Promising Novel Barium Carbonate One-Dimensional Nanostructures and Their Gas Sensing Application: Preparation and Characterization
Abstract
:1. Introduction
2. Materials and Methods
3. Results
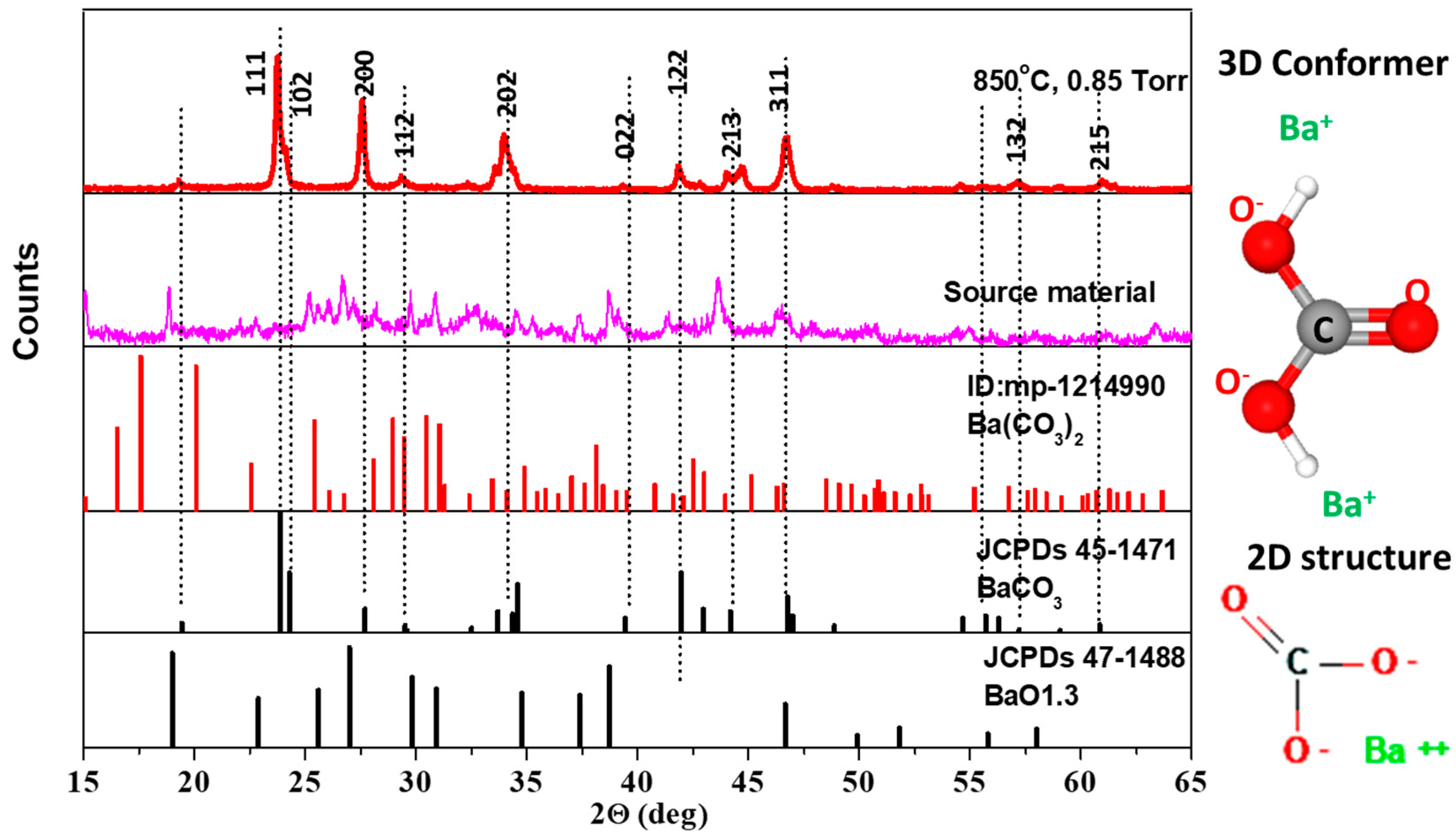
3.1. BaCO3 Characterizations
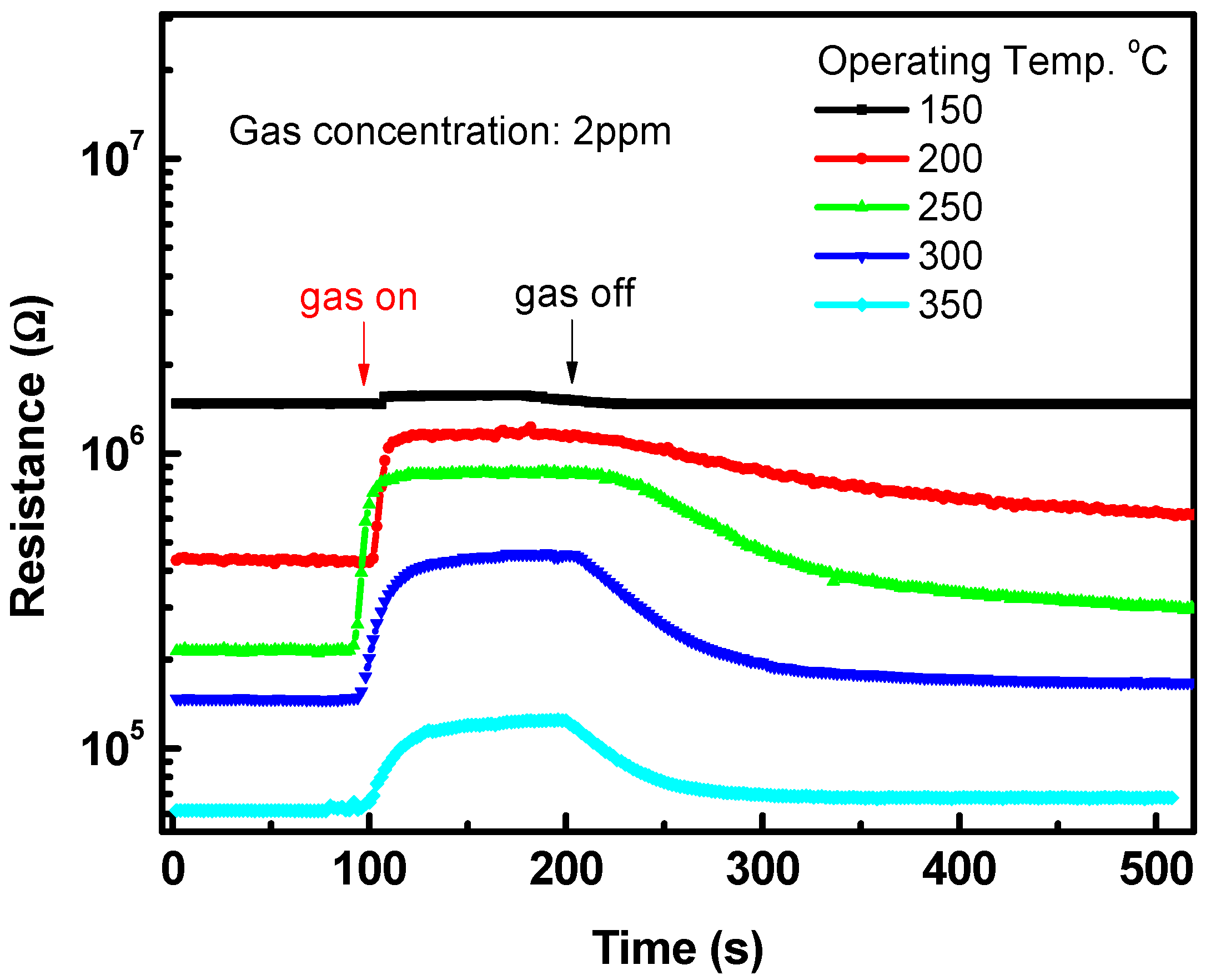
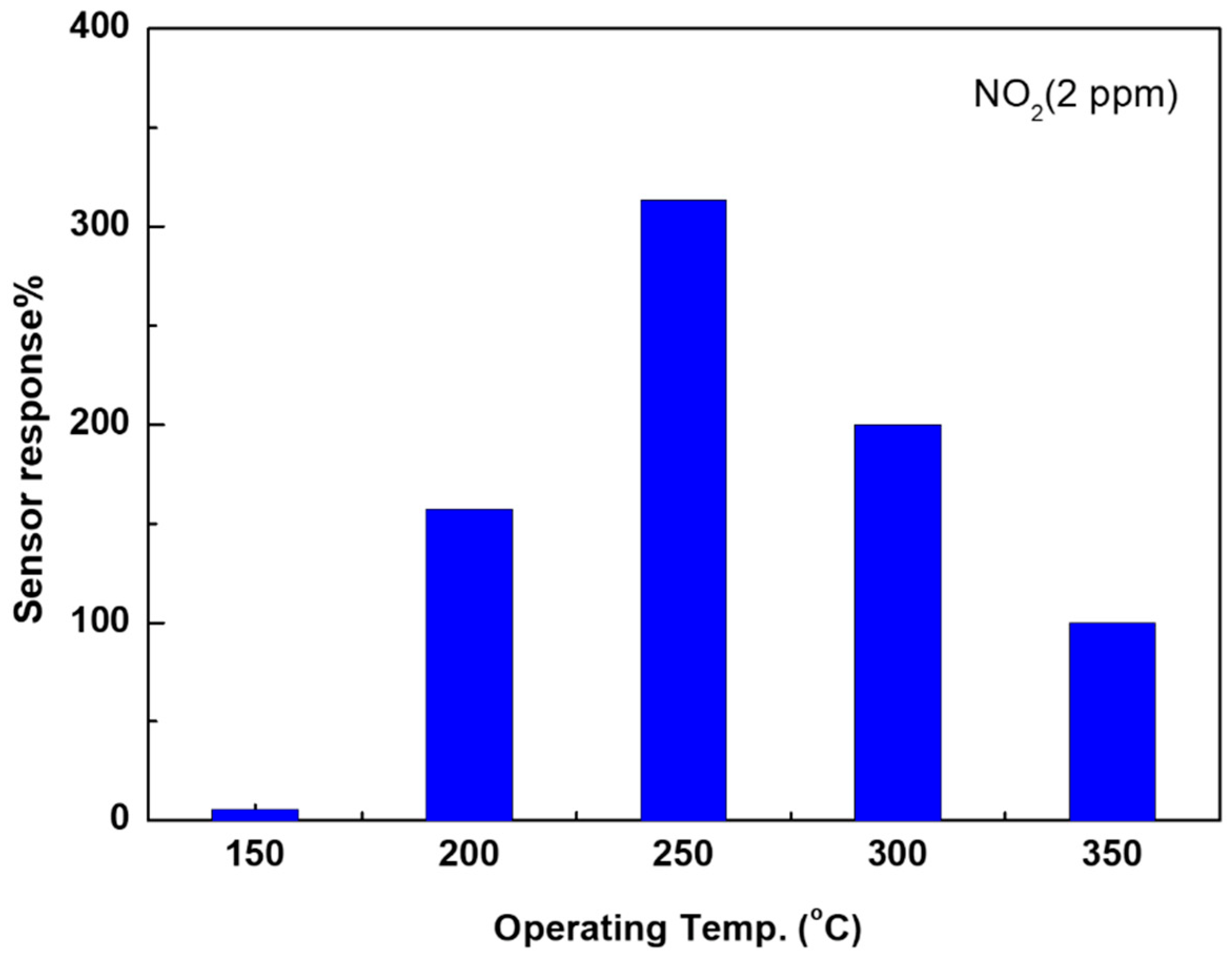
3.2. Sensing Characteristics of NO2 Gas
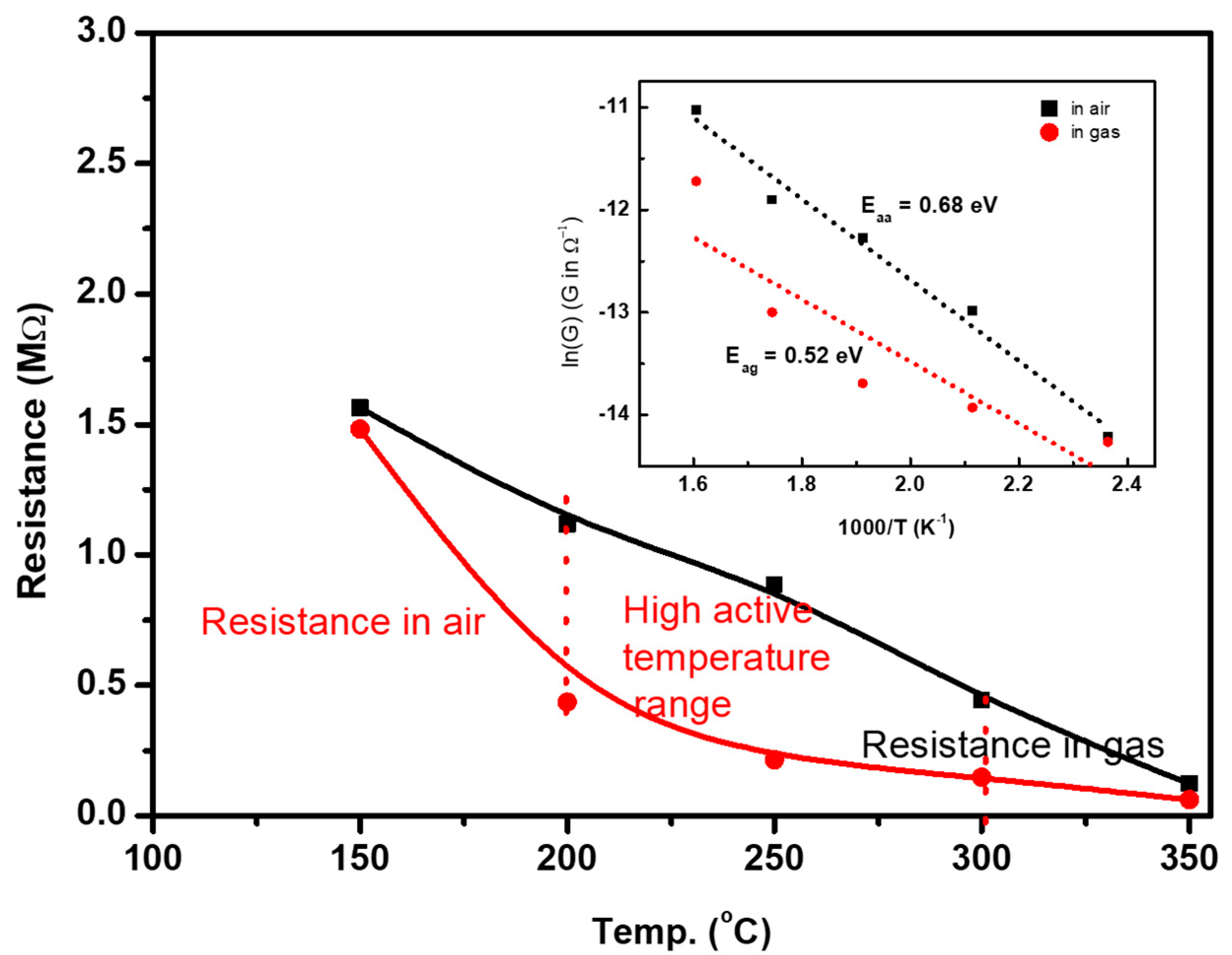
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shaalan, N.M.; Yamazaki, T.; Kikuta, T. Influence of morphology and structure geometry on NO2 gas-sensing characteristics of SnO2 nanostructures synthesized via a thermal evaporation method. Sens. Actuators B Chem. 2011, 153, 11–16. [Google Scholar] [CrossRef]
- Shaalan, N.M.; Yamazaki, T.; Kikuta, T. Effect of micro-electrode geometry on NO2 gas-sensing characteristics of one-dimensional tin dioxide nanostructure microsensors. Sens. Actuators B Chem. 2011, 156, 784–790. [Google Scholar] [CrossRef]
- Rashad, M.; Shaalan, N.M.; Hafiz, M.M. Enhanced photocatalytic of Zno nanostructures via shape controlled platinum thin film. Dig. J. Nanomater. Biostruct. 2015, 10, 823–830. [Google Scholar]
- Zhang, H.; Hu, C.; Zhang, M.; Yang, R.; Zheng, C. Synthesis of BaCO3 nanowires and their humidity sensitive property. J. Nanosci. Nanotechnol. 2011, 11, 10706–10709. [Google Scholar] [CrossRef]
- Li, J.-M.; Wei, D.-P.; Hu, Y.-B.; Fang, J.; Xu, Z.-A. Synthesis of ultrafine green-emitting BaCO3 nanowires with 18.5 nm-diameter by CO2 vapor-assisted electrospinning. CrystEngComm 2014, 16, 964–968. [Google Scholar] [CrossRef]
