One-Step Hydrothermal Synthesis of 3D Interconnected rGO/In2O3 Heterojunction Structures for Enhanced Acetone Detection
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Synthesis
2.2. Materials Characterization
2.3. Sensor Fabrication and Measurement
3. Results and Discussion
3.1. Structural and Morphological Characteristics
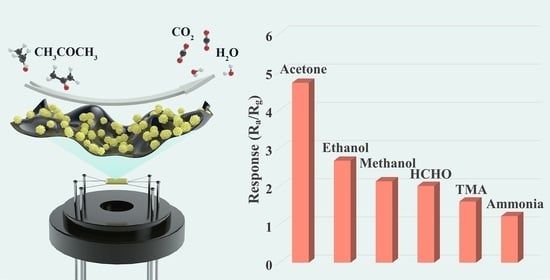
3.2. Gas Sensing Performance
3.3. Acetone-Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, L.; Yuan, K.; Li, Z.; Miao, X.; Wang, J.; Sun, S.; Devi, A.; Lu, H. Highly sensitive and stable MEMS acetone sensors based on well-designed α-Fe2O3/C mesoporous nanorods. J. Colloid Interface Sci. 2022, 622, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Bao, L.; Xu, J.; Wang, D.; Wang, X. Highly sensitive acetone gas sensor based on ultra-low content bimetallic PtCu modified WO3·H2O hollow sphere. Chin. Chem. Lett. 2020, 31, 2041–2044. [Google Scholar] [CrossRef]
- Lee, J.; Choi, Y.; Park, B.; Han, J.; Lee, H.; Park, J.; Lee, W. Precise control of surface oxygen vacancies in ZnO nanoparticles for extremely high acetone sensing response. J. Adv. Ceram. 2022, 11, 769–783. [Google Scholar] [CrossRef]
- Ama, O.; Sadiq, M.; Johnson, M.; Zhang, Q.; Wang, D. Novel 1D/2D KWO/Ti3C2Tx Nanocomposite-Based Acetone Sensor for Diabetes Prevention and Monitoring. Chemosensors 2020, 8, 102. [Google Scholar] [CrossRef]
- Fanget, S.; Hentz, S.; Puget, P.; Arcamone, J.; Matheron, M.; Colinet, E.; Andreucci, P.; Duraffourg, L.; Myers, E.; Roukes, M.L. Gas sensors based on gravimetric detection—A review. Sens. Actuators B 2011, 160, 804–821. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, C. Volatile organic compounds gas sensor based on quartz crystal microbalance for fruit freshness detection: A review. Food Chem. 2021, 334, 127615. [Google Scholar] [CrossRef] [PubMed]
- Avramov, I.D.; Ivanov, G.R. Layer by Layer Optimization of Langmuir–Blodgett Films for Surface Acoustic Wave (SAW) Based Sensors for Volatile Organic Compounds (VOC) Detection. Coatings 2022, 12, 669. [Google Scholar] [CrossRef]
- Kanawade, R.; Kumar, A.; Pawar, D.; Vairagi, K.; Late, D.; Sarkar, S.; Sinha, R.K.; Mondal, S. Negative axicon tip-based fiber optic interferometer cavity sensor for volatile gas sensing. Opt. Express 2019, 27, 7277–7290. [Google Scholar] [CrossRef]
- Dadkhah, M.; Tulliani, J. Nanostructured Metal Oxide Semiconductors towards Greenhouse Gas Detection. Chemosensors 2022, 10, 57. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, J.; Yu, X.; Zhang, W.; Zhang, X. Determination of acetone in human breath by gas chromatography–mass spectrometry and solid-phase microextraction with on-fiber derivatization. J. Chromatogr. B 2004, 810, 269–275. [Google Scholar] [CrossRef]
- Galstyan, V.; Bhandari, M.P.; Sberveglieri, V.; Sberveglieri, G.; Comini, E. Metal Oxide Nanostructures in Food Applications: Quality Control and Packaging. Chemosensors 2018, 6, 16. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Shen, Y.; Zhao, S.; Bai, J.; Gao, S.; Liu, W.; Wei, D.; Meng, D.; San, X. Construction of rGO-SnO2 heterojunction for enhanced hydrogen detection. Appl. Surf. Sci. 2022, 585, 152623. [Google Scholar] [CrossRef]
- Tao, K.; Han, X.; Yin, Q.; Wang, D.; Han, L.; Chen, L. Metal-Organic Frameworks-Derived Porous In2O3 Hollow Nanorod for High-Performance Ethanol Gas Sensor. ChemistrySelect 2017, 2, 10918–10925. [Google Scholar] [CrossRef]
