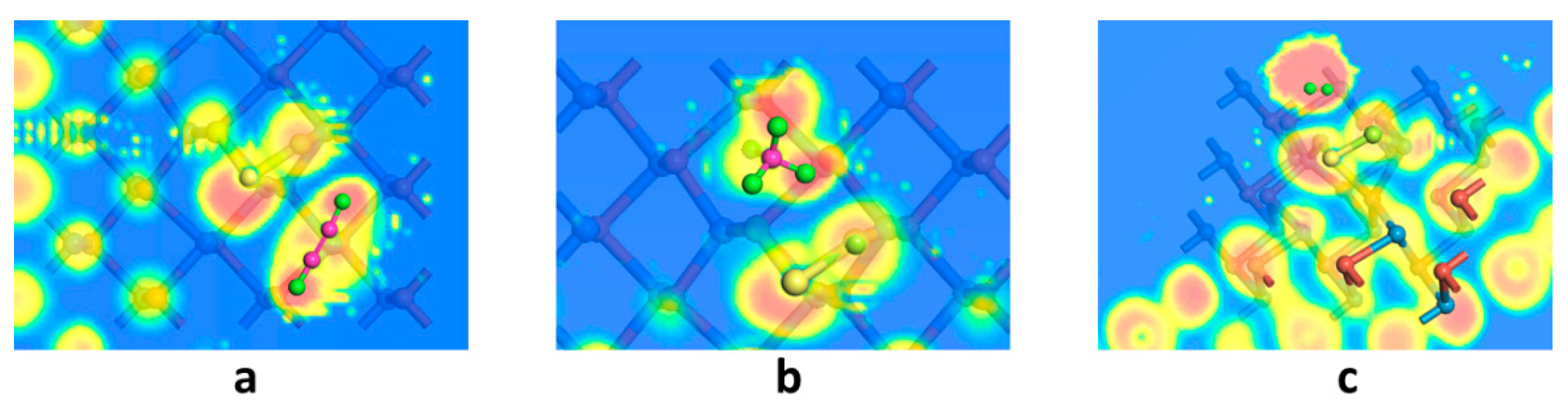
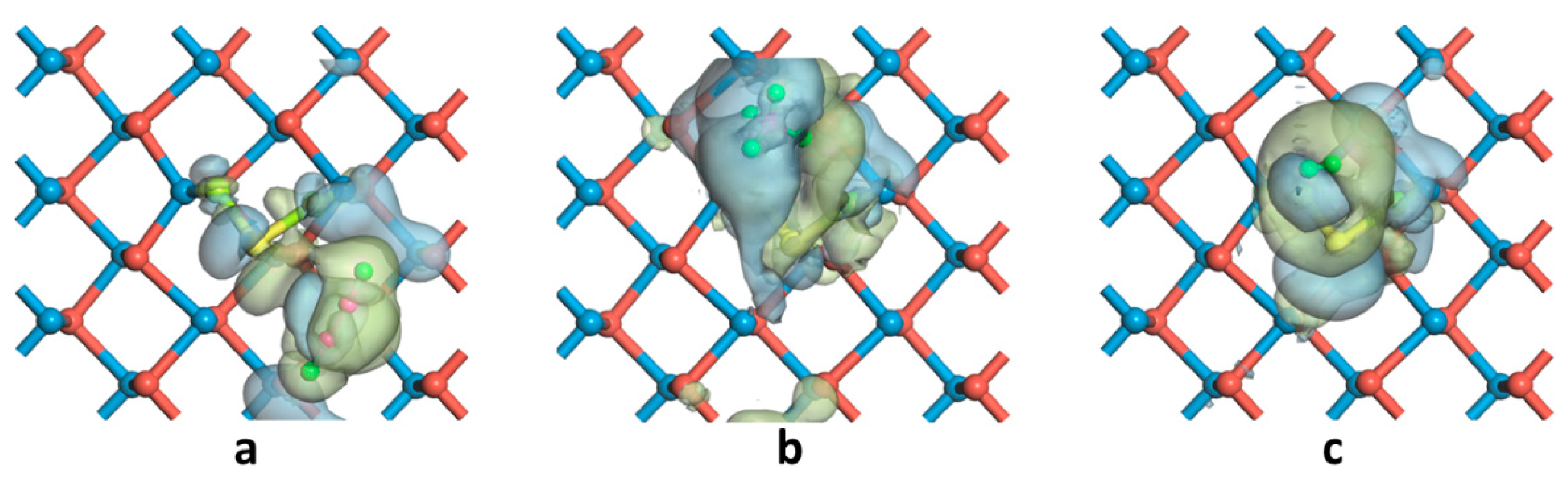
- The SnO2 unit’s doping on the GeSe monolayer surface effectively changed the electron cloud distribution, and it became the catalytic activity center for the adsorption of gas molecules on the GeSe monolayer surface, reducing the gas adsorption barrier on the substrate. At the same time, the SnO2 unit provided adsorption sites for gas molecules and promoted the interaction between gas molecules and the SnO2–GeSe monolayer system.