- Zhu, W.; Cai, C.; Lin, J.; Wang, L.; Chen, L.; Zhuang, Z. Polymer micelle-directed growth of BaCO3 spiral nanobelts. Chem. Commun. 2012, 48, 8544–8546. [Google Scholar] [CrossRef]
- Hertzberg, B.; Sviridov, L.; Stach, E.A.; Gupta, T.; Steingart, D. A Manganese-Doped Barium Carbonate Cathode for Alkaline Batteries. J. Electrochem. Soc. 2014, 161, A835–A840. [Google Scholar] [CrossRef] [Green Version]
- Shahid, T.; Arfan, M.; Zeb, A.; BiBi, T.; Khan, T.M. Preparation and physical properties of functional barium carbonate nanostructures by a facile composite-hydroxide-mediated route. Nanomater. Nanotechnol. 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.Y.; Kang, H.; Choi, N.-J.; Park, K.H.; Lee, H.-K. A carbon dioxide gas sensor based on cobalt oxide containing barium carbonate. Sens. Actuators B Chem. 2017, 248, 987–992. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, Z.; Zhang, X. A novel gaseous acetaldehyde sensor utilizing cataluminescence on nanosized BaCO3. Sens. Actuators B Chem. 2004, 99, 30–35. [Google Scholar] [CrossRef]
- Hong, T.; Brinkman, K.S.; Xia, C. Barium Carbonate Nanoparticles as Synergistic Catalysts for the Oxygen Reduction Reaction on La0.6Sr0.4Co0.2Fe0.8O3−δSolid-Oxide Fuel Cell Cathodes. ChemElectroChem 2016, 3, 805–813. [Google Scholar] [CrossRef]
- Chen, G.; Chen, J.; Peng, J. Homogeneous precipitation synthesis of BaCO3 powders with a needle-like morphology. Green Process. Synth. 2018, 7, 225–230. [Google Scholar] [CrossRef]
