Emerging Strategies Based on Sensors for Chronic Wound Monitoring and Management
Abstract
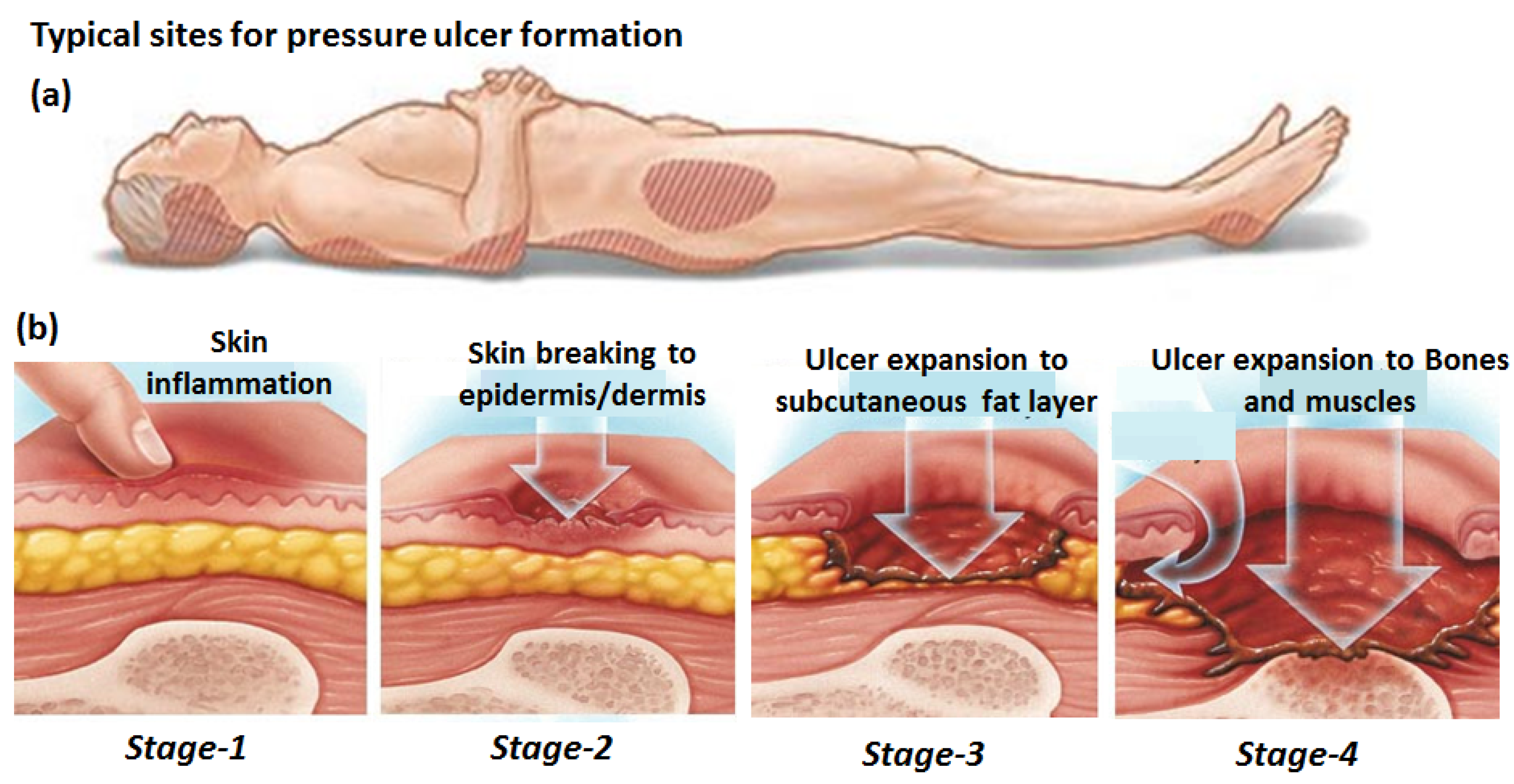
:1. Introduction
2. Chronic Wound Monitoring
2.1. Pressure Monitoring
2.1.1. Different Transduction Mechanisms
2.1.2. Application for Wound Monitoring

2.2. VOC Chemical Monitoring
2.2.1. VOC Biomarkers in a Chronic Wound
2.2.2. Sampling of VOCs at Chronic Wound Sites
2.3. Optical Monitoring
2.3.1. Image Analysis
2.3.2. Spectroscopic Analysis
3. Chronic Wound Management
3.1. Management with Smart Bandages
3.2. Management with Sensors
3.2.1. Management by Monitoring Applied Forces
3.2.2. Management by Monitoring Moisture
3.2.3. Management by Monitoring of a Wound’s pH
3.3. Telemetric Wound Monitoring
4. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sumarno, A.S. Pressure Ulcers: The Core, Care and Cure Approach. Br. J. Community Nurs. 2019, 24, S38–S42. [Google Scholar] [CrossRef] [PubMed]
- National Pressure Ulcer Advisory Panel; European Pressure Ulcer Advisory Panel; Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers: Quick Reference Guide; Cambridge Media: Perth, Australia, 2014. [Google Scholar]
- Staging of Pressure Ulcers (Bed Sores). Available online: https://www.lvlawny.com/post/2017/10/23/staging-of-pressure-ulcers-bed-sores (accessed on 23 October 2017).
