How Meaningful Are Minor Details in the Generation of Nanomodified Electrochemical Enzyme Biosensors? Exploring the Scenario with Sinusoidal Approaches
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Synthesis and Characterization of AuNPs
2.3. Electrochemical Measurements
2.4. Modification of the SNGC Electrode Surface
2.5. Analytical Measurements
2.6. Surface Characterization by SEM
3. Results and Discussion
3.1. Characterization of AuNPs Synthesized by Ultrasound-Assisted Synthesis
3.2. Electrochemical Characterization of the Proposed (Bio)Sensors
3.3. SEM Characterization
3.4. Analytical Performance
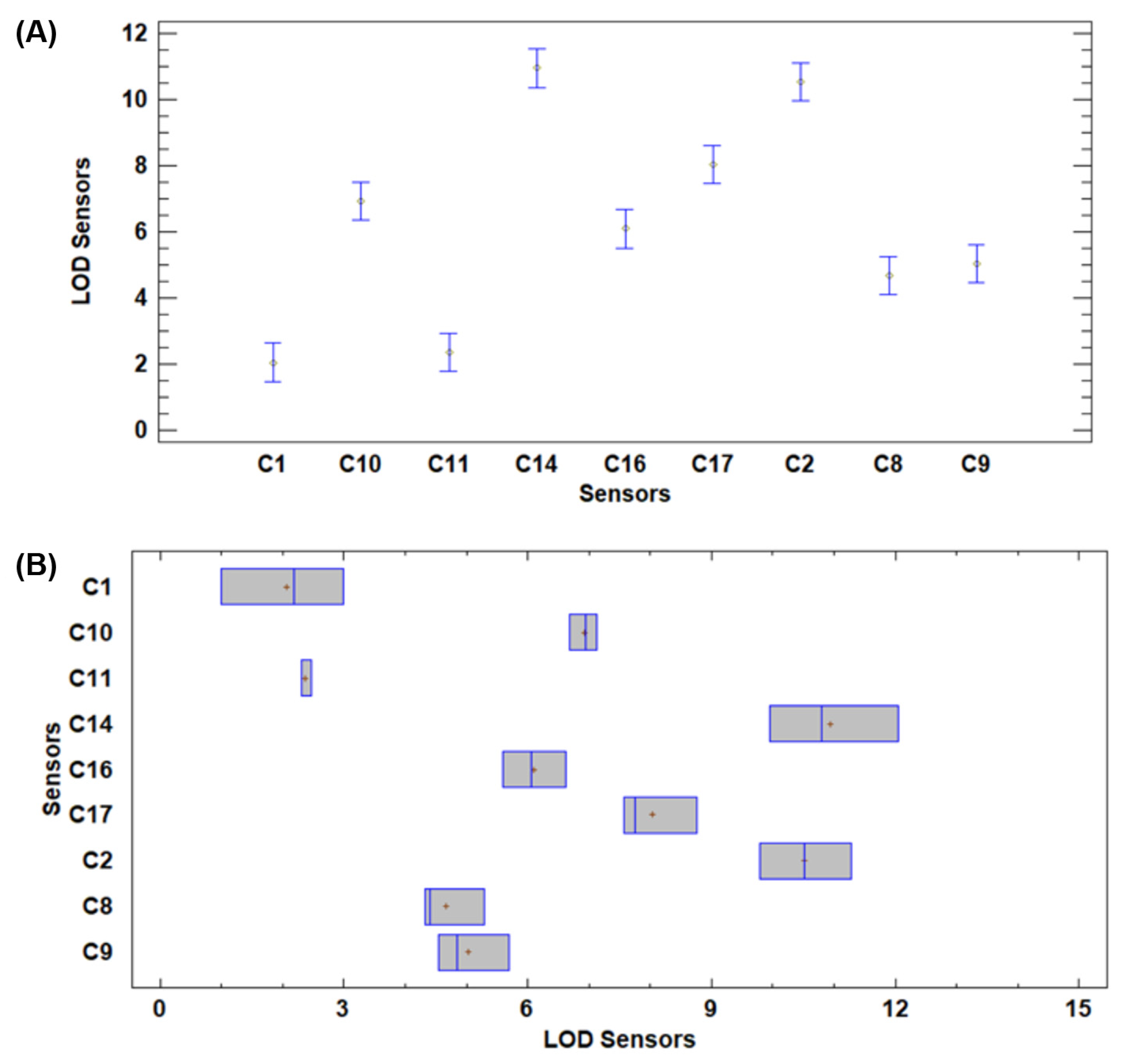
3.4.1. Plain Examination of the Figures of Merit Observed
3.4.2. Statistical Studies of the Data by ANOVA
3.5. Interference Studies
3.6. Real Samples Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, X.; Xia, X.; Du, Y.; Wang, C. Electroless Deposition of Au Nanoparticles on Reduced Graphene Oxide/Polyimide Film for Electrochemical Detection of Hydroquinone and Catechol. Front. Mater. Sci. 2017, 11, 262–270. [Google Scholar] [CrossRef]
- Manan, F.A.A.; Hong, W.W.; Abdullah, J.; Yusof, N.A.; Ahmad, I. Nanocrystalline Cellulose Decorated Quantum Dots Based Tyrosinase Biosensor for Phenol Determination. Mater. Sci. Eng. C 2019, 99, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Kausaite-Minkstimiene, A.; Glumbokaite, L.; Ramanaviciene, A.; Dauksaite, E.; Ramanavicius, A. An Amperometric Glucose Biosensor Based on Poly (Pyrrole-2-Carboxylic Acid)/Glucose Oxidase Biocomposite. Electroanalysis 2018, 30, 1634–1644. [Google Scholar] [CrossRef]
- Xu, H.; Zhou, S.; Jiang, D.; Chen, H.Y. Cholesterol Oxidase/Triton X-100 Parked Microelectrodes for the Detection of Cholesterol in Plasma Membrane at Single Cells. Anal. Chem. 2018, 90, 1054–1058. [Google Scholar] [CrossRef] [Green Version]
- Cunha-Silva, H.; Arcos-Martinez, M.J. Dual Range Lactate Oxidase-Based Screen Printed Amperometric Biosensor for Analysis of Lactate in Diversified Samples. Talanta 2018, 188, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Phetsang, S.; Jakmunee, J.; Mungkornasawakul, P.; Laocharoensuk, R.; Ounnunkad, K. Sensitive Amperometric Biosensors for Detection of Glucose and Cholesterol Using a Platinum/Reduced Graphene Oxide/Poly(3-Aminobenzoic Acid) Film-Modified Screen-Printed Carbon Electrode. Bioelectrochemistry 2019, 127, 125–135. [Google Scholar] [CrossRef]