- Chen, G.; Chang, X.; Chen, J.; Zhao, W.; Peng, J. Investigation of BaCO3 Powders Synthesized by Microwave Homogeneous Precipitation. High Temp. Mater. Process. 2015, 34, 757–764. [Google Scholar] [CrossRef]
- Lee, J.-S.; Lee, J.-H.; Hong, S.-H. NASICON-based amperometric CO2 sensor using Na2CO3–BaCO3 auxiliary phase. Sens. Actuators B Chem. 2003, 96, 663–668. [Google Scholar] [CrossRef]
- Obata, K.; Shimanoe, K.; Miura, N.; Yamazoe, N. NASICON devices attached with Li2CO3-BaCO3 auxiliary phase for CO2 sensing under ambient conditions. J. Mater. Sci. 2003, 38, 4283–4288. [Google Scholar] [CrossRef]
- Matsubara, S.; Kaneko, S.; Morimoto, S.; Shimizu, S.; Ishihara, T.; Takita, Y. A practical capacitive type CO2 sensor using CeO2/BaCO3/CuO ceramics. Sens. Actuators B Chem. 2000, 65, 128–132. [Google Scholar] [CrossRef]
- Usher, T.-M.; Kavey, B.; Caruntu, G.; Page, K. Effect of BaCO3 Impurities on the Structure of BaTiO3 Nanocrystals: Implications for Multilayer Ceramic Capacitors. ACS Appl. Nano Mater. 2020, 3, 9715–9723. [Google Scholar] [CrossRef]
- Kou, L.; Zhang, D.; Liu, D. A Novel Medical E-Nose Signal Analysis System. Sensors 2017, 17, 402. [Google Scholar] [CrossRef] [Green Version]
- Shirui, Z.; Jihua, W.; Daming, D.; Wengang, Z.; Xiande, Z. A Review of Contact Sensors Used for Monitoring Malodorous Gas in Animal Facilities. Adv. Mater. Res. 2013, 629, 655–661. [Google Scholar] [CrossRef]
