Differential Sensing of Antibiotics Using Metal Ions and Gold Nanoclusters Based on TMB–H2O2 System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of AuNCs
2.3. Selection and Optimization of the Reaction System of AuNCs-Metal Ions
2.4. Detection of Antibiotics Based on AuNCs-Metal Ions Reaction System
2.5. Antibiotics Identification
3. Results and Discussion
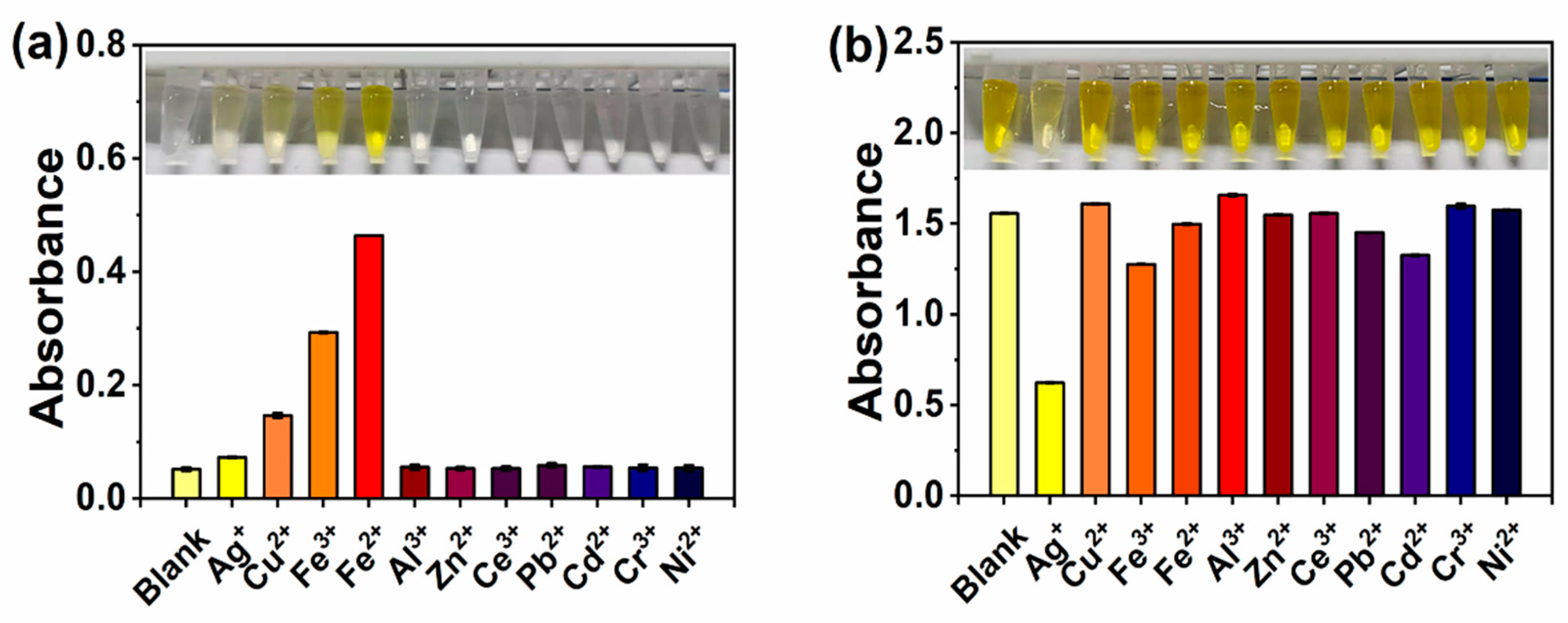
3.1. Effects of Metal Ions on the Catalytic Performance of AuNCs
3.2. Optimization of the Reaction System of AuNCs-Metal Ions
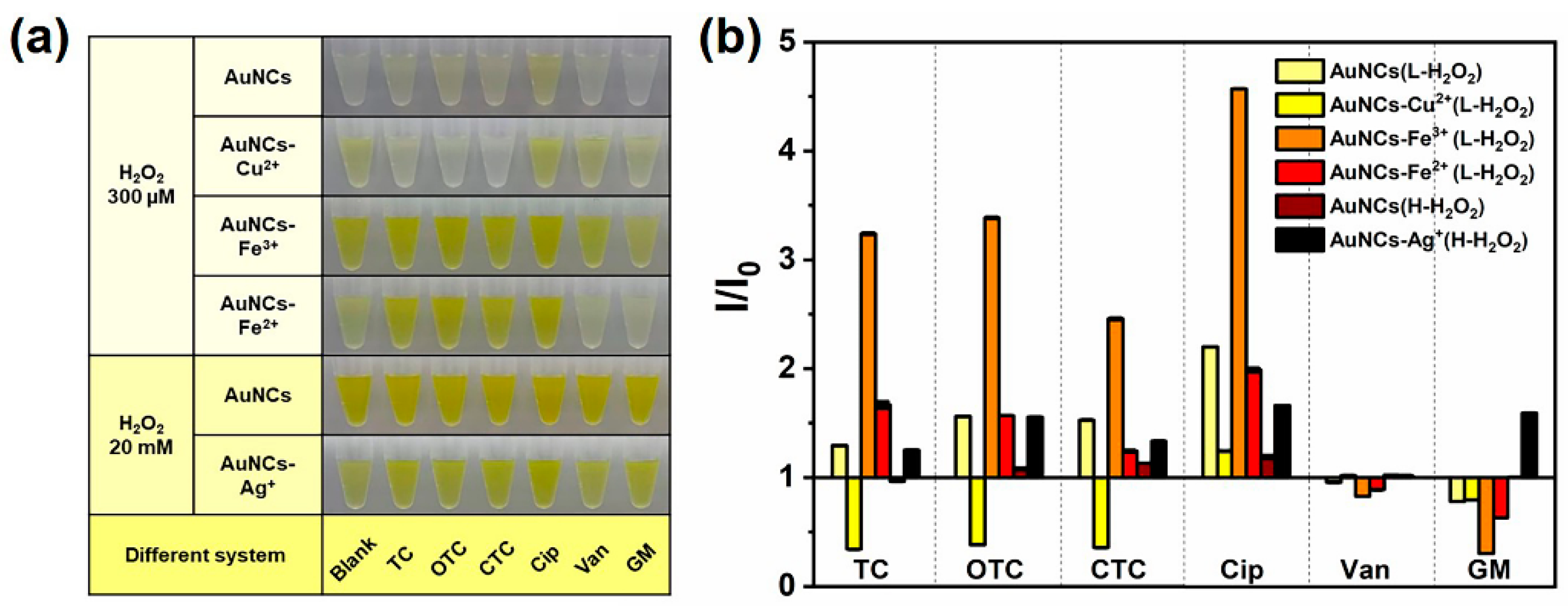
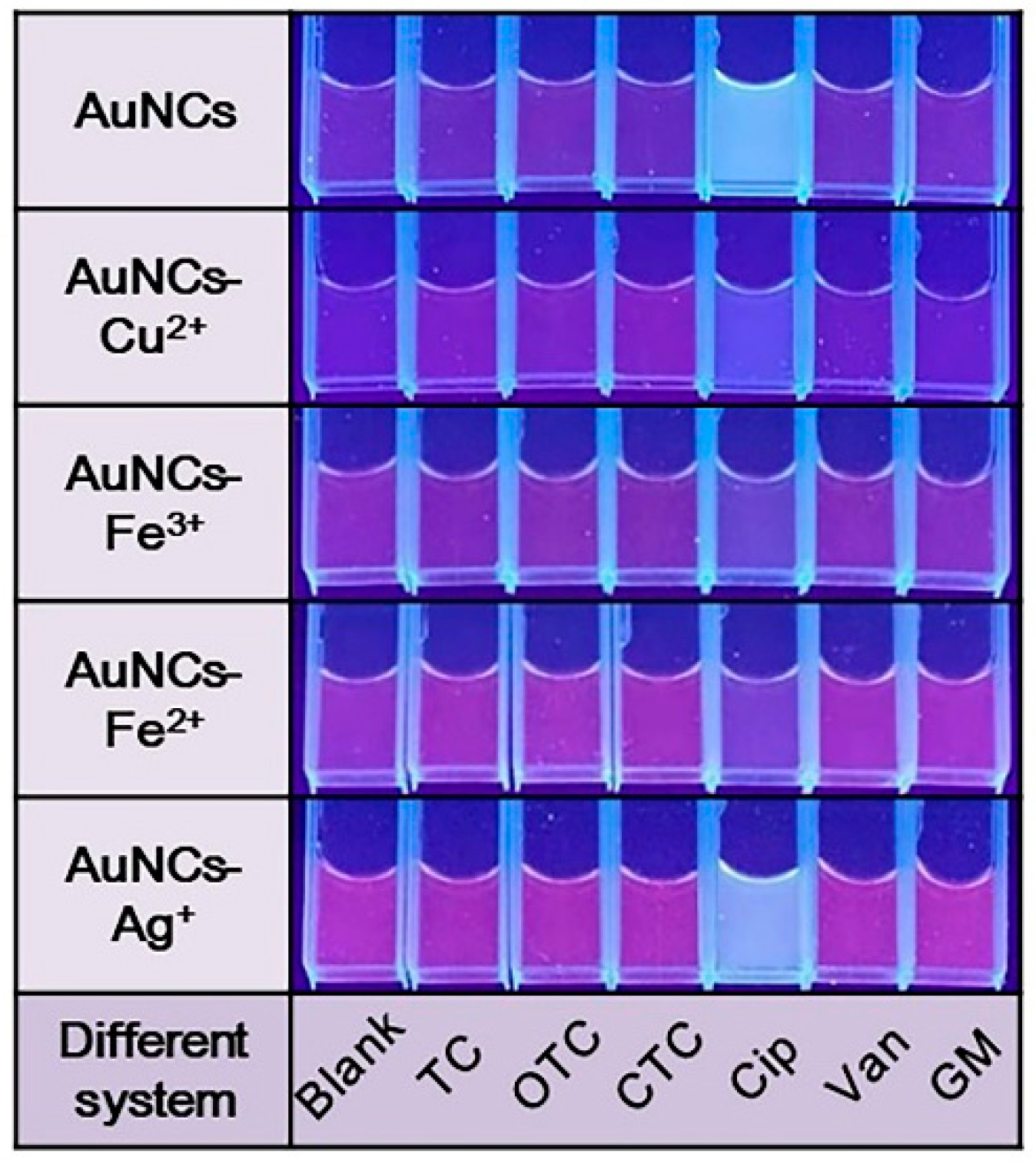
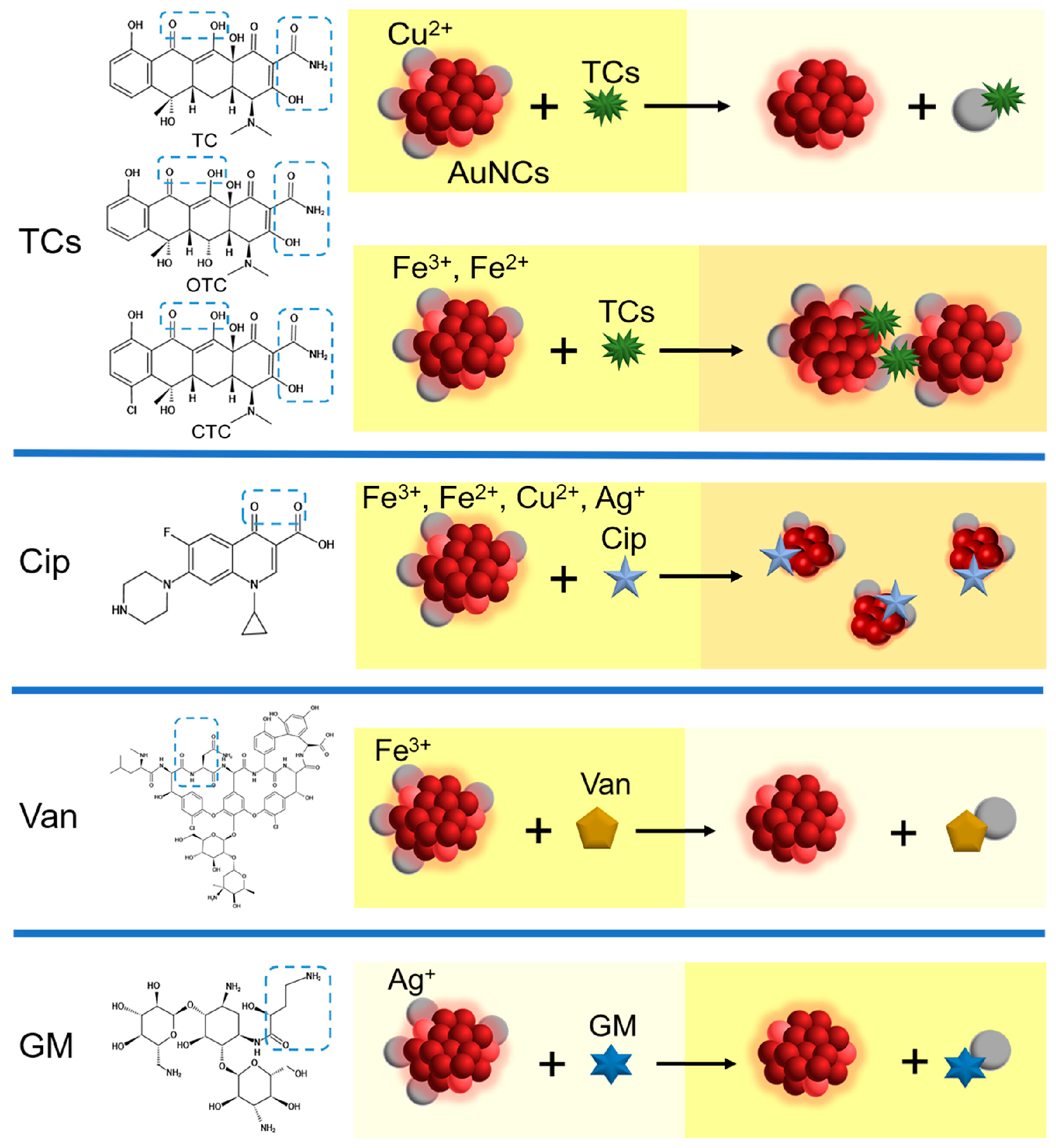
3.3. The Feasibility and Proposed Mechanism of Detecting Antibiotics with AuNCs-Metal Ions Reaction System
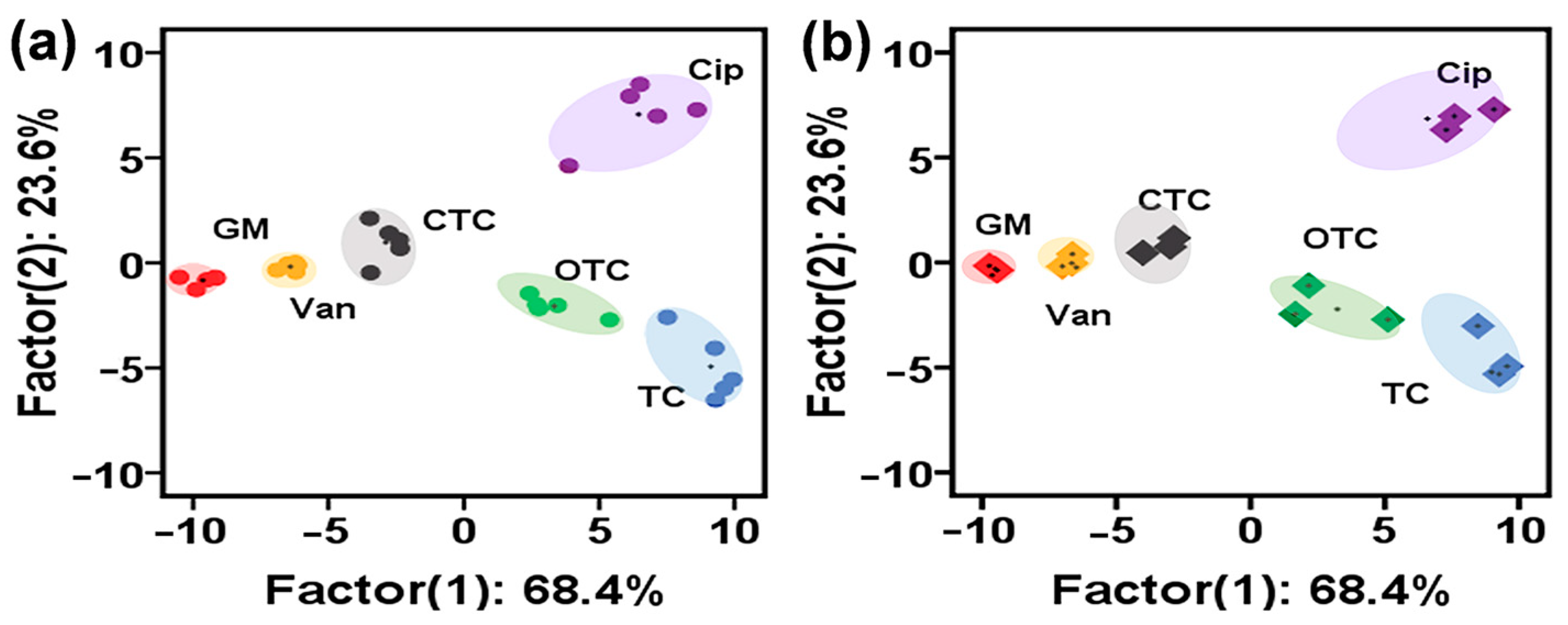
3.4. Detection of Antibiotics with AuNCs-Metal Ions Reaction System
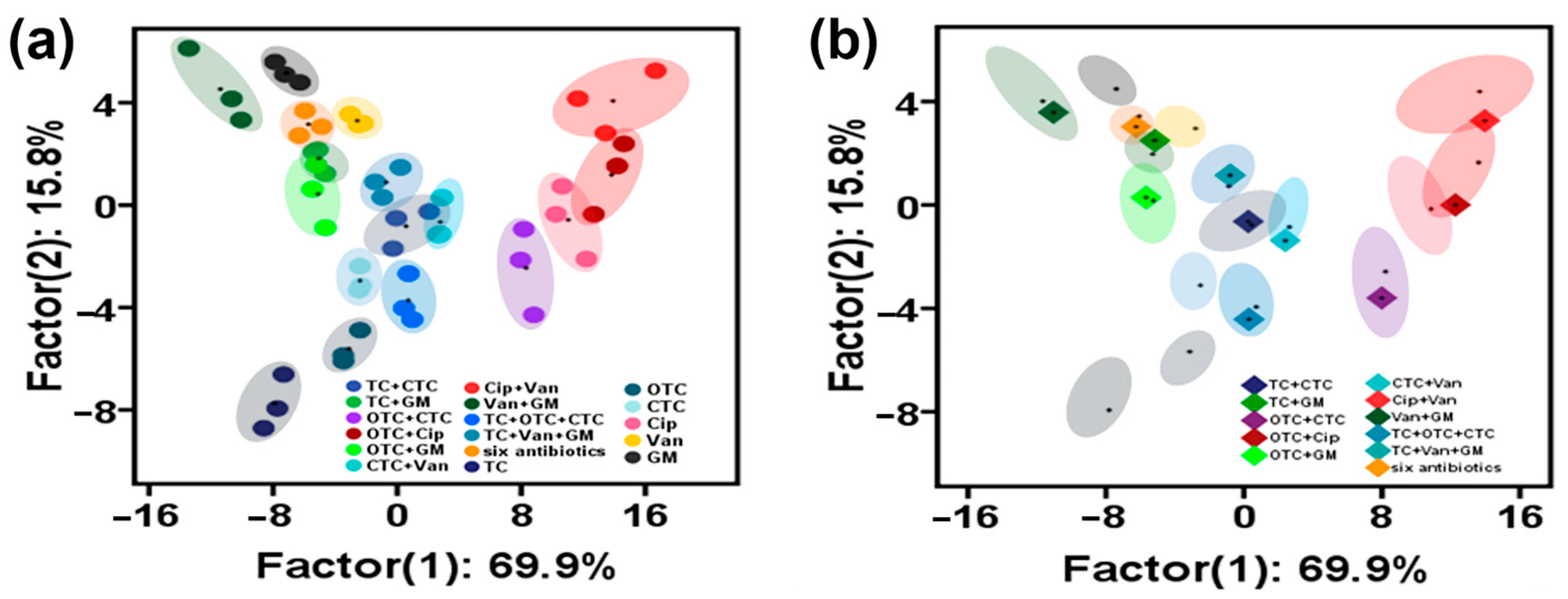
3.5. Classification and Identification of Multicomponent Antibiotics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, D.L.; Zhang, M.; He, L.X.; Zou, H.Y.; Liu, Y.S.; Li, B.B.; Yang, Y.Y.; Liu, C.; He, L.Y.; Ying, G.G. Contamination profile of antibiotic resistance genes in ground water in comparison with surface water. Sci. Total Environ. 2020, 715, 136975. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, M.; Montaseri, M.; Hosseinzadeh, S.; Majlesi, M.; Berizi, E.; Zare, M.; Derakhshan, Z.; Ferrante, M.; Conti, G.O. Antibiotic residues in poultry tissues in iran: A systematic review and meta-analysis. Environ. Res. 2021, 204, 112038. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hou, J.; Wang, X.; Hou, C.; Ji, Z.; He, Q.; Huo, D. A novel fluorescence probe for rapid and sensitive detection of tetracyclines residues based on silicon quantum dots. Spectrochim. Acta A 2020, 240, 118463. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chu, L.; Wojnarovits, L.; Takacs, E. Occurrence and fate of antibiotics, antibiotic resistant genes (ARGs) and antibiotic resistant bacteria (ARB) in municipal wastewater treatment plant: An overview. Sci. Total Environ. 2020, 744, 140997. [Google Scholar] [CrossRef] [PubMed]
- Kovalakova, P.; Cizmas, L.; McDonald, T.J.; Marsalek, B.; Feng, M.; Sharma, V.K. Occurrence and toxicity of antibiotics in the aquatic environment: A review. Chemosphere 2020, 251, 126351. [Google Scholar] [CrossRef]
- Oliver, J.P.; Gooch, C.A.; Lansing, S.; Schueler, J.; Hurst, J.J.; Sassoubre, L.; Crossette, E.M.; Aga, D.S. Invited review: Fate of antibiotic residues, antibiotic-resistant bacteria, and antibiotic resistance genes in US dairy manure management systems. J. Dairy Sci. 2020, 103, 1051–1071. [Google Scholar] [CrossRef]
- Zainab, S.M.; Junaid, M.; Xu, N.; Malik, R.N. Antibiotics and antibiotic resistant genes (ARGs) in groundwater: A global review on dissemination, sources, interactions, environmental and human health risks. Water Res. 2020, 187, 116455. [Google Scholar] [CrossRef]
- Wu, Q.; Shabbir, M.A.B.; Peng, D.; Yuan, Z.; Wang, Y. Microbiological inhibition-based method for screening and identifying of antibiotic residues in milk, chicken egg and honey. Food Chem. 2021, 363, 130074. [Google Scholar] [CrossRef]
- Pham, T.N.M.; Le, T.B.; Le, D.D.; Ha, T.H.; Nguyen, N.S.; Pham, T.D.; Hauser, P.C.; Nguyen, T.A.H.; Mai, T.D. Determination of carbapenem antibiotics using a purpose-made capillary electrophoresis instrument with contactless conductivity detection. J. Pharm. Biomed. 2020, 178, 112906. [Google Scholar] [CrossRef]