- Nagles, E.; García-Beltrán, O.; Calderón, J.A. Evaluation of the Usefulness of a Novel Electrochemical Sensor in Detecting Uric Acid and Dopamine in the Presence of Ascorbic Acid Using a Screen-Printed Carbon Electrode Modified with Single Walled Carbon Nanotubes and Ionic Liquids. Electrochim. Acta 2017, 258, 512–523. [Google Scholar] [CrossRef]
- Cui, L.; Lu, M.; Li, Y.; Tang, B.; Zhang, C. A reusable ratiometric electrochemical biosensor on the basis of the binding of methylene blue to DNA with alternating AT base sequence for sensitive detection of adenosine. Biosens. Electron. B 2018, 102, 87–93. [Google Scholar] [CrossRef]
- Cui, L.; Shen, J.; Li, C.; Cui, P.; Luo, X.; Wang, X.; Zhang, C. Construction of a Dye-Sensitized and Gold plasmon-Enhanced Cathodic Photoelectrochemical Biosensor for Methyltransferase Activity Assay. Anal. Chem. 2021, 93, 10310–10316. [Google Scholar] [CrossRef]
- Attar, A.; Cubillana-Aguilera, L.; Naranjo-Rodríguez, I.; de Cisneros, J.L.H.-H.H.; Palacios-Santander, J.M.; Amine, A. Amperometric Inhibition Biosensors Based on Horseradish Peroxidase and Gold Sononanoparticles Immobilized onto Different Electrodes for Cyanide Measurements. Bioelectrochemistry 2014, 101, 84–91. [Google Scholar] [CrossRef]
- Attar, A.; Amine, A.; Achi, F.; Bacha, S.B.; Cubillana-Aguilera, L.; Palacios-Santander, J.M.; Baraket, A.; Errachid, A. A Novel Amperometric Inhibition Biosensor Based on HRP and Gold Sononanoparticles Immobilised onto Sonogel-Carbon Electrode for the Determination of Sulphides and Gold Sononanoparticles Immobilised Onto. Int. J. Environ. Anal. Chem. 2016, 7319, 1–15. [Google Scholar] [CrossRef]
- Das, P.; Das, M.; Chinnadayyala, S.R.; Singha, I.M.; Goswami, P. Recent Advances on Developing 3rd Generation Enzyme Electrode for Biosensor Applications. Biosens. Bioelectron. 2016, 79, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, W.; Ghica, M.E.; Ajayi, R.F.; Iwuoha, E.I.; Brett, C.M.A. Tyrosinase Based Amperometric Biosensor for Determination of Tyramine in Fermented Food and Beverages with Gold Nanoparticle Doped Poly(8-Anilino-1-Naphthalene Sulphonic Acid) Modified Electrode. Food Chem. 2019, 282, 18–26. [Google Scholar] [CrossRef]
- Lupu, S.; Lete, C.; Javier del Campo, F. Dopamine Electroanalysis Using Electrochemical Biosensors Prepared by a Sinusoidal Voltages Method. Electroanalysis 2015, 27, 1649–1659. [Google Scholar] [CrossRef]
- Buk, V.; Pemble, M.E. A Highly Sensitive Glucose Biosensor Based on a Micro Disk Array Electrode Design Modified with Carbon Quantum Dots and Gold Nanoparticles. Electrochim. Acta 2019, 298, 97–105. [Google Scholar] [CrossRef]