- Liu, M.; Song, P.; Zhong, X.; Yang, Z.; Wang, Q. Facile synthesis of Au-decorated α-Fe2O3/rGO ternary hybrid structure nanocomposites for enhanced triethylamine gas-sensing properties. J. Mater. Sci. Mater. Electron. 2020, 31, 22713–22726. [Google Scholar] [CrossRef]
- Zhao, S.; Shen, Y.; Yan, X.; Zhou, P.; Yin, Y.; Lu, R.; Han, C.; Cui, B.; Wei, D. Complex-surfactant-assisted hydrothermal synthesis of one-dimensional ZnO nanorods for high-performance ethanol gas sensor. Sens. Actuators B 2019, 286, 501–511. [Google Scholar] [CrossRef]
- Wang, X.; Han, W.; Yang, J.; Jiang, B.; Cheng, P.; Wang, Y.; Sun, P.; Zhang, H.; Sun, Y.; Lu, G. Facet effect on diverse WO3 morphologies and ideal work function for ppb-level triethylamine detection. Sens. Actuators B 2022, 363, 131849. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, D.; Hou, X.; Zhang, Y.; Yi, S.; Ji, H.; Wang, Y.; Yin, L.; Sun, J. Microwave-assisted synthesis of hierarchically porous Co3O4/rGO nanocomposite for low-temperature acetone detection. J. Colloid Interface Sci. 2021, 594, 690–701. [Google Scholar] [CrossRef]
- Bai, H.; Guo, H.; Wang, J.; Dong, Y.; Liu, B.; Xie, Z.; Guo, F.; Chen, D.; Zhang, R.; Zheng, Y. A room-temperature NO2 gas sensor based on CuO nanoflakes modified with rGO nanosheets. Sens. Actuators B 2021, 337, 129783. [Google Scholar] [CrossRef]
- Meng, D.; Qiao, T.; Wang, G.; San, X.; Meng, F. One-step synthesis of rGO/V2O5 flower-like microsphere composites with enhanced trimethylamine sensing properties. Mater. Lett. 2021, 299, 130023. [Google Scholar] [CrossRef]
- Meng, F.; Qi, T.; Zhang, J.; Zhu, H.; Yuan, Z.; Liu, C.; Qin, W.; Ding, M. MoS2-Templated Porous Hollow MoO3 Microspheres for Highly Selective Ammonia Sensing via a Lewis Acid-Base Interaction. IEEE Trans. Ind. Electron. 2022, 69, 960–970. [Google Scholar] [CrossRef]
- Choi, J.; Seo, J.; Jeong, H.; Song, K.; Baeck, S.; Shim, S.; Qian, Y. Effects of Field-Effect and Schottky Heterostructure on p-Type Graphene-Based Gas Sensor Modified by n-Type In2O3 and Phenylenediamine. Appl. Surf. Sci. 2022, 578, 152025. [Google Scholar] [CrossRef]
- Liang, T.; Kim, D.; Yoon, J.; Yu, Y. Rapid synthesis of rhombohedral In2O3 nanoparticles via a microwave-assisted hydrothermal pathway and their application for conductometric ethanol sensing. Sens. Actuators B 2021, 346, 130578. [Google Scholar] [CrossRef]
- Zhang, K.; Qin, S.; Tang, P.; Feng, Y.; Li, D. Ultra-sensitive ethanol gas sensors based on nanosheet-assembled hierarchical ZnO-In2O3 heterostructures. J. Hazard. Mater. 2020, 391, 122191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ding, S.; Zhang, Q.; Yi, H.; Liu, Z.; Shi, M.; Guan, R.; Yue, L. Rare earth element-doped porous In2O3 nanosheets for enhanced gas-sensing performance. Rare Met. 2021, 40, 1662–1668. [Google Scholar] [CrossRef]
- Huang, X.; Tang, Z.; Tan, Z.; Sheng, S.; Zhao, Q. Hierarchical In2O3 nanostructures for improved formaldehyde: Sensing performance. J. Mater. Sci. Mater. Electron. 2021, 32, 11857–11864. [Google Scholar] [CrossRef]
- Kohli, N.; Hastir, A.; Kumari, M.; Singh, R.C. Hydrothermally synthesized heterostructures of In2O3/MWCNT as acetone gas sensor. Sens. Actuators A 2020, 314, 112240. [Google Scholar] [CrossRef]