- Jaul, E.; Barron, J.; Rosenzweig, J.P.; Menczel, J. An Overview of Co-Morbidities and the Development of Pressure Ulcers among Older Adults. BMC Geriatr. 2018, 18, 305. [Google Scholar] [CrossRef] [PubMed]
- Shiferaw, W.S.; Akalu, T.Y.; Mulugeta, H.; Aynalem, Y.A. The Global Burden of Pressure Ulcers among Patients with Spinal Cord Injury: A Systematic Review and Meta-Analysis. BMC Musculoskelet. Disord. 2020, 21, 334. [Google Scholar] [CrossRef]
- Courvoisier, D.S.; Righi, L.; Béné, N.; Rae, A.-C.; Chopard, P. Variation in Pressure Ulcer Prevalence and Prevention in Nursing Homes: A Multicenter Study. Appl. Nurs. Res. 2018, 42, 45–50. [Google Scholar] [CrossRef]
- Gottrup, F. A Specialized Wound-Healing Center Concept: Importance of a Multidisciplinary Department Structure and Surgical Treatment Facilities in the Treatment of Chronic Wounds. Am. J. Surg. 2004, 187, S38–S43. [Google Scholar] [CrossRef]
- Li, Z.; Lin, F.; Thalib, L.; Chaboyer, W. Global Prevalence and Incidence of Pressure Injuries in Hospitalised Adult Patients: A Systematic Review and Meta-Analysis. Int. J. Nurs. Stud. 2020, 105, 103546. [Google Scholar] [CrossRef]
- Al Mutairi, K.B.; Hendrie, D. Global Incidence and Prevalence of Pressure Injuries in Public Hospitals: A Systematic Review. Wound Med. 2018, 22, 23–31. [Google Scholar] [CrossRef]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human Skin Wounds: A Major and Snowballing Threat to Public Health and the Economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef]
- Gupta, S.; Sagar, S.; Maheshwari, G.; Kisaka, T.; Tripathi, S. Chronic Wounds: Magnitude, Socioeconomic Burden and Consequences. Wounds Asia 2021, 4, 8–14. [Google Scholar]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound Dressings—A Review. BioMedicine 2015, 5, 22. [Google Scholar] [CrossRef]
- Zeng, Q.; Qi, X.; Shi, G.; Zhang, M.; Haick, H. Wound Dressing: From Nanomaterials to Diagnostic Dressings and Healing Evaluations. ACS Nano 2022, 16, 1708–1733. [Google Scholar] [CrossRef] [PubMed]
- Demarré, L.; Verhaeghe, S.; Annemans, L.; Van Hecke, A.; Grypdonck, M.; Beeckman, D. The Cost of Pressure Ulcer Prevention and Treatment in Hospitals and Nursing Homes in Flanders: A Cost-of-Illness Study. Int. J. Nurs. Stud. 2015, 52, 1166–1179. [Google Scholar] [CrossRef] [PubMed]
- Popov, V.V.; Kudryavtseva, E.V.; Kumar Katiyar, N.; Shishkin, A.; Stepanov, S.I.; Goel, S. Industry 4.0 and Digitalisation in Healthcare. Materials 2022, 15, 2140. [Google Scholar] [CrossRef]
- Walia, G.S.; Wong, A.L.; Lo, A.Y.; Mackert, G.A.; Carl, H.M.; Pedreira, R.A.; Bello, R.; Aquino, C.S.; Padula, W.V.; Sacks, J.M. Efficacy of Monitoring Devices in Support of Prevention of Pressure Injuries. Adv. Skin Wound Care 2016, 29, 567–574. [Google Scholar] [CrossRef]
- Dargaville, T.R.; Farrugia, B.L.; Broadbent, J.A.; Pace, S.; Upton, Z.; Voelcker, N.H. Sensors and Imaging for Wound Healing: A Review. Biosens. Bioelectron. 2013, 41, 30–42. [Google Scholar] [CrossRef]
- Dreifke, M.B.; Jayasuriya, A.A.; Jayasuriya, A.C. Current Wound Healing Procedures and Potential Care. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 48, 651–662. [Google Scholar] [CrossRef]
- Mayet, N.; Choonara, Y.E.; Kumar, P.; Tomar, L.K.; Tyagi, C.; Du Toit, L.C.; Pillay, V. A Comprehensive Review of Advanced Biopolymeric Wound Healing Systems. J. Pharm. Sci. 2014, 103, 2211–2230. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Wang, J. Non-Invasive Wearable Electrochemical Sensors: A Review. Trends Biotechnol. 2014, 32, 363–371. [Google Scholar] [CrossRef]
- Trung, T.Q.; Lee, N.E. Flexible and Stretchable Physical Sensor Integrated Platforms for Wearable Human-Activity Monitoring and Personal Healthcare. Adv. Mater. 2016, 28, 4338–4372. [Google Scholar] [CrossRef]
- Swisher, S.L.; Lin, M.C.; Liao, A.; Leeflang, E.J.; Khan, Y.; Pavinatto, F.J.; Mann, K.; Naujokas, A.; Young, D.; Roy, S.; et al. Impedance Sensing Device Enables Early Detection of Pressure Ulcers In Vivo. Nat. Commun. 2015, 6, 6575. [Google Scholar] [CrossRef]
- Mehmood, N.; Hariz, A.; Templeton, S.; Voelcker, N.H. Calibration of Sensors for Reliable Radio Telemetry in a Prototype Flexible Wound Monitoring Device. Sens. Bio-Sens. Res. 2014, 2, 23–30. [Google Scholar] [CrossRef]
- Mehmood, N.; Hariz, A.; Templeton, S.; Voelcker, N.H. A Flexible and Low Power Telemetric Sensing and Monitoring System for Chronic Wound Diagnostics. Biomed. Eng. Online 2015, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- Hammock, M.L.; Chortos, A.; Tee, B.C.-K.; Tok, J.B.-H.; Bao, Z. 25th Anniversary Article: The Evolution of Electronic Skin (E-Skin): A Brief History, Design Considerations, and Recent Progress. Adv. Mater. 2013, 25, 5997–6038. [Google Scholar] [CrossRef] [PubMed]