- (2)
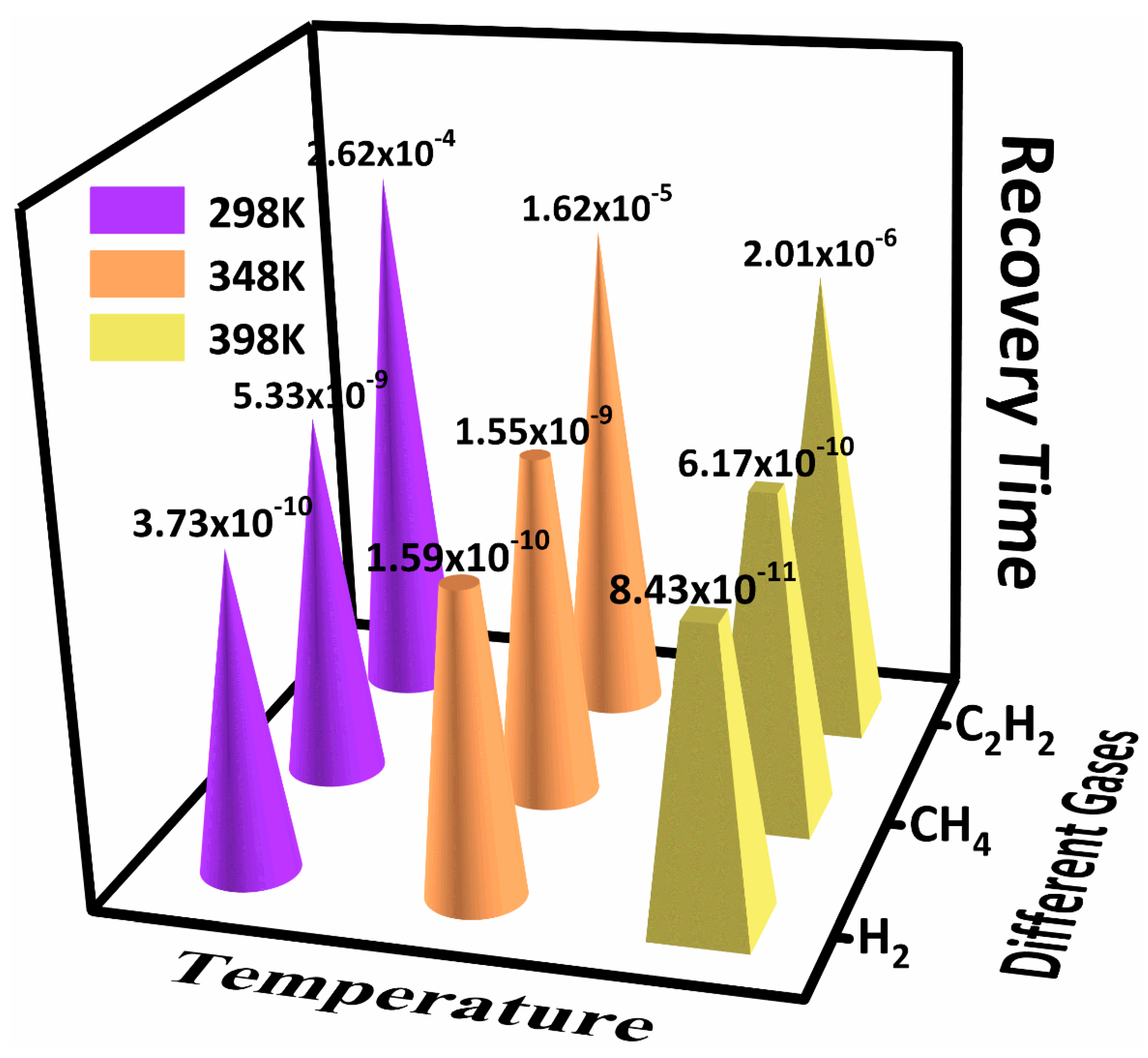
- Three kinds of adsorption systems were optimized based on the density functional theory, and the gas-sensing mechanism of the three gases interacting with the SnO2–GeSe monolayer was obtained. The kind of gas adsorption was physical adsorption, and the intensity and selectivity were C2H2 > CH4 > H2. The recovery time of the order was the opposite. When gas molecules adsorbed on the SnO2–GeSe monolayer surface, C2H2, and H2 gas molecules acted as electron donors, CH4 as an electron acceptor, and the SnO2–GeSe monolayer acted as electron acceptor and electron donor. Macroscopically, there was a significant difference in the system resistance value changes. Therefore, this study provides a theoretical basis for the preparation of the SnO2–GeSe monolayer, explored the potential of the SnO2–GeSe monolayer as a gas sensor for dissolved gases or other uses in insulating materials, and provided a new method of and idea for the development of other gas sensors.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, J.; Xu, M.; Cespedes, M.; Kauffman, M. Data Center Power System Stability—Part I: Power Supply Impedance Modeling. CSEE J. Power Energy Syst. 2022, 8, 403–419. [Google Scholar]
- Haes Alhelou, H.; Hamedani-Golshan, M.E.; Njenda, T.C.; Siano, P. A survey on power system blackout and cascading events: Research motivations and challenges. Energies 2019, 12, 682. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Yang, Y.; Blaabjerg, F. Power electronics: The enabling technology for renewable energy integration. CSEE J. Power Energy Syst. 2021, 8, 39–52. [Google Scholar]
- Yang, J.; Lu, W.; Liu, X. Prediction of Top Oil Temperature for Oil-immersed Transformers Based on PSO-LSTM. In Proceedings of the 2021 4th International Conference on Energy, Electrical and Power Engineering (CEEPE), Chengdu, China, 20–23 September 2017; pp. 278–283. [Google Scholar]
- Zheng, H.; Zhang, C.; Zhang, Y.; Liu, J.; Zhang, E.; Shi, Z.; Shao, G.; Shi, K.; Guo, J.; Zhang, C. Optimization of ethanol detection by automatic headspace method for cellulose insulation aging of oil-immersed transformers. Polymers 2020, 12, 1567. [Google Scholar] [CrossRef]
- Liu, J.; Fan, X.; Zhang, C.; Lai, C.S.; Zhang, Y.; Zheng, H.; Lai, L.L.; Zhang, E. Moisture diagnosis of transformer oil-immersed insulation with intelligent technique and frequency-domain spectroscopy. IEEE Trans. Ind. Inform. 2020, 17, 4624–4634. [Google Scholar] [CrossRef]
- Arguence, O.; Cadoux, F. Sizing power transformers in power systems planning using thermal rating. Int. J. Electr. Power Energy Syst. 2020, 118, 105781. [Google Scholar] [CrossRef]