- Shaalan, N.; Hamad, D. Low-temperature hydrogen sensor based on sputtered tin dioxide nanostructures through slow deposition rate. Appl. Surf. Sci. 2022, 598, 153857. [Google Scholar] [CrossRef]
- Li, G.; Xia, Y.; Tian, Y.; Wu, Y.; Liu, J.; He, Q.; Chen, D. Review—Recent Developments on Graphene-Based Electrochemical Sensors toward Nitrite. J. Electrochem. Soc. 2019, 166, B881–B895. [Google Scholar] [CrossRef]
- Zhang, Y.; Nie, J.; Wei, H.; Xu, H.; Wang, Q.; Cong, Y.; Tao, J.; Chu, L.; Zhou, Y.; Wu, X. Electrochemical detection of nitrite ions using Ag/Cu/MWNT nanoclusters electrodeposited on a glassy carbon electrode. Sens. Actuators B Chem. 2018, 258, 1107–1116. [Google Scholar] [CrossRef]
- Lee, S.W.; Jung, H.G.; Jang, J.W.; Park, D.; Lee, D.; Kim, I.; Kim, Y.; Cheong, D.Y.; Hwang, K.S.; Lee, G.; et al. Graphene-Based Electronic Textile Sheet for Highly Sensitive Detection of NO2 and NH3. Sens. Actuators B Chem. 2021, 345, 130361. [Google Scholar] [CrossRef]
- Ali, S.; Gupta, A.; Shafiei, M.; Langford, S. Recent Advances in Perylene Diimide-Based Active Materials in Electrical Mode Gas Sensing. Chemosensors 2021, 9, 30. [Google Scholar] [CrossRef]
- Jarvis, D.; Adamkiewicz, G.; Heroux, M.-E.; Rapp, R.; Kelly, F.; Jarvis, D.; Adamkiewicz, G.; Heroux, M.-E.; Rapp, R.; Kelly, F. Nitrogen Dioxide. In WHO Guidelines for Indoor Air Quality: Selected Pollutants; World Health Organization: Geneva, Switzerland, 2010; Volume 5. [Google Scholar]