- Tung, T.T.; Robert, C.; Castro, M.; Feller, J.F.; Kim, T.Y.; Suh, K.S. Enhancing the Sensitivity of Graphene/Polyurethane Nanocomposite Flexible Piezo-Resistive Pressure Sensors with Magnetite Nano-Spacers. Carbon 2016, 108, 450–460. [Google Scholar] [CrossRef]
- Woo, S.-J.; Kong, J.-H.; Kim, D.-G.; Kim, J.-M. A Thin All-Elastomeric Capacitive Pressure Sensor Array Based on Micro-Contact Printed Elastic Conductors. J. Mater. Chem. C 2014, 2, 4415–4422. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Z.; Li, X.; Lin, Y.; Luo, N.; Long, M.; Zhao, N.; Xu, J.-B. Flexible Piezoelectric-Induced Pressure Sensors for Static Measurements Based on Nanowires/Graphene Heterostructures. ACS Nano 2017, 11, 4507–4513. [Google Scholar] [CrossRef]
- Maheshwari, V.; Saraf, R.F. High-Resolution Thin-Film Device to Sense Texture by Touch. Science 2006, 312, 1501–1504. [Google Scholar] [CrossRef]
- Wu, X.; Han, Y.; Zhang, X.; Zhou, Z.; Lu, C. Large-Area Compliant, Low-Cost, and Versatile Pressure-Sensing Platform Based on Microcrack-Designed Carbon Black@Polyurethane Sponge for Human-Machine Interfacing. Adv. Funct. Mater. 2016, 26, 6246–6256. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Yang, T.; Li, X.; Zang, X.; Zhu, M.; Wang, K.; Wu, D.; Zhu, H. Wearable and Highly Sensitive Graphene Strain Sensors for Human Motion Monitoring. Adv. Funct. Mater. 2014, 24, 4666–4670. [Google Scholar] [CrossRef]
- Yan, C.; Wang, J.; Kang, W.; Cui, M.; Wang, X.; Foo, C.Y.; Chee, K.J.; Lee, P.S. Highly Stretchable Piezoresistive Graphene-Nanocellulose Nanopaper for Strain Sensors. Adv. Mater. 2014, 26, 2022–2027. [Google Scholar] [CrossRef]
- Park, J.; Lee, Y.; Hong, J.; Ha, M.; Jung, Y.D.; Lim, H.; Kim, S.Y.; Ko, H. Giant Tunneling Piezoresistance of Composite Elastomers with Interlocked Microdome Arrays for Ultrasensitive and Multimodal Electronic Skins. ACS Nano 2014, 8, 4689–4697. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Chortos, A.; Yu, G.; Wang, Y.; Isaacson, S.; Allen, R.; Shi, Y.; Dauskardt, R.; Bao, Z. An Ultra-Sensitive Resistive Pressure Sensor Based on Hollow-Sphere Microstructure Induced Elasticity in Conducting Polymer Film. Nat. Commun. 2014, 5, 3002. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Schwalb, W.; Wang, Y.; Chen, Y.; Tang, Y.; Si, J.; Shirinzadeh, B.; Cheng, W. A Wearable and Highly Sensitive Pressure Sensor with Ultrathin Gold Nanowires. Nat. Commun. 2014, 5, 3132. [Google Scholar] [CrossRef]
- Cheng, M.-Y.; Lin, C.-L.; Lai, Y.-T.; Yang, Y.-J. A Polymer-Based Capacitive Sensing Array for Normal and Shear Force Measurement. Sensors 2010, 10, 10211–10225. [Google Scholar] [CrossRef] [PubMed]
- Frutiger, A.; Muth, J.T.; Vogt, D.M.; Mengüç, Y.; Campo, A.; Valentine, A.D.; Walsh, C.J.; Lewis, J.A. Capacitive Soft Strain Sensors via Multicore-Shell Fiber Printing. Adv. Mater. 2015, 27, 2440–2446. [Google Scholar] [CrossRef]
- Lipomi, D.J.; Vosgueritchian, M.; Tee, B.C.K.; Hellstrom, S.L.; Lee, J.; Fox, C.H.; Bao, Z. Skin-like Pressure and Strain Sensors Based on Transparent Elastic Films of Carbon Nanotubes. Nat. Nanotechnol. 2011, 6, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Zhang, F.; Huang, D.; Gao, X.; Di, C.; Zhu, D. Flexible Suspended Gate Organic Thin-Film Transistors for Ultra-Sensitive Pressure Detection. Nat. Commun. 2015, 6, 6269. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Dong, L.; Han, X.; Du, W.; Zhai, J.; Pan, C.; Wang, Z.L. Self-Powered High-Resolution and Pressure-Sensitive Triboelectric Sensor Matrix for Real-Time Tactile Mapping. Adv. Mater. 2016, 28, 2896–2903. [Google Scholar] [CrossRef]
- Mannsfeld, S.C.B.; Tee, B.C.-K.; Stoltenberg, R.M.; Chen, C.V.H.-H.; Barman, S.; Muir, B.V.O.; Sokolov, A.N.; Reese, C.; Bao, Z. Highly Sensitive Flexible Pressure Sensors with Microstructured Rubber Dielectric Layers. Nat. Mater. 2010, 9, 859–864. [Google Scholar] [CrossRef]
- Hu, W.; Niu, X.; Zhao, R.; Pei, Q. Elastomeric Transparent Capacitive Sensors Based on an Interpenetrating Composite of Silver Nanowires and Polyurethane. Appl. Phys. Lett. 2013, 102, 083303. [Google Scholar] [CrossRef]
- Graz, I.; Kaltenbrunner, M.; Keplinger, C.; Schwödiauer, R.; Bauer, S.; Lacour, S.P.; Wagner, S. Flexible Ferroelectret Field-Effect Transistor for Large-Area Sensor Skins and Microphones. Appl. Phys. Lett. 2006, 89, 073501. [Google Scholar] [CrossRef]
- Wang, Z.L.; Song, J. Piezoelectric Nanogenerators Based on Zinc Oxide Nanowire Arrays. Science 2006, 312, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Park, K.I.; Son, J.H.; Hwang, G.T.; Jeong, C.K.; Ryu, J.; Koo, M.; Choi, I.; Lee, S.H.; Byun, M.; Wang, Z.L.; et al. Highly-Efficient, Flexible Piezoelectric PZT Thin Film Nanogenerator on Plastic Substrates. Adv. Mater. 2014, 26, 2514–2520. [Google Scholar] [CrossRef] [PubMed]