- Ayalew, Z.; Kobayashi, K.; Matsumoto, S.; Kato, M. Dissolved gas analysis (DGA) of arc discharge fault in transformer insulation oils (ester and mineral oils). In Proceedings of the 2018 IEEE Electrical Insulation Conference (EIC), San Antonio, TX, USA, 17–20 June 2018; pp. 150–153. [Google Scholar]
- Sami, S.M.; Bhuiyan, M.I.H. An EMD-based Method for the Detection of Power Transformer Faults with a Hierarchical Ensemble Classifier. In Proceedings of the 2020 11th International Conference on Electrical and Computer Engineering (ICECE), Dhaka, Bangladesh, 17–19 December 2020; pp. 206–209. [Google Scholar]
- Mharakurwa, E.T.; Nyakoe, G.N.; Akumu, A. Power transformer fault severity estimation based on dissolved gas analysis and energy of fault formation technique. J. Electr. Comput. Eng. 2019, 2019, 9674054. [Google Scholar] [CrossRef] [Green Version]
- Rezaie, S.; Bafghi, Z.G.; Manavizadeh, N.; Kordmahale, S.B. Highly Sensitive Detection of Dissolved Gases in Transformer Oil With Carbon-Doped ZnO Nanotube: A DFT Study. IEEE Sens. J. 2021, 22, 82–89. [Google Scholar] [CrossRef]
- Naganathan, G.S.; Senthilkumar, M.; Aiswariya, S.; Muthulakshmi, S.; Riyasen, G.S.; Priyadharshini, M.M. Internal fault diagnosis of power transformer using artificial neural network. Mater. Today Proc. 2021; in press. [Google Scholar]
- Rodríguez, J.; Contreras, J.; Gaytán, C. Evaluation and Interpretation of Dissolved Gas Analysis of Soybean-Based Natural Ester Insulating Liquid. IEEE Trns. Dielectr. Electr. Insul. 2021, 28, 1343–1348. [Google Scholar] [CrossRef]
- Zope, N.; Ali, S.I.; Padmanaban, S.; Bhaskar, M.S.; Mihet-Popa, L. Analysis of 132kV/33kV 15MVA power transformer dissolved gas using transport-X Kelman Kit through Duval’s triangle and Roger’s Ratio prediction. In Proceedings of the 2018 IEEE International Conference on Industrial Technology (ICIT), Lyon, France, 20–22 February 2018; pp. 1160–1164. [Google Scholar]
- Pei, L.; Hongbo, L.; Nannan, G.; Yan, Z.; Yanyan, Z.; Ying, P.; Qinghua, Y. Case Analysis of 220 kV Oil-immersed Current Transformer Defect. In Proceedings of the 2020 5th Asia Conference on Power and Electrical Engineering (ACPEE), Chengdu, China, 4–7 June 2020; pp. 2178–2182. [Google Scholar]
- Shutenko, O.; Kulyk, O. Analysis of gas content in oil-filled equipment with low energy density discharges. Int. J. Electr. Eng. Inform. 2020, 12, 258–277. [Google Scholar] [CrossRef]
