Quantitative Correlation between Adsorbed and Condensed Water Mass with Response Galvanic Current Detected at the Micron Gap of Galvanic-Coupled Arrays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of MSC
2.2. Detection of Adsorbed Water Molecules
2.3. Detection of Condensed Water Droplets
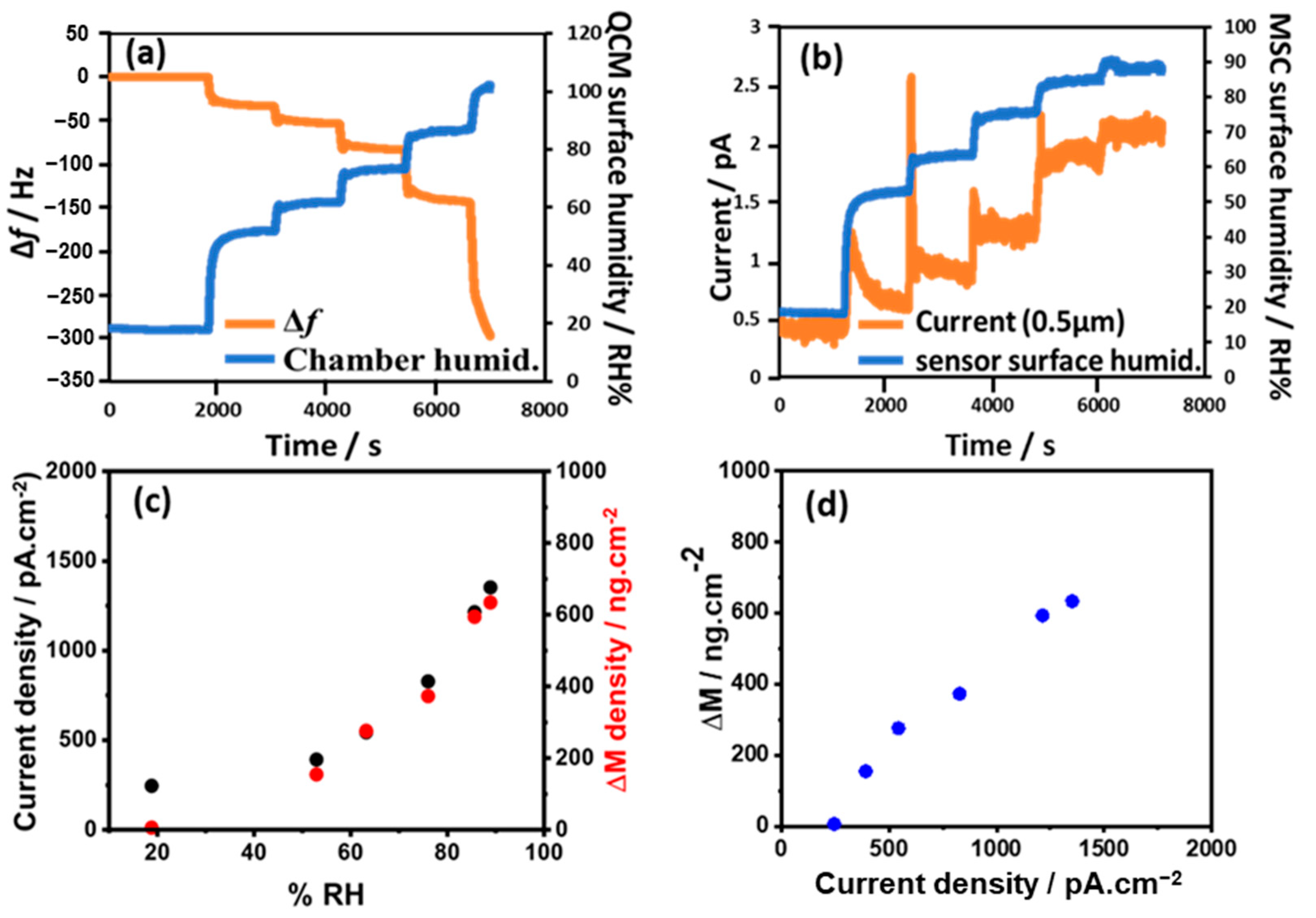
3. Results and Discussion
3.1. Detection of Adsorbed Water Molecules
3.2. Detection of Condensed Water Droplets
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eden, M.A.; Hill, R.A.; Beresford, R.; Stewart, A. The influence of inoculum concentration, relative humidity, and temperature on infection of greenhouse tomatoes by Botrytis Cinerea. Plant Pathol. 1996, 45, 795. [Google Scholar] [CrossRef]
- Lang, C.; Hübert, T.; Quaas, H.; Linke, M.; Herppich, W.B. On line measurement of humidity in the agri food-chain. Acta Hortic. 2010, 858, 413. [Google Scholar] [CrossRef]
- Tételin, A.; Pellet, C.; Laville, C.; N’Kaoua, G. Fast response humidity sensors for a medical microsystem. Sens. Actuators B Chem. 2003, 91, 211–218. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, Y.; Chen, M.-Q.; Xia, F. Research Advances in Microfiber Humidity Sensors. Small 2018, 14, 1800524. [Google Scholar] [CrossRef]
- Ali, S.; Jameel, M.A.; Harrison, C.J.; Gupta, A.; Shafiei, M.; Langford, S.J. Nanoporous naphthalene diimide surface enhances humidity and ammonia sensing at room temperature. Sens. Actuators B Chem. 2022, 351, 130972. [Google Scholar] [CrossRef]
- Grassian, V.L. Surface science of complex environmental interfaces: Oxide and carbonate surfaces in dynamic equilibrium with water vapor. Surf. Sci. 2008, 602, 2955–2962. [Google Scholar] [CrossRef]
- Baltrusaitis, J.; Grassian, V.L. Calcite (101¯4) surface in humid environments. Surf. Sci. 2009, 603, L99–L104. [Google Scholar] [CrossRef]
- Rother, G.; Gautam, S.; Liu, T.; Cole, R.D.; Busch, A.; Stack, A.G. Molecular Structure of Adsorbed Water Phases in Silica Nanopores. J. Phys. Chem. C 2022, 126, 2885−2895. [Google Scholar] [CrossRef]
- Wang, H.W.; Wesolowski, D.J.; Proffen, T.E.; Vlcek, L.; Wang, W.; Allard, L.F.; Kolesnikov, A.I.; Feygenson, M.; Anovitz, L.M.; Paul, R.L. Structure and Stability of SnO2 Nanocrystals and Surface Bound Water Species. J. Am. Chem. Soc. 2013, 135, 6885−6895. [Google Scholar] [CrossRef]