- Gillespie-Bennett, J.; Pierse, N.; Wickens, K.; Crane, J.; Howden-Chapman, P.; Shields, H.; Viggers, H.; Free, S.; Phipps, R.; Fjallstrom, P.; et al. The respiratory health effects of nitrogen dioxide in children with asthma. Eur. Respir. J. 2011, 38, 303–309. [Google Scholar] [CrossRef] [Green Version]
- US Department of Health and Human Services. Agency for Toxic Substances and Disease Registry. In Nitrogen Oxides (NO, NO 2, and others) CAS 10102-43-9; UN 1660 (NO) CAS 10102-44-0; UN 1067 (NO 2) UN 1975 (Mixture); ATSDR: Atlanda, GA, USA, 1975; Volume 1660. [Google Scholar]
- Chauhan, A.J.; Krishna, M.T.; Frew, A.J.; Holgate, S.T. Exposure to nitrogen dioxide (NO2) and respiratory disease risk. Rev. Environ. Health 1998, 13, 73–90. [Google Scholar]
- Shivakumar, M.; Nagashree, K.L.; Manjappa, S.; Dharmaprakash, M.S. Electrochemical Detection of Nitrite Using Glassy Carbon Electrode Modified with Silver Nanospheres (AgNS) Obtained by Green Synthesis Using Pre-hydrolysed Liquor. Electroanalysis 2017, 29, 1434–1442. [Google Scholar] [CrossRef]
- Mo, R.; Wang, X.; Yuan, Q.; Yan, X.; Su, T.; Feng, Y.; Lv, L.; Zhou, C.; Hong, P.; Sun, S.; et al. Electrochemical Determination of Nitrite by Au Nanoparticle/Graphene-Chitosan Modified Electrode. Sensors 2018, 18, 1986. [Google Scholar] [CrossRef] [Green Version]
- Antao, S.M.; Hassan, I. BaCO3: High-temperature crystal structures and the Pmcn→R3m phase transition at 811 °C. Phys. Chem. Miner. 2007, 34, 573–580. [Google Scholar] [CrossRef]
- Shackelford, J.F. Introduction to Materials Science for Engineers; Pearson Education, Inc., New Jersey Library: Upper Saddle River, NJ, USA, 2003; p. 463. [Google Scholar]