- Balaraman, S.; Madavan, R.; Vedhanayaki, S.; Saroja, S.; Srinivasan, M.; Stonier, A.A. Fault Diagnosis and Asset Management of Power Transformer Using Adaptive Boost Machine Learning Algorithm. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1055, 012133. [Google Scholar] [CrossRef]
- Hashtroudi, H.; Yu, A.; Juodkazis, S.; Shafiei, M. Two-Dimensional Dy2O3-Pd-PDA/rGO Heterojunction Nanocomposite: Synergistic Effects of Hybridisation, UV Illumination and Relative Humidity on Hydrogen Gas Sensing. Chemosensors 2022, 10, 78. [Google Scholar] [CrossRef]
- Li, T.; Yin, W.; Gao, S.; Sun, Y.; Xu, P.; Wu, S.; Kong, H.; Yang, G.; Wei, G. The Combination of Two-Dimensional Nanomaterials with Metal Oxide Nanoparticles for Gas Sensors: A Review. Nanomaterials 2022, 12, 982. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Yoon, Y.S.; Kim, D.-J. Two-dimensional transition metal dichalcogenides and metal oxide hybrids for gas sensing. ACS Sens. 2018, 3, 2045–2060. [Google Scholar] [CrossRef]
- Wang, C.; Li, R.; Feng, L.; Xu, J. The SnO2/MXene Composite Ethanol Sensor Based on MEMS Platform. Chemosensors 2022, 10, 109. [Google Scholar] [CrossRef]
- Faglia, G.; Ferroni, M.; Dang, T.T.L.; Donarelli, M.; Rigoni, F.; Baratto, C. Vertically coupling ZnO nanorods onto MoS2 flakes for optical gas sensing. Chemosensors 2020, 8, 19. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.; Yao, L.; Cui, X.; Zhao, R.; Chen, T.; Xiao, X.; Wang, Y. A two-dimensional Ti3C2TX MXene@TiO2/MoS2 heterostructure with excellent selectivity for the room temperature detection of ammonia. J. Mater. Chem. A 2022, 10, 5505–5519. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Zeng, W. Preparation and Application of 2D MXene-Based Gas Sensors: A Review. Chemosensors 2021, 9, 225. [Google Scholar] [CrossRef]
- Maity, A.; Raychaudhuri, A.; Ghosh, B. High sensitivity NH3 gas sensor with electrical readout made on paper with perovskite halide as sensor material. Sci. Rep. 2019, 9, 7777. [Google Scholar] [CrossRef] [Green Version]
- Hou, C.; Tai, G.; Liu, Y.; Liu, X. Borophene gas sensor. Nano Res. 2022, 15, 2537–2544. [Google Scholar] [CrossRef]
- Qiao, X.; Xu, Y.; Yang, K.; Ma, J.; Li, C.; Wang, H.; Jia, L. Mo doped BiVO4 gas sensor with high sensitivity and selectivity towards H2S. Chem. Eng. J. 2020, 395, 125144. [Google Scholar] [CrossRef]
- Shakeel, A.; Rizwan, K.; Farooq, U.; Iqbal, S.; Altaf, A.A. Advanced polymeric/inorganic nanohybrids: An integrated platform for gas sensing applications. Chemosphere 2022, 294, 133772. [Google Scholar] [CrossRef]