- Pi, Z.; Zhang, J.; Wen, C.; Zhang, Z.B.; Wu, D. Flexible Piezoelectric Nanogenerator Made of Poly(Vinylidenefluoride-Co-Trifluoroethylene) (PVDF-TrFE) Thin Film. Nano Energy 2014, 7, 33–41. [Google Scholar] [CrossRef]
- Dagdeviren, C.; Su, Y.; Joe, P.; Yona, R.; Liu, Y.; Kim, Y.-S.; Huang, Y.Y.; Damadoran, A.R.; Xia, J.; Martin, L.W.; et al. Conformable Amplified Lead Zirconate Titanate Sensors with Enhanced Piezoelectric Response for Cutaneous Pressure Monitoring. Nat. Commun. 2014, 5, 4496. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Dong, L.; Zhu, G.; Niu, S.; Yu, R.; Yang, Q.; Liu, Y.; Wang, Z.L. High-Resolution Electroluminescent Imaging of Pressure Distribution Using a Piezoelectric Nanowire LED Array. Nat. Photonics 2013, 7, 752–758. [Google Scholar] [CrossRef]
- Martínez-Nova, A.; Cuevas-García, J.C.; Pascual-Huerta, J.; Sánchez-Rodríguez, R. BioFoot® In-Shoe System: Normal Values and Assessment of the Reliability and Repeatability. Foot 2007, 17, 190–196. [Google Scholar] [CrossRef]
- Rosenbaum, D.; Becker, H.-P. Plantar Pressure Distribution Measurements. Technical Background and Clinical Applications. Foot Ankle Surg. 1997, 3, 1–14. [Google Scholar] [CrossRef]
- Shu, L.; Hua, T.; Wang, Y.; Li, Q.; Feng, D.D.; Tao, X. In-Shoe Plantar Pressure Measurement and Analysis System Based on Fabric Pressure Sensing Array. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 767–775. [Google Scholar] [CrossRef]
- Gerlach, C.; Krumm, D.; Illing, M.; Lange, J.; Kanoun, O.; Odenwald, S.; Hubler, A. Printed MWCNT-PDMS-Composite Pressure Sensor System for Plantar Pressure Monitoring in Ulcer Prevention. IEEE Sens. J. 2015, 15, 3647–3656. [Google Scholar] [CrossRef]
- Hou, C.; Wang, H.; Zhang, Q.; Li, Y.; Zhu, M. Highly Conductive, Flexible, and Compressible All-Graphene Passive Electronic Skin for Sensing Human Touch. Adv. Mater. 2014, 26, 5018–5024. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Kim, J.H.; Kim, J.; Choi, S.; Lee, J.; Park, I.; Hyeon, T.; Kim, D.-H. Reverse-Micelle-Induced Porous Pressure-Sensitive Rubber for Wearable Human-Machine Interfaces. Adv. Mater. 2014, 26, 4825–4830. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.; Lee, G.-Y.; Kim, T.; Kim, S.M.; Kim, H.N.; Ahn, S.-H.; Suh, K.-Y. A Flexible and Highly Sensitive Strain-Gauge Sensor Using Reversible Interlocking of Nanofibres. Nat. Mater. 2012, 11, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, H.; Vosgueritchian, M.; Cheon, S.; Kim, H.; Koo, J.H.; Kim, T.R.; Lee, S.; Schwartz, G.; Chang, H.; et al. Stretchable Energy-Harvesting Tactile Electronic Skin Capable of Differentiating Multiple Mechanical Stimuli Modes. Adv. Mater. 2014, 26, 7324–7332. [Google Scholar] [CrossRef] [PubMed]
- Choong, C.L.; Shim, M.B.; Lee, B.S.; Jeon, S.; Ko, D.S.; Kang, T.H.; Bae, J.; Lee, S.H.; Byun, K.E.; Im, J.; et al. Highly Stretchable Resistive Pressure Sensors Using a Conductive Elastomeric Composite on a Micropyramid Array. Adv. Mater. 2014, 26, 3451–3458. [Google Scholar] [CrossRef]
- Viry, L.; Levi, A.; Totaro, M.; Mondini, A.; Mattoli, V.; Mazzolai, B.; Beccai, L. Flexible Three-Axial Force Sensor for Soft and Highly Sensitive Artificial Touch. Adv. Mater. 2014, 26, 2659–2664. [Google Scholar] [CrossRef]
- Lou, Z.; Chen, S.; Wang, L.; Jiang, K.; Shen, G. An Ultra-Sensitive and Rapid Response Speed Graphene Pressure Sensors for Electronic Skin and Health Monitoring. Nano Energy 2016, 23, 7–14. [Google Scholar] [CrossRef]
- Park, J.; Lee, Y.; Hong, J.; Lee, Y.; Ha, M.; Jung, Y.; Lim, H.; Kim, S.Y.; Ko, H. Tactile-Direction-Sensitive and Stretchable Electronic Skins Based on Human-Skin-Inspired Interlocked Microstructures. ACS Nano 2014, 8, 12020–12029. [Google Scholar] [CrossRef]
- Sekitani, T.; Someya, T. Stretchable Organic Integrated Circuits for Large-Area Electronic Skin Surfaces. MRS Bull. 2012, 37, 236–245. [Google Scholar] [CrossRef]
- Ge, J.; Sun, L.; Zhang, F.-R.; Zhang, Y.; Shi, L.-A.; Zhao, H.-Y.; Zhu, H.-W.; Jiang, H.-L.; Yu, S.-H. A Stretchable Electronic Fabric Artificial Skin with Pressure-, Lateral Strain-, and Flexion-Sensitive Properties. Adv. Mater. 2016, 28, 722–728. [Google Scholar] [CrossRef]
- Rajala, S.; Mattila, R.; Kaartinen, I.; Lekkala, J. Designing, Manufacturing and Testing of a Piezoelectric Polymer Film In-Sole Sensor for Plantar Pressure Distribution Measurements. IEEE Sens. J. 2017, 17, 6798–6805. [Google Scholar] [CrossRef]
- Bhang, S.H.; Jang, W.S.; Han, J.; Yoon, J.-K.; La, W.-G.; Lee, E.; Kim, Y.S.; Shin, J.-Y.; Lee, T.-J.; Baik, H.K.; et al. Zinc Oxide Nanorod-Based Piezoelectric Dermal Patch for Wound Healing. Adv. Funct. Mater. 2017, 27, 1603497. [Google Scholar] [CrossRef]
- Yi, Q.; Pei, X.; Das, P.; Qin, H.; Lee, S.W.; Esfandyarpour, R. A Self-Powered Triboelectric MXene-Based 3D-Printed Wearable Physiological Biosignal Sensing System for on-Demand, Wireless, and Real-Time Health Monitoring. Nano Energy 2022, 101, 107511. [Google Scholar] [CrossRef]