- Gupta Chatterjee, S.; Chatterjee, S.; Ray, A.; Chakraborty, A. Graphene–metal oxide nanohybrids for toxic gas sensor: A review. Sens. Actuators B 2015, 221, 1170–1181. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Liu, Q.; Chen, R.; Zhang, H.; Yu, J.; Song, D.; Li, J.; Zhang, M.; Wang, J. Template-free synthesis of rGO decorated hollow Co3O4 nano/microspheres for ethanol gas sensor. Ceram. Int. 2018, 44, 21091–21098. [Google Scholar] [CrossRef]
- Ren, H.; Gu, C.; Joo, S.W.; Zhao, J.; Sun, Y.; Huang, J. Effective hydrogen gas sensor based on NiO@rGO nanocomposite. Sens. Actuators B 2018, 266, 506–513. [Google Scholar] [CrossRef]
- de Lima, B.; Komorizono, A.; Ndiaye, A.; Bernardi, M.B.; Brunet, J.; Mastelaro, V. Tunning the Gas Sensing Properties of rGO with In2O3 Nanoparticles. Surfaces 2022, 5, 127–142. [Google Scholar] [CrossRef]
- Dong, X.; Han, Q.; Kang, Y.; Li, H.; Huang, X.; Fang, Z.; Yuan, H.; Elzatahry, A.A.; Chi, Z.; Wu, G.; et al. Rational construction and triethylamine sensing performance of foam shaped α-MoO3@SnS2 nanosheets. Chin. Chem. Lett. 2022, 33, 567–572. [Google Scholar] [CrossRef]
- Liu, J.-B.; Hu, J.-Y.; Liu, C.; Tan, Y.-M.; Peng, X.; Zhang, Y. Mechanically exfoliated MoS2 nanosheets decorated with SnS2 nanoparticles for high-stability gas sensors at room temperature. Rare Met. 2021, 40, 1536–1544. [Google Scholar] [CrossRef]
- Meng, F.; Li, X.; Yuan, Z.; Lei, Y.; Qi, T.; Li, J. Ppb-Level Xylene Gas Sensors Based on Co3O4 Nanoparticle-Coated Reduced Graphene Oxide(rGO) Nanosheets Operating at Low Temperature. IEEE Trans. Instrum. Meas. 2021, 70, 1–10. [Google Scholar] [CrossRef]
- Hung, C.; Dat, D.; Van Duy, N.; Van Quang, V.; Van Toan, N.; Van Hieu, N.; Hoa, N. Facile synthesis of ultrafine rGO/WO3 nanowire nanocomposites for highly sensitive toxic NH3 gas sensors. Mater. Res. Bull. 2020, 125, 110810. [Google Scholar] [CrossRef]
- Lin, G.; Wang, H.; Lai, X.; Yang, R.; Zou, Y.; Wan, J.; Liu, D.; Jiang, H.; Hu, Y. Co3O4/N-doped RGO nanocomposites derived from MOFs and their highly enhanced gas sensing performance. Sens. Actuators B 2020, 303, 127219. [Google Scholar] [CrossRef]
- Zuo, J.; Tavakoli, S.; Mathavakrishnan, D.; Ma, T.; Lim, M.; Rotondo, B.; Pauzauskie, P.; Pavinatto, F.; MacKenzie, D. Additive Manufacturing of a Flexible Carbon Monoxide Sensor Based on a SnO2-Graphene Nanoink. Chemosensors 2020, 8, 36. [Google Scholar] [CrossRef]
- Cao, P.; Cai, Y.; Pawar, D.; Navale, S.; Rao, C.; Han, S.; Xu, W.; Fang, M.; Liu, X.; Zeng, Y.; et al. Down to ppb level NO2 detection by ZnO/rGO heterojunction based chemiresistive sensors. Chem. Eng. J. 2020, 401, 125491. [Google Scholar] [CrossRef]
- Mishra, R.K.; Murali, G.; Kim, T.-H.; Kim, J.H.; Lim, Y.J.; Kim, B.-S.; Sahay, P.P.; Lee, S.H. Nanocube In2O3@RGO heterostructure based gas sensor for acetone and formaldehyde detection. RSC Adv. 2017, 7, 38714–38724. [Google Scholar] [CrossRef] [Green Version]
- Hummers, W.; Offeman, R. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Yang, W.; Wan, P.; Zhou, X.; Hu, J.; Guan, Y.; Feng, L. Additive-Free Synthesis of In2O3 Cubes Embedded into Graphene Sheets and Their Enhanced NO2 Sensing Performance at Room Temperature. ACS Appl. Mater. Interfaces 2014, 6, 21093–21100. [Google Scholar] [CrossRef]