- Stuart, K.T. Chapter: Semiconductor Materials. In Microelectronics, 2nd ed.; Whitaker, J.C., Ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar] [CrossRef]
- Anderson, J.H., Jr.; Parks, G.A. Electrical conductivity of silica gel in the presence of adsorbed water. J. Phys. Chem. 1968, 72, 3662–3668. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, C. Humidity Sensors: A Review of Materials and Mechanisms. Sens. Lett. 2005, 3, 274–295. [Google Scholar] [CrossRef] [Green Version]
- Shaalan, N.; Yamazaki, T.; Kikuta, T. Synthesis of metal and metal oxide nanostructures and their application for gas sensing. Mater. Chem. Phys. 2011, 127, 143–150. [Google Scholar] [CrossRef]
- Dai, Z.; Pan, Z.; Wang, Z.L. Novel Nanostructures of Functional Oxides Synthesized by Thermal Evaporation. Adv. Funct. Mater. 2003, 13, 9–24. [Google Scholar] [CrossRef]
- Kresse, R.; Baudis, U.; Jäger, P.; Riechers, H.H.; Wagner, H.; Winkler, J.; Wolf, H.U. Barium and Barium Compounds. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar] [CrossRef] [Green Version]
- Patterson, A.L. The Scherrer Formula for X-ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Broqvist, P.; Panas, I.; Grönbeck, H. Toward a Realistic Description of NOx Storage in BaO: The Aspect of BaCO3. J. Phys. Chem. B 2005, 109, 9613–9621. [Google Scholar] [CrossRef]
- Sawicki, B.; Tomaszewicz, E.; Piątkowska, M.; Groń, T.; Duda, H.; Górny, K. Correlation between the Band-Gap Energy and the Electrical Conductivity in MPr2W_2O10Tungstates (Where M = Cd, Co, Mn). Acta Phys. Pol. A 2016, 129, 94–96. [Google Scholar] [CrossRef]