- Deng, H.-T.; Wang, Z.-Y.; Wang, Y.-L.; Wen, D.-L.; Zhang, X.-S. Integrated Hybrid Sensing and Microenergy for Compact Active Microsystems. Microsyst. Nanoeng. 2022, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Jose, M.; Bronckaers, A.; Kumar, R.S.N.; Reenaers, D.; Vandenryt, T.; Thoelen, R.; Deferme, W. Stretchable Printed Device for the Simultaneous Sensing of Temperature and Strain Validated in a Mouse Wound Healing Model. Sci. Rep. 2022, 12, 10138. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.-L.; Deng, H.-T.; Liu, X.; Li, G.-K.; Zhang, X.-R.; Zhang, X.-S. Wearable Multi-Sensing Double-Chain Thermoelectric Generator. Microsyst. Nanoeng. 2020, 6, 68. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Chen, S.; Dong, X.; Lin, Y.; Liu, L. Flexible Piezoresistive Sensors Based on “Dynamic Bridging Effect” of Silver Nanowires toward Graphene. Carbon 2017, 113, 395–403. [Google Scholar] [CrossRef]
- Lee, S.; Reuveny, A.; Reeder, J.; Lee, S.; Jin, H.; Liu, Q.; Yokota, T.; Sekitani, T.; Isoyama, T.; Abe, Y.; et al. A Transparent Bending-Insensitive Pressure Sensor. Nat. Nanotechnol. 2016, 11, 472–478. [Google Scholar] [CrossRef]
- Persano, L.; Dagdeviren, C.; Su, Y.; Zhang, Y.; Girardo, S.; Pisignano, D.; Huang, Y.; Rogers, J.A. High Performance Piezoelectric Devices Based on Aligned Arrays of Nanofibers of Poly(Vinylidenefluoride-Co-Trifluoroethylene). Nat. Commun. 2013, 4, 1633. [Google Scholar] [CrossRef]
- Chortos, A.; Liu, J.; Bao, Z. Pursuing Prosthetic Electronic Skin. Nat. Mater. 2016, 15, 937–950. [Google Scholar] [CrossRef]
- Kim, J.; Lee, M.; Shim, H.J.; Ghaffari, R.; Cho, H.R.; Son, D.; Jung, Y.H.; Soh, M.; Choi, C.; Jung, S.; et al. Stretchable Silicon Nanoribbon Electronics for Skin Prosthesis. Nat. Commun. 2014, 5, 5747. [Google Scholar] [CrossRef] [PubMed]
- Nassar, J.M.; Cordero, M.D.; Kutbee, A.T.; Karimi, M.A.; Sevilla, G.A.T.; Hussain, A.M.; Shamim, A.; Hussain, M.M. Paper Skin Multisensory Platform for Simultaneous Environmental Monitoring. Adv. Mater. Technol. 2016, 1, 1600004. [Google Scholar] [CrossRef]
- Gillard, N.; Leong, A.; Departe, J.P.; Kerdraon, J.; Allegre, W. Early detection of pressure ulcers from a simulation of temporal pressure data with reperfusion. In Proceedings of the JETSAN 2021—Colloque en Télésanté et Dispositifs Biomédicaux, Toulouse, France, 20–21 May 2021. [Google Scholar]
- Zlatkis, A.; Brazell, R.S.; Poole, C.F. The Role of Organic Volatile Profiles in Clinical Diagnosis. Clin. Chem. 1981, 27, 789–797. [Google Scholar] [CrossRef]
- Galassetti, P.R.; Novak, B.; Nemet, D.; Rose-Gottron, C.; Cooper, D.M.; Meinardi, S.; Newcomb, R.; Zaldivar, F.; Blake, D.R. Breath Ethanol and Acetone as Indicators of Serum Glucose Levels: An Initial Report. Diabetes Technol. Ther. 2005, 7, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Daneshkhah, A.; Siegel, A.P.; Agarwal, M. Chapter 23—Volatile Organic Compounds: Potential Biomarkers for Improved Diagnosis and Monitoring of Diabetic Wounds. In Wound Healing, Tissue Repair, and Regeneration in Diabetes; Bagchi, D., Das, A., Roy, S., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 491–512. ISBN 978-0-12-816413-6. [Google Scholar]
- Hu, W.; Wu, W.; Jian, Y.; Haick, H.; Zhang, G.; Qian, Y.; Yuan, M.; Yao, M. Volatolomics in Healthcare and Its Advanced Detection Technology. Nano Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Di Natale, C.; Macagnano, A.; Paolesse, R.; Tarizzo, E.; Mantini, A.; D’Amico, A. Human Skin Odor Analysis by Means of an Electronic Nose. Sens. Actuators B Chem. 2000, 65, 216–219. [Google Scholar] [CrossRef]
- Ashrafi, M.; Bates, M.; Baguneid, M.; Alonso-Rasgado, T.; Rautemaa-Richardson, R.; Bayat, A. Volatile Organic Compound Detection as a Potential Means of Diagnosing Cutaneous Wound Infections. Wound Repair Regen. 2017, 25, 574–590. [Google Scholar] [CrossRef]
- Dini, F.; Capuano, R.; Strand, T.; Ek, A.-C.; Lindgren, M.; Paolesse, R.; Di Natale, C.; Lundström, I. Volatile Emissions from Compressed Tissue. PLoS ONE 2013, 8, e69271. [Google Scholar] [CrossRef]
- Chesham, J.S.; Platt, D.J. Patterns of Wound Colonisation in Patients with Peripheral Vascular Disease. J. Infect. 1987, 15, 21–26. [Google Scholar] [CrossRef]
- Shirasu, M.; Nagai, S.; Hayashi, R.; Ochiai, A.; Touhara, K. Dimethyl Trisulfide as a Characteristic Odor Associated with Fungating Cancer Wounds. Biosci. Biotechnol. Biochem. 2009, 73, 2117–2120. [Google Scholar] [CrossRef]
- Romanelli, M.; Gaggio, G.; Coluccia, M.; Piaggesi, A. Technological Advances in Wound Bed Measurements. Wounds 2002, 14, 58–66. [Google Scholar]