- Ravikumar, A.; Panneerselvam, P. A novel fluorescent sensing platform based on metal-polydopamine frameworks for the dual detection of kanamycin and oxytetracycline. Analyst 2019, 144, 2337–2344. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, L.; Shen, G.; Zhang, D.; Xie, J.; Mamut, A.; Huang, W.; Zhou, S. Colorimetric determination of ofloxacin using unmodified aptamers and the aggregation of gold nanoparticles. Microchim. Acta 2018, 185, 355. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Chen, X.; Liu, Y.; Li, Q.; Zeng, Z.; Maiyalagan, T.; Mao, S. Nanocomposites of Zr(IV)-based metal-organic frameworks and reduced graphene oxide for electrochemically sensing ciprofloxacin in water. Acs Appl. Nano Mater. 2019, 2, 2367–2376. [Google Scholar] [CrossRef]
- Acaroz, U.; Dietrich, R.; Knauer, M.; Märtlbauer, E. Development of a generic enzyme-immunoassay for the detection of fluoro(quinolone)-residues in foodstuffs based on a highly sensitive monoclonal antibody. Food Anal. Method. 2019, 13, 780–792. [Google Scholar] [CrossRef]
- Soheili, V.; Taghdisi, S.M.; Hassanzadeh Khayyat, M.; Fazly Bazzaz, B.S.; Ramezani, M.; Abnous, K. Colorimetric and ratiometric aggregation assay for streptomycin using gold nanoparticles and a new and highly specific aptamer. Microchim. Acta 2016, 183, 1687–1697. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, H.C.; Liu, J.; Wang, J.P. A receptor-based chemiluminescence enzyme linked immunosorbent assay for determination of tetracyclines in milk. Anal. Biochem. 2019, 564–565, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Lin, H.; Lin, J.; Yao-Say Solomon Adade, S.; Chen, Q.; Xue, Z.; Chan, C. Non-destructive detection of multi-component heavy metals in corn oil using nano-modified colorimetric sensor combined with near-infrared spectroscopy. Food Control 2022, 133, 108640. [Google Scholar] [CrossRef]