- Ruhland, B.; Becker, T.; Müller, G. Gas-kinetic interactions of nitrous oxides with SnO2 surfaces. Sens. Actuators B Chem. 1998, 50, 85–94. [Google Scholar] [CrossRef]
- Sergent, N.; Epifani, M.; Comini, E.; Faglia, G.; Pagnier, T. Interactions of nanocrystalline tin oxide powder with NO2: A Raman spectroscopic study. Sens. Actuators B Chem. 2007, 126, 1–5. [Google Scholar] [CrossRef]
- Kim, S.; Kim, D.-H.; Park, S.-G. Highly sensitive and on-site NO2 SERS sensors operated under ambient conditions. Analyst 2018, 143, 3006–3010. [Google Scholar] [CrossRef]
- Harrar, J.E.; Rigdon, L.P.; Rice, S.F. Raman spectral study of solutions of N2O4 and N2O5 in nitric acid. J. Raman Spectrosc. 1997, 28, 891–899. [Google Scholar] [CrossRef]
- Shaalan, N.; Yamazaki, T.; Kikuta, T. NO2 response enhancement and anomalous behavior of n-type SnO2 nanowires functionalized by Pd nanodots. Sens. Actuators B Chem. 2012, 166-167, 671–677. [Google Scholar] [CrossRef]
- Shaalan, N.M.; Morsy, A.E.A.; Abdel-Rahim, M.A.; Rashad, M. Simple preparation of Ni/CuO nanocomposites with superior sensing activity toward the detection of methane gas. Appl. Phys. A 2021, 127, 1–12. [Google Scholar] [CrossRef]
- Wang, X. Chapter 4—Lacustrine Shale Gas Occurrence Characteristics. In Lacustrine Shale Gas; Gulf Professional Publishing: Houston, TX, USA, 2017; pp. 179–241. [Google Scholar] [CrossRef]