- Çakıroğlu, B.; Demirci, Y.C.; Gökgöz, E.; Özacar, M. A Photoelectrochemical Glucose and Lactose Biosensor Consisting of Gold Nanoparticles, MnO2 and g-C3N4 Decorated TiO2. Sens. Actuators B Chem. 2019, 282, 282–289. [Google Scholar] [CrossRef]
- Xuan, X.; Yoon, H.S.; Park, J.Y. A Wearable Electrochemical Glucose Sensor Based on Simple and Low-Cost Fabrication Supported Micro-Patterned Reduced Graphene Oxide Nanocomposite Electrode on Flexible Substrate. Biosens. Bioelectron. 2018, 109, 75–82. [Google Scholar] [CrossRef]
- Stasyuk, N.; Gayda, G.; Zakalskiy, A.; Zakalska, O.; Serkiz, R.; Gonchar, M. Amperometric Biosensors Based on Oxidases and PtRu Nanoparticles as Artificial Peroxidase. Food Chem. 2019, 285, 213–220. [Google Scholar] [CrossRef]
- Chauhan, N.; Tiwari, S.; Narayan, T.; Jain, U. Bienzymatic Assembly Formed @ Pt Nano Sensing Framework Detecting Acetylcholine in Aqueous Phase. Appl. Surf. Sci. 2019, 474, 154–160. [Google Scholar] [CrossRef]
- Uribe, P.A.; Ortiz, C.C.; Centeno, D.A.; Castillo, J.J.; Blanco, S.I.; Gutierrez, J.A. Self-Assembled Pt Screen Printed Electrodes with a Novel Peroxidase Panicum Maximum and Zinc Oxide Nanoparticles for H2O2 Detection. Colloids Surf. A Physicochem. Eng. Asp. 2019, 561, 18–24. [Google Scholar] [CrossRef]
- Malekzad, H.; Sahandi Zangabad, P.; Mirshekari, H.; Karimi, M.; Hamblin, M.R. Noble Metal Nanoparticles in Biosensors: Recent Studies and Applications. Nanotechnol. Rev. 2017, 6, 301–329. [Google Scholar] [CrossRef] [PubMed]
- Lanzellotto, C.; Favero, G.; Antonelli, M.L.; Tortolini, C.; Cannistraro, S.; Coppari, E.; Mazzei, F. Nanostructured Enzymatic Biosensor Based on Fullerene and Gold Nanoparticles: Preparation, Characterization and Analytical Applications. Biosens. Bioelectron. 2014, 55, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chou, Y.; Tsai, J.; Huang, T. Applied Sciences Tyrosinase/Chitosan/Reduced Graphene Oxide Modified Screen-Printed Carbon Electrode for Sensitive and Interference-Free Detection of Dopamine. Appl. Sci. 2019, 9, 662. [Google Scholar] [CrossRef] [Green Version]
- Barkauskas, J.; Mikoliunaite, L.; Paklonskaite, I.; Genys, P.; Petroniene, J.J.; Morkvenaite-Vilkonciene, I.; Ramanaviciene, A.; Samukaite-Bubniene, U.; Ramanavicius, A. Single-Walled Carbon Nanotube Based Coating Modified with Reduced Graphene Oxide for the Design of Amperometric Biosensors. Mater. Sci. Eng. C 2019, 98, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Canbay, E.; Akyilmaz, E. Design of a Multiwalled Carbon Nanotube-Nafion-Cysteamine Modified Tyrosinase Biosensor and Its Adaptation of Dopamine Determination. Anal. Biochem. 2014, 444, 8–15. [Google Scholar] [CrossRef]