- Wahlgren, R.V. Atmospheric water vapour processor designs for potable water production: A review. Water Res. 2001, 35, 1–22. [Google Scholar] [CrossRef]
- Milani, D.; Abbas, A.; Vassallo, A.; Chiesa, M.; Bakri, D.A. Evaluation of using thermoelectric coolers in a dehumidification system to generate freshwater from ambient air. Chem. Eng. Sci. 2011, 66, 2491–2501. [Google Scholar] [CrossRef]
- Hong, K.; Webb, R.L. Performance of dehumidifying heat exchangers with and without wetting coatings. J. Heat Transf. 1999, 121, 1018–1026. [Google Scholar] [CrossRef]
- Parekh, S.; Farid, M.M.; Selman, J.R.; Al-hallaj, S. Solar desalination with a humidification-dehumidification technique: A comprehensive technical review. Desalination 2004, 160, 167–186. [Google Scholar] [CrossRef]
- Pérez-Lombard, L.; Ortiz, J.; Pout, C. A review on buildings energy consumption information. Energy Build. 2008, 40, 394–398. [Google Scholar] [CrossRef]
- Gast, T.; Talebi, R. Detection of adsorbed or condensed water films with the aid of thermal oscillations. Thermochim. Acta 1987, 119, 37–45. [Google Scholar] [CrossRef]
- Pandey, N.K.; Shakya, V.; Mishra, S. Characterization and Humidity Sensing Application of WO3-SnO2 Nanocomposite. IOSR J. Appl. Phys. 2013, 4, 10–17. [Google Scholar] [CrossRef]
- Crochemorea, G.B.; Itoa, A.R.P.; Goularta, C.A.; De Souza, D.P.F. Identification of Humidity Sensing Mechanism in MgAl2 O4 by Impedance Spectroscopy as Function of Relative Humidity. Mater. Res. 2018, 21, e20170729. [Google Scholar]
- Kawakita, J.; Chikyow, T. Detection of Micro/Nano Droplet by Galvanic-Coupled Arrays. ECS Trans. 2017, 75, 51–59. [Google Scholar] [CrossRef]
- Mekawy, M.; Terada, E.; Inoue, S.; Sakamoto, Y.; Kawakita, J. Quantitative Correlation of Droplet on Galvanic-Coupled Arrays with Response Current by Image Processing. ACS Omega 2021, 6, 30818–30825. [Google Scholar] [CrossRef]
- Mekawy, M.; Kawakita, J. Recent Sensing Technologies of Imperceptible Water in Atmosphere. Chemosensors 2022, 10, 112. [Google Scholar] [CrossRef]