- Flores, R.M. Chapter 4—Coalification, Gasification, and Gas Storage. In Coal and Coalbed Gas Fueling the Future; Elsevier: Boston, MA, USA, 2013; pp. 167–233. [Google Scholar] [CrossRef]



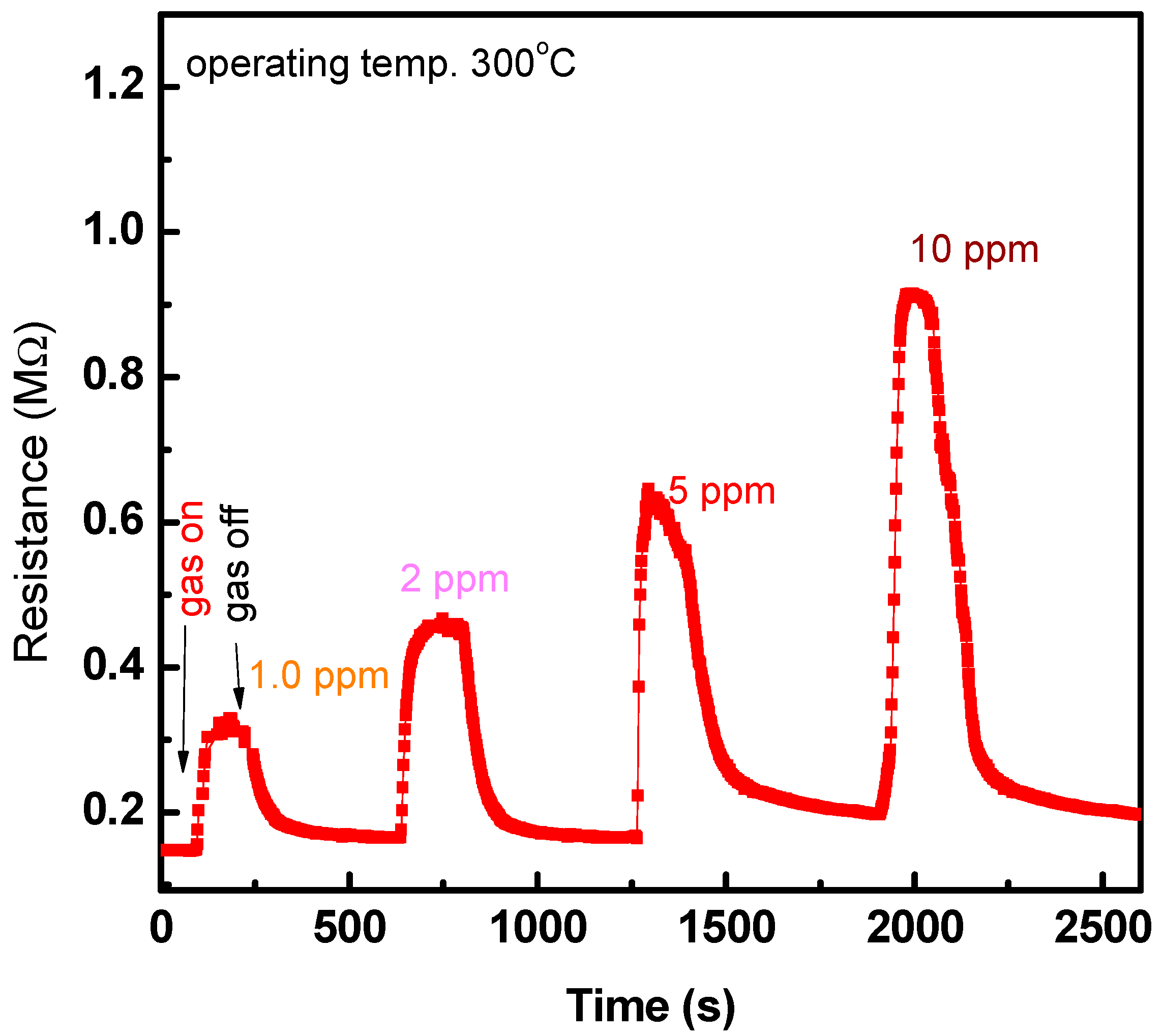
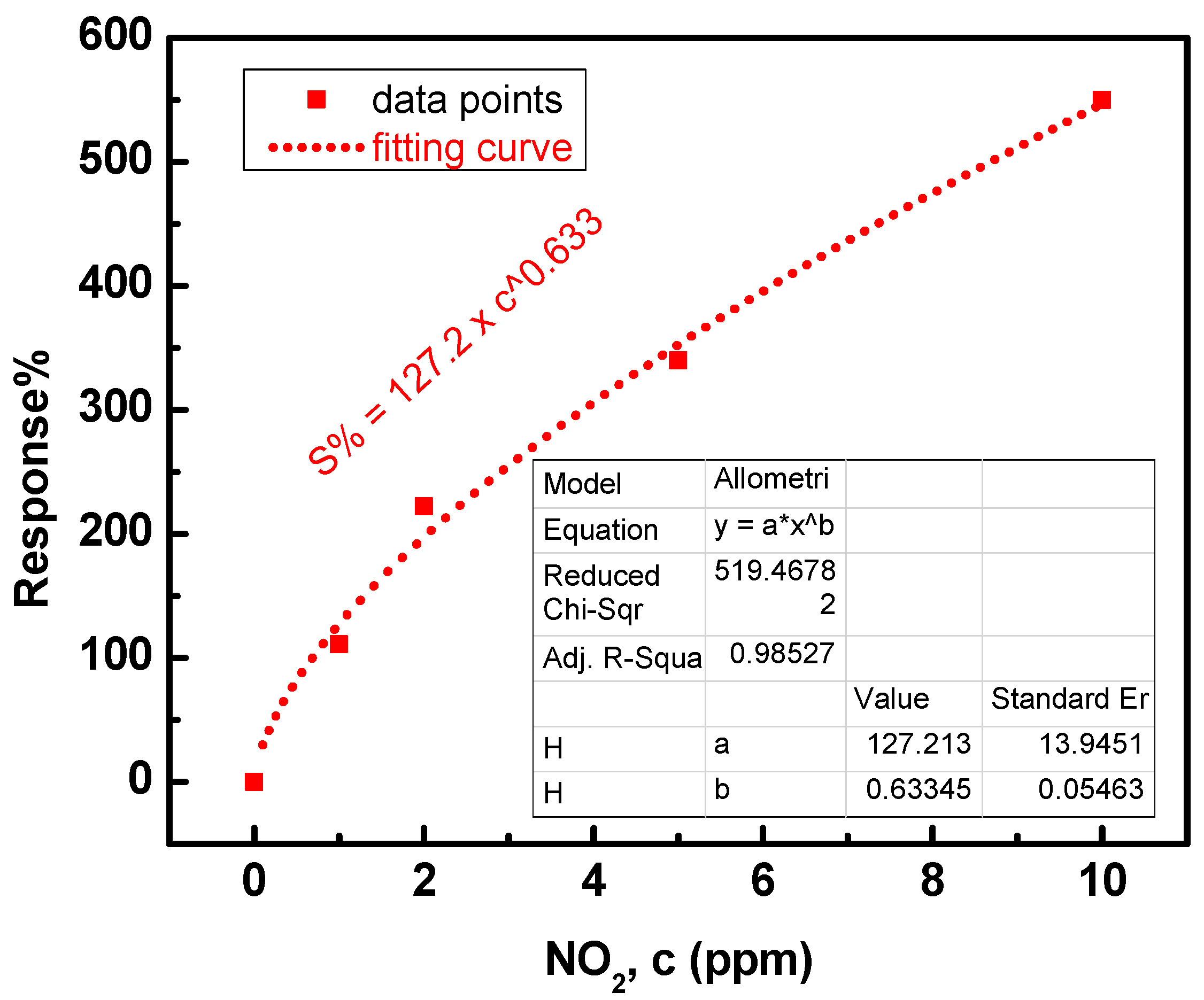

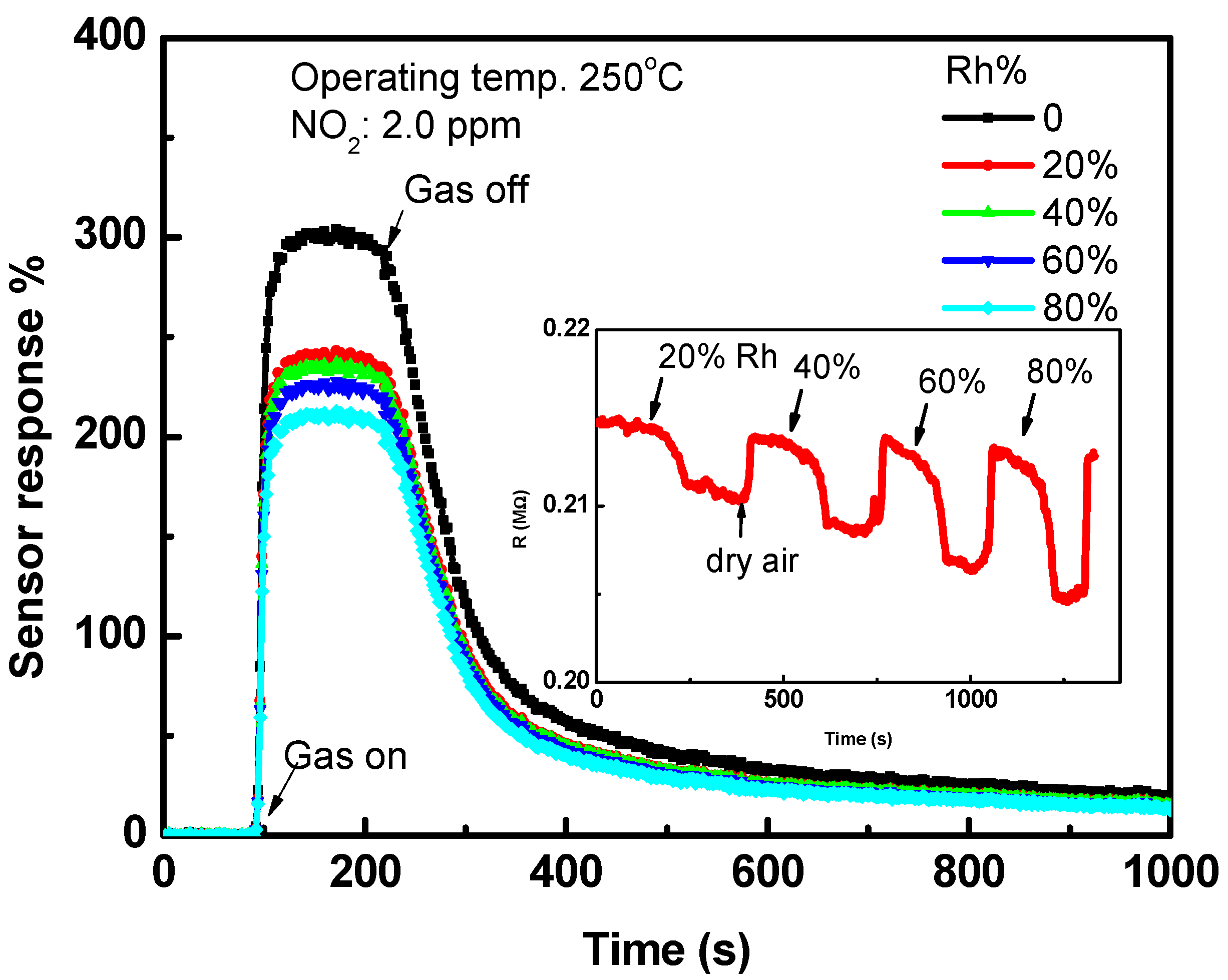


| Parameter | Operating Temp. (°C) | ||||
|---|---|---|---|---|---|
| 150 | 200 | 250 | 300 | 350 | |
| Sensor response% | 6 | 157 | 313 | 200 | 100 |
| Response time constant (s) | 6 | 8 | 9 | 10 | 20 |
| Recovery time constant (s) | 30 | 150 | 100 | 60 | 40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaalan, N.M. Promising Novel Barium Carbonate One-Dimensional Nanostructures and Their Gas Sensing Application: Preparation and Characterization. Chemosensors 2022, 10, 230. https://doi.org/10.3390/chemosensors10060230
Shaalan NM. Promising Novel Barium Carbonate One-Dimensional Nanostructures and Their Gas Sensing Application: Preparation and Characterization. Chemosensors. 2022; 10(6):230. https://doi.org/10.3390/chemosensors10060230
Chicago/Turabian StyleShaalan, Nagih M. 2022. "Promising Novel Barium Carbonate One-Dimensional Nanostructures and Their Gas Sensing Application: Preparation and Characterization" Chemosensors 10, no. 6: 230. https://doi.org/10.3390/chemosensors10060230
APA StyleShaalan, N. M. (2022). Promising Novel Barium Carbonate One-Dimensional Nanostructures and Their Gas Sensing Application: Preparation and Characterization. Chemosensors, 10(6), 230. https://doi.org/10.3390/chemosensors10060230