- White, J.; Bocarsly, A. Enhanced Carbon Dioxide Reduction Activity on Indium-Based Nanoparticles. J. Electrochem. Soc. 2016, 163, H410–H416. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, S.; Fu, H.; Wang, Y.; Yu, K.; Wang, L. Facile Design and Hydrothermal Synthesis of In2O3 Nanocube Polycrystals with Superior Triethylamine Sensing Properties. ACS Omega 2020, 5, 11466–11472. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Feng, L.; He, S.; Liu, L.; Liu, S. Density Gradient Strategy for Preparation of Broken In2O3 Microtubes with Remarkably Selective Detection of Triethylamine Vapor. ACS Appl. Mater. Interfaces 2018, 10, 27131–27140. [Google Scholar] [CrossRef] [PubMed]
- Rodwihok, C.; Wongratanaphisan, D.; Thi Ngo, Y.; Khandelwal, M.; Hur, S.; Chung, J. Effect of GO Additive in ZnO/rGO Nanocomposites with Enhanced Photosensitivity and Photocatalytic Activity. Nanomaterials 2019, 9, 1441. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Wan, G.; Peng, X.; Dou, Z.; Li, X.; Wang, K.; Lin, S.; Wang, G. Fabrication of carbon-coated NiO supported on graphene for high performance supercapacitors. RSC Adv. 2016, 6, 14199–14204. [Google Scholar] [CrossRef]
- Yan, H.; Song, P.; Zhang, S.; Zhang, J.; Yang, Z.; Wang, Q. Au nanoparticles modified MoO3 nanosheets with their enhanced properties for gas sensing. Sens. Actuators B 2016, 236, 201–207. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, Q.; Pan, Y.; Huang, F.; Tang, L.; Liu, C.; Cheng, Z.; Wang, P.; Ma, J.; Ding, M. Single-Atom Tailoring of Two-Dimensional Atomic Crystals Enables Highly Efficient Detection and Pattern Recognition of Chemical Vapors. ACS Sens. 2022, 7, 1533–1543. [Google Scholar] [CrossRef]
- Chaloeipote, G.; Prathumwan, R.; Subannajui, K.; Wisitsoraat, A.; Wongchoosuk, C. 3D printed CuO semiconducting gas sensor for ammonia detection at room temperature. Mater. Sci. Semicond. Process. 2021, 123, 105546. [Google Scholar] [CrossRef]
- Chang, X.; Li, K.; Qiao, X.; Xiong, Y.; Xia, F.; Xue, Q. ZIF-8 derived ZnO polyhedrons decorated with biomass derived nitrogen-doped porous carbon for enhanced acetone sensing. Sens. Actuators B 2021, 330, 129366. [Google Scholar] [CrossRef]
- Qin, Q.; Zhu, X.; Zhang, X. Synthesis of α-Fe2O3 hollow spheres with rapid response and excellent selectivity towards acetone. Mater. Sci. Eng. B 2022, 275, 115482. [Google Scholar] [CrossRef]
- Zhu, H.; Haidry, A.A.; Wang, Z.; Ji, Y. Improved acetone sensing characteristics of TiO2 nanobelts with Ag modification. J. Alloys Comp. 2021, 887, 161312. [Google Scholar] [CrossRef]
- Wang, G.; Fu, Z.; Wang, T.; Lei, W.; Sun, P.; Sui, Y.; Zou, B. A rational design of hollow nanocages Ag@CuO-TiO2 for enhanced acetone sensing performance. Sens. Actuators B 2019, 295, 70–78. [Google Scholar] [CrossRef]
- Wang, Q.; Cheng, X.; Wang, Y.; Yang, Y.; Su, Q.; Li, J.; An, B.; Luo, Y.; Wu, Z.; Xie, E. Sea urchins-like WO3 as a material for resistive acetone gas sensors. Sens. Actuators B 2022, 355, 131262. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, C.; Wang, Y.; Xu, L.; Dang, F.; Lv, L.; Li, X. Enhanced acetone sensing properties based on in situ growth SnO2 nanotube arrays. Nanotechnology 2021, 32, 245503. [Google Scholar] [CrossRef]
- Ueda, T.; Boehme, I.; Hyodo, T.; Shimizu, Y.; Weimar, U.; Barsan, N. Enhanced NO2-Sensing Properties of Au-Loaded Porous In2O3 Gas Sensors at Low Operating Temperatures. Chemosensors 2020, 8, 72. [Google Scholar] [CrossRef]