- Yusuf, N.; Omar, M.; Zakaria, A.; Abdullah, A.A.; Kamarudin, L.M.; Shakaff, A.Y.M.; Masnan, M.J.; Zakaria, N.Z.I.; Yeap, E.J.; Othman, A.; et al. Diagnosis of Bacteria for Diabetic Foot Infection Using Electronic Nose Technology. In Proceedings of the 2013 IEEE Conference on Wireless Sensor (ICWISE), Kuching, Malaysia, 2–4 December 2013; pp. 114–118. [Google Scholar] [CrossRef]
- Yan, J.; Duan, S.; Huang, T.; Wang, L. Hybrid Feature Matrix Construction and Feature Selection Optimization-Based Multi-Objective QPSO for Electronic Nose in Wound Infection Detection. Sens. Rev. 2016, 36, 23–33. [Google Scholar] [CrossRef]
- Ousey, K.; Roberts, D.; Gefen, A. Early Identification of Wound Infection: Understanding Wound Odour. J. Wound Care 2017, 26, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Akhmetova, A.; Saliev, T.; Allan, I.U.; Illsley, M.J.; Nurgozhin, T.; Mikhalovsky, S. A Comprehensive Review of Topical Odor-Controlling Treatment Options for Chronic Wounds. J. Wound Ostomy Cont. Nurs. 2016, 43, 598–609. [Google Scholar] [CrossRef]
- Kataoka, H.; Saito, K.; Kato, H.; Masuda, K. Noninvasive Analysis of Volatile Biomarkers in Human Emanations for Health and Early Disease Diagnosis. Bioanalysis 2013, 5, 1443–1459. [Google Scholar] [CrossRef]
- Ruzsanyi, V.; Mochalski, P.; Schmid, A.; Wiesenhofer, H.; Klieber, M.; Hinterhuber, H.; Amann, A. Ion Mobility Spectrometry for Detection of Skin Volatiles. J. Chromatogr. B 2012, 911, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.N.; Riazanskaia, S.; Cheung, W.; Xu, Y.; Goodacre, R.; Thomas, C.L.P.; Baguneid, M.S.; Bayat, A. Novel Noninvasive Identification of Biomarkers by Analytical Profiling of Chronic Wounds Using Volatile Organic Compounds. Wound Repair Regen. 2010, 18, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Dutkiewicz, E.P.; Chiu, H.Y.; Urban, P.L. Probing Skin for Metabolites and Topical Drugs with Hydrogel Micropatches. Anal. Chem. 2017, 89, 2664–2670. [Google Scholar] [CrossRef]
- Jiang, R.; Cudjoe, E.; Bojko, B.; Abaffy, T.; Pawliszyn, J. A Non-Invasive Method for in Vivo Skin Volatile Compounds Sampling. Anal. Chim. Acta 2013, 804, 111–119. [Google Scholar] [CrossRef]
- Riazanskaia, S.; Blackburn, G.; Harker, M.; Taylor, D.; Thomas, C.L.P. The Analytical Utility of Thermally Desorbed Polydimethylsilicone Membranes for In-Vivo Sampling of Volatile Organic Compounds in and on Human Skin. Analyst 2008, 133, 1020–1027. [Google Scholar] [CrossRef]
- Tran, M.T. Development of Nanocomposite Quantum Resistive Sensors for the Prevention of Bedsores. Ph.D. Thesis, University of South Brittany (UBS), Lorient, France, 2018. [Google Scholar]
- Bouvree, A.; Feller, J.F.; Castro, M.; Grohens, Y.; Rinaudo, M. Conductive Polymer Nano-BioComposites (CPC): Chitosan-Carbon Nanoparticle a Good Candidate to Design Polar Vapour Sensors. Sens. Actuators B Chem. 2009, 138, 138–147. [Google Scholar] [CrossRef]
- Kumar, B.; Feller, J.F.; Castro, M.; Lu, J. Conductive Bio-Polymer Nano-Composites (CPC): Chitosan-Carbon Nanotube Transducers Assembled via Spray Layer-by-Layer for Volatile Organic Compound Sensing. Talanta 2010, 81, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Sachan, A.; Castro, M.; Choudhary, V.; Feller, J.-F. Influence of Water Molecules on the Detection of Volatile Organic Compounds (VOC) Cancer Biomarkers by Nanocomposite Quantum Resistive Vapor Sensors VQRS. Chemosensors 2018, 6, 64. [Google Scholar] [CrossRef]
- Burns, M.; Enderle, J.; Rosow, E.; Zhu, Q. Development of a Wound Assessment System for Quantitative Chronic Wound Monitoring. In Proceedings of the IEEE 28th Annual Northeast Bioengineering Conference, Philadelphia, PA, USA, 21 April 2002; pp. 7–8. [Google Scholar] [CrossRef]
- Rajendran, P.J.; Leachtenauer, J.; Kell, S.; Turner, B.; Newcomer, C.; Lyder, C.; Alwan, M. Improving the Detection of Stage I Pressure Ulcers by Enhancing Digital Color Images. In Proceedings of the 28th Annual International Conference of the IEEE Engineering in Medicine & Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 5206–5209. [Google Scholar] [CrossRef]
- Wannous, H.; Treuillet, S.; Lucas, Y. Supervised Tissue Classification from Color Images for a Complete Wound Assessment Tool. In Proceedings of the 29th Annual International Conference of the IEEE Engineering in Medicine & Biology Society, Lyon, France, 22–26 August 2007; pp. 6031–6034. [Google Scholar] [CrossRef]
- Thawer, H.A.; Houghton, P.E.; Woodbury, M.G.; Keast, D.; Campbell, K. A Comparison of Computer-Assisted and Manual Wound Size Measurement. Ostomy Wound Manag. 2002, 48, 46–53. [Google Scholar]
- Papazoglou, E.S.; Zubkov, L.; Mao, X.; Neidrauer, M.; Rannou, N.; Weingarten, M.S. Image Analysis of Chronic Wounds for Determining the Surface Area. Wound Repair Regen. 2010, 18, 349–358. [Google Scholar] [CrossRef]