- Yu, Y.; Naik, S.S.; Oh, Y.; Theerthagiri, J.; Lee, S.J.; Choi, M.Y. Lignin-mediated green synthesis of functionalized gold nanoparticles via pulsed laser technique for selective colorimetric detection of lead ions in aqueous media. J. Hazard. Mater. 2021, 420, 126585. [Google Scholar] [CrossRef]
- Unnikrishnan, B.; Lien, C.W.; Chu, H.W.; Huang, C.C. A review on metal nanozyme-based sensing of heavy metal ions: Challenges and future perspectives. J. Hazard. Mater. 2021, 401, 123397. [Google Scholar] [CrossRef]
- Zhong, Y.H.; He, Y.; Zhou, H.Q.; Zheng, S.L.; Zeng, Q.; Chung, L.H.; Liao, W.M.; He, J. Enhanced stability and colorimetric detection on Ag(I) ions of a methylthio-functionalized Zn(II) metal-organic framework. J. Mater. Chem. C 2021, 9, 5088–5092. [Google Scholar] [CrossRef]
- Chen, Z.; Qian, S.; Chen, J.; Cai, J.; Wu, S.; Cai, Z. Protein-templated gold nanoclusters based sensor for off-on detection of ciprofloxacin with a high selectivity. Talanta 2012, 94, 240–245. [Google Scholar] [CrossRef]
- Nilghaz, A.; Lu, X. Detection of antibiotic residues in pork using paper-based microfluidic device coupled with filtration and concentration. Anal. Chim. Acta 2019, 1046, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Yan, H.; Liu, P.; Yao, Y.; Wu, Y.; Zhang, J.; Li, H.; Gong, X.; Chang, J. An ultra-sensitive and colorimetric sensor for copper and iron based on glutathione-functionalized gold nanoclusters. Anal. Chim. Acta 2016, 948, 73–79. [Google Scholar] [CrossRef]
- Ma, S.; Wang, J.; Yang, G.; Yang, J.; Ding, D.; Zhang, M. Copper(II) ions enhance the peroxidase-like activity and stability of keratin-capped gold nanoclusters for the colorimetric detection of glucose. Microchim. Acta 2019, 186, 271. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, J.; Mu, K.; Liu, S.; Yang, G.; Zhang, M.; Yang, J. Copper (II) ion-modified gold nanoclusters as peroxidase mimetics for the colorimetric detection of pyrophosphate. Sensors 2021, 21, 5538. [Google Scholar] [CrossRef]