- Sosa, A.N.; Santana, J.E.; Miranda, Á.; Pérez, L.A.; Rurali, R.; Cruz-Irisson, M. Transition metal-decorated germanene for NO, N2 and O2 sensing: A DFT study. Surf. Interfaces 2022, 30, 101886. [Google Scholar] [CrossRef]
- Zhou, K.L.; Wang, Z.; Han, C.B.; Ke, X.; Wang, C.; Jin, Y.; Zhang, Q.; Liu, J.; Wang, H.; Yan, H. Platinum single-atom catalyst coupled with transition metal/metal oxide heterostructure for accelerating alkaline hydrogen evolution reaction. Nat. Commun. 2021, 12, 3783. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hu, Y.; Liu, F.; Liu, H.; Xu, X.; Xue, Y.; Zhang, J.; Li, Y.; Tang, C. Electronic structure modulation of CoSe2 nanowire arrays by tin doping toward efficient hydrogen evolution. Int. J. Hydrog. Energy 2021, 46, 17133–17142. [Google Scholar] [CrossRef]
- Fan, H.; Mao, P.; Sun, H.; Wang, Y.; Mofarah, S.S.; Koshy, P.; Arandiyan, H.; Wang, Z.; Liu, Y.; Shao, Z. Recent advances of metal telluride anodes for high-performance lithium/sodium-ion batteries. Mater. Horiz. 2022, 9, 524–546. [Google Scholar] [CrossRef]
- Hu, Z.; Ding, Y.; Hu, X.; Zhou, W.; Yu, X.; Zhang, S. Recent progress in 2D group IV–IV monochalcogenides: Synthesis, properties and applications. Nanotechnology 2019, 30, 252001. [Google Scholar] [CrossRef]
- Liu, X.; Jia, S.; Yang, M.; Tang, Y.; Wen, Y.; Chu, S.; Wang, J.; Shan, B.; Chen, R. Activation of subnanometric Pt on Cu-modified CeO2 via redox-coupled atomic layer deposition for CO oxidation. Nat. Commun. 2020, 11, 4240. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Y.; Chen, D.; Miao, J.; Zhi, X.; Deng, S.; Lin, S.; Jin, H.; Cui, D. Nitrogen Dioxide Gas Sensor Based on Ag-Doped Graphene: A First-Principle Study. Chemosensors 2021, 9, 227. [Google Scholar] [CrossRef]
- Wang, G.; Yu, J.; Zheng, K.; Huang, Y.; Li, X.; Chen, X.; Tao, L.-Q. A monolayer composite of h-BN doped by a nano graphene domain: As sensitive material for SO2 gas detection. IEEE Electron Device Lett. 2020, 41, 1404–1407. [Google Scholar] [CrossRef]
- Peng, Z.; Tao, L.-Q.; Wang, G.; Zhang, F.; Sun, H.; Zhu, C.; Zou, S.; Yu, J.; Chen, X. The promotion of sulfuric vacancy in two-dimensional molybdenum disulfide on the sensing performance of SF6 decomposition components. Appl. Surf. Sci. 2022, 571, 151377. [Google Scholar] [CrossRef]
- Wang, G.; Zheng, K.; Huang, Y.; Yu, J.; Wu, H.; Chen, X.; Tao, L.-Q. An investigation of the positive effects of doping an Al atom on the adsorption of CO2 on BN nanosheets: A DFT study. Phys. Chem. Chem. Phys. 2020, 22, 9368–9374. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Tao, L.-Q.; Li, T.; Gao, X.; Wang, G.; Peng, Z.; Zhu, C.; Zou, S.; Gui, Y.; Xia, S.-Y. Sensing Characteristics of Toxic C₄F₇N Decomposition Products on Metallic-Nanoparticle Co-Doped BN Monolayer: A First Principles Study. IEEE Sens. J. 2021, 21, 13082–13089. [Google Scholar] [CrossRef]