- Dhane, D.M.; Krishna, V.; Achar, A.; Bar, C.; Sanyal, K.; Chakraborty, C. Spectral Clustering for Unsupervised Segmentation of Lower Extremity Wound Beds Using Optical Images. J. Med. Syst. 2016, 40, 207. [Google Scholar] [CrossRef]
- Meier, R.J.; Schreml, S.; Wang, X.D.; Landthaler, M.; Babilas, P.; Wolfbeis, O.S. Simultaneous Photographing of Oxygen and PH in Vivo Using Sensor Films. Angew. Chem. Int. Ed. 2011, 50, 10893–10896. [Google Scholar] [CrossRef]
- Sowa, M.G.; Friesen, J.R. In Vivo Tissue Analysis by Near-Infrared Spectroscopy. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2000; ISBN 9780470027318. [Google Scholar]
- Sen, C.K. Wound Healing Essentials: Let There Be Oxygen. Wound Repair Regen. 2009, 17, 1–18. [Google Scholar] [CrossRef]
- Papazoglou, E.S.; Weingarten, M.S.; Zubkov, L.; Neidrauer, M.; Pourrezaei, K. Assessment of Diabetic Foot Ulcers with Diffuse near Infrared Methodology. In Proceedings of the 2008 8th IEEE International Conference on BioInformatics and BioEngineering, Athens, Greece, 8–10 October 2008; pp. 1–5. [Google Scholar] [CrossRef]
- Neidrauer, M.; Zubkov, L.; Weingarten, M.S.; Pourrezaei, K.; Papazoglou, E.S. Evaluation of Chronic Diabetic Wounds with the Near Infrared Wound Monitor. In XII Mediterranean Conference on Medical and Biological Engineering and Computing 2010, Proceedings of the MEDICON 2010, Chalkidiki, Greece, 27–30 May 2010; Springer: Berlin/Heidelberg, Germany, 2010; pp. 487–489. [Google Scholar]
- Weingarten, M.S.; Neidrauer, M.; Mateo, A.; Mao, X.; McDaniel, J.E.; Jenkins, L.; Bouraee, S.; Zubkov, L.; Pourrezaei, K.; Papazoglou, E.S. Prediction of Wound Healing in Human Diabetic Foot Ulcers by Diffuse Near-Infrared Spectroscopy: A Pilot Study. Wound Repair Regen. 2010, 18, 180–185. [Google Scholar] [CrossRef]
- Van Haren, R.M.; Ryan, M.L.; Thorson, C.M.; Namias, N.; Livingstone, A.S.; Proctor, K.G. Bilateral Near-Infrared Spectroscopy for Detecting Traumatic Vascular Injury. J. Surg. Res. 2013, 184, 526–532. [Google Scholar] [CrossRef]
- Grosenick, D.; Wabnitz, H.; Ebert, B. Recent Advances in Contrast-Enhanced near Infrared Diffuse Optical Imaging of Diseases Using Indocyanine Green. J. Near Infrared Spectrosc. 2012, 20, 203–221. [Google Scholar] [CrossRef]
- Alander, J.T.; Kaartinen, I.; Laakso, A.; Pätilä, T.; Spillmann, T.; Tuchin, V.V.; Venermo, M.; Välisuo, P. A Review of Indocyanine Green Fluorescent Imaging in Surgery. Int. J. Biomed. Imaging 2012, 2012, 940585. [Google Scholar] [CrossRef] [PubMed]
- Sowa, M.G.; Kuo, W.C.; Ko, A.C.T.; Armstrong, D.G. Review of Near-Infrared Methods for Wound Assessment. J. Biomed. Opt. 2016, 21, 091304. [Google Scholar] [CrossRef]
- Hope-Ross, M.; Yannuzzi, L.A.; Gragoudas, E.S.; Guyer, D.R.; Slakter, J.S.; Sorenson, J.A.; Krupsky, S.; Orlock, D.A.; Puliafito, C.A. Adverse Reactions Due to Indocyanine Green. Ophthalmology 1994, 101, 529–533. [Google Scholar] [CrossRef]
- Wu, P.; Fisher, A.C.; Foo, P.P.; Queen, D.; Gaylor, J.D.S. In Vitro Assessment of Water Vapour Transmission of Synthetic Wound Dressings. Biomaterials 1995, 16, 171–175. [Google Scholar] [CrossRef]
- Milne, S.D.; Seoudi, I.; Al Hamad, H.; Talal, T.K.; Anoop, A.A.; Allahverdi, N.; Zakaria, Z.; Menzies, R.; Connolly, P. A Wearable Wound Moisture Sensor as an Indicator for Wound Dressing Change: An Observational Study of Wound Moisture and Status. Int. Wound J. 2016, 13, 1309–1314. [Google Scholar] [CrossRef]
- Schneider, L.A.; Korber, A.; Grabbe, S.; Dissemond, J. Influence of PH on Wound-Healing: A New Perspective for Wound-Therapy? Arch. Dermatol. Res. 2007, 298, 413–420. [Google Scholar] [CrossRef]
- Mariani, F.; Serafini, M.; Gualandi, I.; Arcangeli, D.; Decataldo, F.; Possanzini, L.; Tessarolo, M.; Tonelli, D.; Fraboni, B.; Scavetta, E. Advanced Wound Dressing for Real-Time PH Monitoring. ACS Sens. 2021, 6, 2366–2377. [Google Scholar] [CrossRef]
- Mehmood, N.; Hariz, A.; Fitridge, R.; Voelcker, N.H. Applications of Modern Sensors and Wireless Technology in Effective Wound Management. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2014, 102, 885–895. [Google Scholar] [CrossRef]
- Matzeu, G.; Losacco, M.; Parducci, E.; Pucci, A.; Dini, V.; Romanelli, M.; Di Francesco, F. Skin Temperature Monitoring by a Wireless Sensor. In Proceedings of the IECON 2011—37th Annual Conference of the IEEE Industrial Electronics Society, Melbourne, Australia, 7–10 November 2011; pp. 3533–3535. [Google Scholar] [CrossRef]
- Milici, S.; Amendola, S.; Bianco, A.; Marrocco, G. Epidermal RFID Passive Sensor for Body Temperature Measurements. In Proceedings of the 2014 IEEE RFID Technology and Applications Conference (RFID-TA), Tampere, Finland, 8–9 September 2014; pp. 140–144. [Google Scholar] [CrossRef]