- Song, Y.; Qiao, J.; Liu, W.; Qi, L. Enhancement of gold nanoclusters-based peroxidase nanozymes for detection of tetracycline. Microchem. J. 2020, 157, 104871. [Google Scholar] [CrossRef]
- Cowger, T.A.; Yang, Y.; Rink, D.E.; Todd, T.; Chen, H.; Shen, Y.; Yan, Y.; Xie, J. Protein-adsorbed magnetic-nanoparticle-mediated assay for rapid detection of bacterial antibiotic resistance. Bioconjug. Chem. 2017, 28, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Tomita, S.; Niwa, O.; Kurita, R. Artificial modification of an enzyme for construction of cross-reactive polyion complexes to fingerprint signatures of proteins and mammalian cells. Anal. Chem. 2016, 88, 9079–9086. [Google Scholar] [CrossRef]
- Yan, P.; Li, X.; Dong, Y.; Li, B.; Wu, Y. A pH-based sensor array for the detection and identification of proteins using CdSe/ZnS quantum dots as an indicator. Analyst 2019, 144, 2891–2897. [Google Scholar] [CrossRef]
- Mao, Y.; Cui, S.; Li, W.; Fan, X.; Liu, Y.; Xu, S.; Luo, X. A lab-on-a-carbon nanodot sensor array for simultaneous pattern recognition of multiple antibiotics. Sensor. Actuat. B-Chem. 2019, 296, 126694. [Google Scholar] [CrossRef]
- Yan, P.; Ding, Z.; Li, X.; Dong, Y.; Fu, T.; Wu, Y. Colorimetric sensor array based on wulff-type boronate functionalized AgNPs at various pH for bacteria identification. Anal. Chem. 2019, 91, 12134–12137. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zhu, N.; Zou, Y.; Wu, X.; Qu, G.; Shi, J. A novel, enzyme-linked immunosorbent assay based on the catalysis of AuNCs@BSA-induced signal amplification for the detection of dibutyl phthalate. Talanta 2018, 179, 64–69. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, Z. Colorimetric detection of Hg2+ by Au nanoparticles formed by H2O2 reduction of HAuCl4 using Au nanoclusters as the catalyst. Sensor. Actuat. B-Chem. 2017, 241, 1063–1068. [Google Scholar] [CrossRef]
- Li, S.; Zhang, L.; Jiang, Y.; Zhu, S.; Lv, X.; Duan, Z.; Wang, H. In-site encapsulating gold “nanowires” into hemin-coupled protein scaffolds through biomimetic assembly towards the nanocomposites with strong catalysis, electrocatalysis, and fluorescence properties. Nanoscale 2017, 9, 16005–16011. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, A.; Zhang, Y.; Bao, R.; Tian, X.; Li, J. Electro-Fenton degradation of antibiotic ciprofloxacin (CIP): Formation of Fe3+-CIP chelate and its effect on catalytic behavior of Fe2+/Fe3+ and CIP mineralization. Electrochim. Acta 2017, 256, 185–195. [Google Scholar] [CrossRef]
- Puicharla, R.; Mohapatra, D.P.; Brar, S.K.; Drogui, P.; Auger, S.; Surampalli, R.Y. A persistent antibiotic partitioning and co-relation with metals in wastewater treatment plant-chlortetracycline. J. Environ. Chem. Engl. 2014, 2, 1596–1603. [Google Scholar] [CrossRef]
- Yu, M.; Zhu, Z.; Wang, H.; Li, L.; Fu, F.; Song, Y.; Song, E. Antibiotics mediated facile one-pot synthesis of gold nanoclusters as fluorescent sensor for ferric ions. Biosens. Bioelectron. 2017, 91, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Qiu, S.; Wu, K.; Jia, M.; Wang, F.; Gu, C.; Zhang, A.; Jiang, X. The effect of Cu2+ chelation on the direct photolysis of oxytetracycline: A study assisted by spectroscopy analysis and DFT calculation. Environ. Pollut. 2016, 214, 831–839. [Google Scholar] [CrossRef]