- Peng, Z.; Tao, L.-Q.; Zheng, K.; Yu, J.; Wang, G.; Sun, H.; Zhu, C.; Zou, S.; Chen, X. Gas Sensor Based on Semihydrogenated and Semifluorinated h-BN for SF₆ Decomposition Components Detection. IEEE Trans. Electron Devices 2021, 68, 1878–1885. [Google Scholar] [CrossRef]
- Xia, S.-Y.; Tao, L.-Q.; Jiang, T.; Sun, H.; Li, J. Rh-doped h-BN monolayer as a high sensitivity SF6 decomposed gases sensor: A DFT study. Appl. Surf. Sci. 2021, 536, 147965. [Google Scholar] [CrossRef]
- Zhang, F.; Qiu, J.; Guo, H.; Wu, L.; Zhu, B.; Zheng, K.; Li, H.; Wang, Z.; Chen, X.; Yu, J. Theoretical investigations of novel Janus Pb2SSe monolayer as a potential multifunctional material for piezoelectric, photovoltaic, and thermoelectric applications. Nanoscale 2021, 13, 15611–15623. [Google Scholar] [CrossRef] [PubMed]
- Badr, E.A.; Bedair, M.; Shaban, S.M. Adsorption and performance assessment of some imine derivatives as mild steel corrosion inhibitors in 1.0 M HCl solution by chemical, electrochemical and computational methods. Mater. Chem. Phys. 2018, 219, 444–460. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Z.; Chen, D.; Cui, H.; Tang, J. Adsorption behaviour of SO2 and SOF2 gas on Rh-doped BNNT: A DFT study. Mol. Phys. 2020, 118, e1580394. [Google Scholar] [CrossRef]
- Zhao, J.; Buldum, A.; Han, J.; Lu, J.P. Gas molecule adsorption in carbon nanotubes and nanotube bundles. Nanotechnology 2002, 13, 195. [Google Scholar] [CrossRef]
- Azam, M.A.; Alias, F.M.; Tack, L.W.; Seman, R.N.A.R.; Taib, M.F.M. Electronic properties and gas adsorption behaviour of pristine, silicon-, and boron-doped (8, 0) single-walled carbon nanotube: A first principles study. J. Mol. Graph. 2017, 75, 85–93. [Google Scholar] [CrossRef]
- Shukri, M.; Saimin, M.; Yaakob, M.; Yahya, M.; Taib, M. Structural and electronic properties of CO and NO gas molecules on Pd-doped vacancy graphene: A first principles study. Appl. Surf. Sci. 2019, 494, 817–828. [Google Scholar] [CrossRef]
- Segall, M.; Lindan, P.J.; Probert, M.A.; Pickard, C.J.; Hasnip, P.J.; Clark, S.; Payne, M. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys.-Condes. Matter 2002, 14, 2717. [Google Scholar] [CrossRef]