- Occhiuzzi, C.; Ajovalasit, A.; Sabatino, M.A.; Dispenza, C.; Marrocco, G. RFID Epidermal Sensor Including Hydrogel Membranes for Wound Monitoring and Healing. In Proceedings of the 2015 IEEE International Conference on RFID (RFID), San Diego, CA, USA, 15–17 April 2015; pp. 182–188. [Google Scholar]
- Kassal, P.; Zubak, M.; Scheipl, G.; Mohr, G.J.; Steinberg, M.D.; Murković Steinberg, I. Smart Bandage with Wireless Connectivity for Optical Monitoring of PH. Sens. Actuators B Chem. 2017, 246, 455–460. [Google Scholar] [CrossRef]
- Farooqui, M.F.; Shamim, A. Low Cost Inkjet Printed Smart Bandage for Wireless Monitoring of Chronic Wounds. Sci. Rep. 2016, 6, 28949. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Lu, Y.; Cheng, C.; Li, X.; Xu, J.; Liu, Z.; Liu, J.; Liu, G.; Shi, Z.; Chen, Z.; et al. Battery-Free and Wireless Smart Wound Dressing for Wound Infection Monitoring and Electrically Controlled On-Demand Drug Delivery. Adv. Funct. Mater. 2021, 31, 2100852. [Google Scholar] [CrossRef]
- Chakraborty, C.; Gupta, B.; Ghosh, S.K. A Review on Telemedicine-Based WBAN Framework for Patient Monitoring. Telemed. e-Health 2013, 19, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Zgheib, R.; Bastide, R.; Conchon, E. A Semantic Web-of-Things Architecture for Monitoring the Risk of Bedsores. In Proceedings of the 2015 International Conference on Computational Science and Computational Intelligence (CSCI), Las Vegas, NV, USA, 7–9 December 2015; pp. 318–323. [Google Scholar] [CrossRef]
- Texier, I.; Xydis, S.; Soudris, D.; Marcoux, P.; Pham, P.; Muller, M.; Correvon, M.; Dudnik, G.; Voirin, G.; Kristenssen, J.; et al. SWAN-iCare Project: Towards Smart Wearable and Autonomous Negative Pressure Device for Wound Monitoring and Therapy. In Proceedings of the 2014 4th International Conference on Wireless Mobile Communication and Healthcare—“Transforming Healthcare Through Innovations in Mobile and Wireless Technologies” (MOBIHEALTH 2014), Athens, Greece, 3–5 November 2014; pp. 357–360. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, T.; Zhao, C.; Li, J.; Huang, R.; Zhang, Q.; Li, Y.; Li, X. An Integrated Smart Sensor Dressing for Real-Time Wound Microenvironment Monitoring and Promoting Angiogenesis and Wound Healing. Front. Cell Dev. Biol. 2021, 9, 701525. [Google Scholar] [CrossRef] [PubMed]












| Type of Sensor | Materials and Methods | Sensitive Factors | Working Structure and Range | Ref. |
|---|---|---|---|---|
| Capacitive | PDMS or CNT ink-doped PDMS Micro-contact printing | Bending, stretching, twisting, and folding | 4 × 4 pressure sensors array Highly linear, twisting up to 360° | [27] |
| Piezoresistive | PDMS-PEDOT: PSS or PUD Drop casting | Normal pressure, pulse wave | Sensitivity: 10.3 kPa−1 Limit detection: 23 Pa | [57] |
| Capacitive | PDMS-copper- or tin-woven fluorosilicone | Tactile, normal pressure, heartbeat | Sensing range: 100 Pa–400 kPa Tangential sensitivity: 0.3 N−1 | [58] |
| Piezoresistive | PDMS- Reverse micelle-MWCNT Nozzle jet printing | Strain, normal pressure, bending | Low-pressure regime: 0.25 kPa | [54] |
| Piezoresistive | PDMS-PVDF@rGO Electrospinning | Normal pressure, bending, and torsion | Sensitivity: 15.6 kPa−1 | [59] |
| Piezoresistive | Microdome-patterned MWNT–PDMS Micromold casting | Normal pressure, shear, stretch, bending, and twisting | 3 × 3 sensor arrays Sensing range: from 100 Pa to 25 kPa | [60] |
| Piezoresistive | AgNW-APTES Dip coating | Pressure, lateral strain, and flexion | 10 × 10 sensor arrays Pressure sensitivity: 4.29 N−1 | [61] |
| Piezoresistive | PDMS-Pt-PU nanohairs | Normal pressure, shear, torsion | Network of 64 pixels GF: ~11.5 (pressure), ~0.75 (shear), and ~8.53 (torsion) | [62] |
| Piezoelectric | Polyvinylidenefluoride | Normal pressure | 58–486 kPa | [63] |
| Piezoelectric | Zinc oxide nanorod | NA | NA | [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, M.-T.; Kumar, A.; Sachan, A.; Castro, M.; Allegre, W.; Feller, J.-F. Emerging Strategies Based on Sensors for Chronic Wound Monitoring and Management. Chemosensors 2022, 10, 311. https://doi.org/10.3390/chemosensors10080311
Tran M-T, Kumar A, Sachan A, Castro M, Allegre W, Feller J-F. Emerging Strategies Based on Sensors for Chronic Wound Monitoring and Management. Chemosensors. 2022; 10(8):311. https://doi.org/10.3390/chemosensors10080311
Chicago/Turabian StyleTran, Manh-Trung, Abhishek Kumar, Abhishek Sachan, Mickaël Castro, Willy Allegre, and Jean-François Feller. 2022. "Emerging Strategies Based on Sensors for Chronic Wound Monitoring and Management" Chemosensors 10, no. 8: 311. https://doi.org/10.3390/chemosensors10080311
APA StyleTran, M.-T., Kumar, A., Sachan, A., Castro, M., Allegre, W., & Feller, J.-F. (2022). Emerging Strategies Based on Sensors for Chronic Wound Monitoring and Management. Chemosensors, 10(8), 311. https://doi.org/10.3390/chemosensors10080311