- Wang, Y.W.; Tang, H.; Wu, D.; Liu, D.; Liu, Y.; Cao, A.; Wang, H. Enhanced bactericidal toxicity of silver nanoparticles by the antibiotic gentamicin. Environ. Sci. Nano 2016, 3, 788–798. [Google Scholar] [CrossRef]
- Wei, X.; Chen, J.; Xie, Q.; Zhang, S.; Li, Y.; Zhang, Y.; Xie, H. Photochemical behavior of antibiotics impacted by complexation effects of concomitant metals: A case for ciprofloxacin and Cu(II). Environ. Sci. Proc. Imp. 2015, 17, 1220–1227. [Google Scholar] [CrossRef]
- Pereira, J.H.O.S.; Queirós, D.B.; Reis, A.C.; Nunes, O.C.; Borges, M.T.; Boaventura, R.A.R.; Vilar, V.J.P. Process enhancement at near neutral pH of a homogeneous photo-fenton reaction using ferricarboxylate complexes: Application to oxytetracycline degradation. Chem. Eng. J. 2014, 253, 217–228. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Yao, H.; Sun, P.; Li, D.; Huang, C.H. Transformation of tetracycline antibiotics and Fe(II) and Fe(III) species induced by their complexation. Environ. Sci. Technol. 2016, 50, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Pan, X.; Mu, W.; Li, C.; Han, X. Detection of tetracycline in water using glutathione-protected fluorescent gold nanoclusters. Anal. Sci. 2019, 35, 367–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, B.; Zheng, X.; Li, H.; Ding, L.; Wang, F.; Guo, D.-Y.; Yang, W.; Pan, Q. A highly stable, rapid and sensitive fluorescent probe for ciprofloxacin based on Al3+-enhanced fluorescence of gold nanoclusters. Sensor. Actuat. B-Chem. 2021, 346, 130502. [Google Scholar] [CrossRef]
- Wei, W.; He, J.; Wang, Y.; Kong, M. Ratiometric method based on silicon nanodots and Eu3+ system for highly-sensitive detection of tetracyclines. Talanta 2019, 204, 491–498. [Google Scholar] [CrossRef]

| Antibiotics | Original (nM) | Spiked (nM) | Found (nM) | Recovery (%) |
|---|---|---|---|---|