- Nowak-Król, A.; Shoyama, K.; Stolte, M.; Wurthner, F. Naphthalene and perylene diimides—Better alternatives to fullerenes for organic electronics? Chem. Commun. 2018, 54, 13763. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Li, X.; Li, W.; Gou, C.; Zheng, M.; Zhang, Y.; Chen, Z.; Hong, Y. High-sensitive humidity sensor based on MoS2/graphene oxide quantum dot nanocomposite. Mater. Chem. Phys. 2022, 287, 126146. [Google Scholar] [CrossRef]
- Kubota, Y.; Mishra, V.L.; Sakamoto, Y.; Kawakita, J. Micro/nano galvanic-coupled arrays for early and initial detection and prediction of dew condensation. Sens. Actuators A 2020, 303, 111838. [Google Scholar] [CrossRef]
- Mekawy, M.; Noguchi, H.; Kawakita, J. Quantitative and Qualitative Studies for Real Monitoring of Interfacial Molecular Water. J. Colloid Interface Sci. 2022, 613, 311–319. [Google Scholar] [CrossRef]
- Kubota, Y.; Satoh, N.; Mekawy, M.; Sakamoto, Y.; Kawakita, J. Control of Heat Capacity of Moisture Sensor by Galvanic Arrays with Micro/Nano Gap toward Accurate Detection of Dew Condensation on Target. J. Electrochem. Soc. 2021, 168, 067522. [Google Scholar] [CrossRef]
- Terada, E.; Mekawy, M.; Sakamoto, Y.; Kawakita, J. Relation between Water Status on Micro/Nano Gap between Galvanic Arrays and Flowing Current Around 100% in Relative Humidity. J. Electrochem. Soc. 2021, 168, 047512. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mekawy, M.; Terada, E.; Kawakita, J. Quantitative Correlation between Adsorbed and Condensed Water Mass with Response Galvanic Current Detected at the Micron Gap of Galvanic-Coupled Arrays. Chemosensors 2022, 10, 300. https://doi.org/10.3390/chemosensors10080300
Mekawy M, Terada E, Kawakita J. Quantitative Correlation between Adsorbed and Condensed Water Mass with Response Galvanic Current Detected at the Micron Gap of Galvanic-Coupled Arrays. Chemosensors. 2022; 10(8):300. https://doi.org/10.3390/chemosensors10080300
Chicago/Turabian StyleMekawy, Moataz, Eiji Terada, and Jin Kawakita. 2022. "Quantitative Correlation between Adsorbed and Condensed Water Mass with Response Galvanic Current Detected at the Micron Gap of Galvanic-Coupled Arrays" Chemosensors 10, no. 8: 300. https://doi.org/10.3390/chemosensors10080300