- Gu, D.; Liu, W.; Wang, J.; Yu, J.; Zhang, J.; Huang, B.; Rumyantseva, M.N.; Li, X. Au Functionalized SnS2 Nanosheets Based Chemiresistive NO2 Sensors. Chemosensors 2022, 10, 165. [Google Scholar] [CrossRef]
- Lv, L.; Cheng, P.; Zhang, Y.; Zhang, Y.; Lei, Z.; Wang, Y.; Xu, L.; Weng, Z.; Li, C. Ultra-high response acetone gas sensor based on ZnFe2O4 pleated hollow microspheres prepared by green NaCl template. Sens. Actuators B 2022, 358, 131490. [Google Scholar] [CrossRef]
- Nakate, U.T.; Yu, Y.T.; Park, S. High performance acetaldehyde gas sensor based on p-n heterojunction interface of NiO nanosheets and WO3 nanorods. Sens. Actuators B 2021, 344, 130264. [Google Scholar] [CrossRef]
- Wan, K.; Wang, D.; Wang, F.; Li, H.; Xu, J.; Wang, X.; Yang, J. Hierarchical In2O3@SnO2 Core-Shell Nanofiber for High Efficiency Formaldehyde Detection. ACS Appl. Mater. Interfaces 2019, 11, 45214–45225. [Google Scholar] [CrossRef]
- Qiu, Y.; Wang, Y.; Song, C. Facile synthesis of W18O49/Graphene nanocomposites for highly sensitive ethanol gas sensors. Colloids Surf. A 2021, 616, 126300. [Google Scholar] [CrossRef]
- Cheng, M.; Wu, Z.; Liu, G.; Zhao, L.; Gao, Y.; Zhang, B.; Liu, F.; Yan, X.; Liang, X.; Sun, P.; et al. Highly sensitive sensors based on quasi-2D rGO/SnS2 hybrid for rapid detection of NO2 gas. Sens. Actuators B 2019, 291, 216–225. [Google Scholar] [CrossRef]
- Bai, S.; Tian, K.; Zhao, Y.; Feng, Y.; Luo, R.; Li, D.; Chen, A. ZnO/BiFeO3 heterojunction interface modulation and rGO modification for detection of triethylamine. J. Mater. Chem. C 2022, 10, 8015–8023. [Google Scholar] [CrossRef]









| Sensing Materials | Temp. (°C) | Con. (ppm) | Response (Ra/Rg) | Res./Rec. Time (s) | Detection Limit | Ref. |
|---|---|---|---|---|---|---|
| ZnO/NPC | 350 | 100 | 25.47 | 3/150 | 1 ppm | [49] |
| α-Fe2O3-24 | 220 | 100 | 46.6 | 1/15 | 1 ppm | [50] |
| Ag-TiO2 nanobelts | 260 | 500 | 28.25 | 6/8 | 10 ppm | [51] |
| Ag@CuO-TiO2 | 200 | 100 | 6.2 | 9/56 | 1 ppm | [52] |
| WO3 | 200 | 100 | 28.7 | 3/113 | 2 ppm | [53] |
| SnO2 nanotubes | 325 | 100 | 20.3 | 66/15 | 5 ppm | [54] |
| In2O3 | 200 | 10 | 2.37 | 8/44 | 1 ppm | This work |
| 5-rGO/In2O3 | 150 | 10 | 5.57 | 3/18 | 0.5 ppm | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
San, X.; Zhang, Y.; Zhang, L.; Wang, G.; Meng, D.; Cui, J.; Jin, Q. One-Step Hydrothermal Synthesis of 3D Interconnected rGO/In2O3 Heterojunction Structures for Enhanced Acetone Detection. Chemosensors 2022, 10, 270. https://doi.org/10.3390/chemosensors10070270
San X, Zhang Y, Zhang L, Wang G, Meng D, Cui J, Jin Q. One-Step Hydrothermal Synthesis of 3D Interconnected rGO/In2O3 Heterojunction Structures for Enhanced Acetone Detection. Chemosensors. 2022; 10(7):270. https://doi.org/10.3390/chemosensors10070270
Chicago/Turabian StyleSan, Xiaoguang, Yue Zhang, Lei Zhang, Guosheng Wang, Dan Meng, Jia Cui, and Quan Jin. 2022. "One-Step Hydrothermal Synthesis of 3D Interconnected rGO/In2O3 Heterojunction Structures for Enhanced Acetone Detection" Chemosensors 10, no. 7: 270. https://doi.org/10.3390/chemosensors10070270
APA StyleSan, X., Zhang, Y., Zhang, L., Wang, G., Meng, D., Cui, J., & Jin, Q. (2022). One-Step Hydrothermal Synthesis of 3D Interconnected rGO/In2O3 Heterojunction Structures for Enhanced Acetone Detection. Chemosensors, 10(7), 270. https://doi.org/10.3390/chemosensors10070270