| TC | not detected | 80 | 82.85 | 103.56 |
| not detected | 800 | 868.04 | 108.51 | |
| not detected | 8000 | 7695.51 | 96.19 | |
| OTC | not detected | 80 | 76.80 | 96.00 |
| not detected | 800 | 748.15 | 93.52 | |
| not detected | 8000 | 7907.34 | 98.84 | |
| CTC | not detected | 80 | 84.58 | 105.72 |
| not detected | 800 | 859.27 | 107.41 | |
| not detected | 8000 | 8088.48 | 101.12 | |
| Cip | not detected | 80 | 82.18 | 102.72 |
| not detected | 800 | 834.51 | 104.31 | |
| not detected | 8000 | 8693.04 | 108.67 | |
| Van | not detected | 80 | 85.33 | 106.66 |
| not detected | 800 | 847.50 | 105.94 | |
| not detected | 8000 | 7892.36 | 98.65 | |
| GM | not detected | 80 | 85.56 | 106.93 |
| not detected | 800 | 747.14 | 93.39 | |
| not detected | 8000 | 7787.14 | 97.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Wang, J.; Hu, Y.; Shi, Y.; Yang, J.; Zhang, M. Differential Sensing of Antibiotics Using Metal Ions and Gold Nanoclusters Based on TMB–H2O2 System. Chemosensors 2022, 10, 222. https://doi.org/10.3390/chemosensors10060222
Liu S, Wang J, Hu Y, Shi Y, Yang J, Zhang M. Differential Sensing of Antibiotics Using Metal Ions and Gold Nanoclusters Based on TMB–H2O2 System. Chemosensors. 2022; 10(6):222. https://doi.org/10.3390/chemosensors10060222
Chicago/Turabian StyleLiu, Suqin, Jinjie Wang, Yue Hu, Yunjing Shi, Jingxia Yang, and Min Zhang. 2022. "Differential Sensing of Antibiotics Using Metal Ions and Gold Nanoclusters Based on TMB–H2O2 System" Chemosensors 10, no. 6: 222. https://doi.org/10.3390/chemosensors10060222
APA StyleLiu, S., Wang, J., Hu, Y., Shi, Y., Yang, J., & Zhang, M. (2022). Differential Sensing of Antibiotics Using Metal Ions and Gold Nanoclusters Based on TMB–H2O2 System. Chemosensors, 10(6), 222. https://doi.org/10.3390/chemosensors10060222