- Delley, B. Time dependent density functional theory with DMol3. J. Phys. Condes. Matter 2010, 22, 384208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Cao, Y.; Yang, Z.; Wu, J. Nanoheterostructure construction and DFT study of Ni-doped In2O3 nanocubes/WS2 hexagon nanosheets for formaldehyde sensing at room temperature. ACS Appl. Mater. Interfaces 2020, 12, 11979–11989. [Google Scholar] [CrossRef]
- Liu, Z.; Gui, Y.; Xu, L.; Chen, X. Adsorption and sensing performances of transition metal (Ag, Pd, Pt, Rh, and Ru) modified WSe2 monolayer upon SF6 decomposition gases (SOF2 and SO2F2). Appl. Surf. Sci. 2022, 581, 152365. [Google Scholar] [CrossRef]
- Wang, X.; Gui, Y.; Sun, N.; Ding, Z.; Chen, X. A DFT calculation: Gas sensitivity of defect GeSe to air decomposition products (CO, NO and NO2). IEEE Sens. J. 2022. [Google Scholar] [CrossRef]
- Pan, Q.; Li, T.; Zhang, D. Ammonia gas sensing properties and density functional theory investigation of coral-like Au-SnSe2 Schottky junction. Sens. Actuator B-Chem. 2021, 332, 129440. [Google Scholar] [CrossRef]
- Liu, Y.; Gui, Y.; Xu, L.; Chen, X. Adsorption property of Co, Rh, and Pd-embedded g-C3N4 monolayer to SO2F2 gas. J. Mater. Res. Technol.-JMRT 2021, 15, 4790–4799. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, J.; Li, P.; Cao, Y. Room-temperature SO2 gas-sensing properties based on a metal-doped MoS nanoflower: An experimental and density functional theory investigation. J. Mater. Chem. A 2017, 5, 20666–20677. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Q.; Li, P.; Pang, M.; Luo, Y. Fabrication of Pd-decorated MoSe2 nanoflowers and density functional theory simulation toward ammonia sensing. IEEE Electron Device Lett. 2019, 40, 616–619. [Google Scholar] [CrossRef]
- Hu, X.; Gui, Y.; Zhu, S.; Chen, X. First-principles study of the adsorption behavior and sensing properties of C2H4 and C2H6 molecules on (CuO/TiO2)n (n = 1–3) cluster modified MoTe2 monolayer. Surf. Interfaces 2022, 31, 102003. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.-Y.; Liang, S.; Yang, Z.; Jin, L.; Tan, Y.; Huang, Z. Gas-Sensing Properties of Dissolved Gases in Insulating Material Adsorbed on SnO2–GeSe Monolayer. Chemosensors 2022, 10, 212. https://doi.org/10.3390/chemosensors10060212
Guo L-Y, Liang S, Yang Z, Jin L, Tan Y, Huang Z. Gas-Sensing Properties of Dissolved Gases in Insulating Material Adsorbed on SnO2–GeSe Monolayer. Chemosensors. 2022; 10(6):212. https://doi.org/10.3390/chemosensors10060212
Chicago/Turabian StyleGuo, Liang-Yan, Suning Liang, Zhi Yang, Lingfeng Jin, Yaxiong Tan, and Zhengyong Huang. 2022. "Gas-Sensing Properties of Dissolved Gases in Insulating Material Adsorbed on SnO2–GeSe Monolayer" Chemosensors 10, no. 6: 212. https://doi.org/10.3390/chemosensors10060212