- Salazar, P.; Martín, M.; González-Mora, J.L. In Situ Electrodeposition of Cholesterol Oxidase-Modified Polydopamine Thin Film on Nanostructured Screen Printed Electrodes for Free Cholesterol Determination. J. Electroanal. Chem. 2019, 837, 191–199. [Google Scholar] [CrossRef]
- Lete, C.; López-Iglesias, D.; García-Guzmán, J.J.; Leau, S.; Stanciu, A.; Marin, M.; Palacios-Santander, J.M.; Lupu, S.; Cubillana-Aguilera, L. A Sensitive Electrochemical Sensor Based on Sonogel-Carbon Material Enriched with Gold Nanoparticles for Melatonin Determination. Sensors 2021, 22, 120. [Google Scholar] [CrossRef]
- Ghanam, A.; Haddour, N.; Mohammadi, H.; Amine, A.; Sabac, A.; Buret, F. A membrane-less Glucose/O2 non-enzymatic fuel cell based on bimetallic Pd-Au nanostructure anode and air-breathing cathode: Towards micro-power applications at neutral pH. Bionsens. Bioelectron. 2022, 210, 114335. [Google Scholar] [CrossRef]
- ElFadil, D.; Palmieri, S.; Silveri, F.; Della Pelle, F.; Sergi, M.; Del Carlo, M.; Amine, A.; Compagnone, D. Fast sonochemical molecularly imprinted polymer synthesis for selective electrochemical determination of maleic hydrazide. Microchem. J. 2022, 180, 107634. [Google Scholar] [CrossRef]
- Ghanam, A.; Haddour, N.; Mohammadi, H.; Amine, A.; Sabac, A.; Buret, F. Nanoporous Cauliflower-like Pd-Loaded Functionalized Carbon Nanotubes as an Enzyme-Free Electrocatalyst for Glucose Sensing at Neutral pH: Mechanism Study. Sensors 2022, 22, 2706. [Google Scholar] [CrossRef]
- García-Guzmán, J.J.; López-Iglesias, D.; Cubillana-Aguilera, L.; Bellido-Milla, D.; Palacios-Santander, J.M.; Marin, M.; Grigorescu, S.D.; Lete, C.; Lupu, S. Silver Nanostructures-Poly(3,4-Ethylenedioxythiophene) Sensing Material Prepared by Sinusoidal Voltage Procedure for Detection of Antioxidants. Electrochim. Acta 2021, 393. [Google Scholar] [CrossRef]
- García-Guzmán, J.J.; López-Iglesias, D.; Cubillana-Aguilera, L.; Lete, C.; Lupu, S.; Palacios-Santander, J.M.; Bellido-Milla, D. Assessment of the Polyphenol Indices and Antioxidant Capacity for Beers and Wines Using a Tyrosinase-Based Biosensor Prepared by Sinusoidal Current Method. Sensors 2019, 19, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García Guzmán, J.J.; Aguilera, L.C.; Milla, D.B.; Rodríguez, I.N.; Lete, C.; Palacios Santander, J.M.; Lupu, S. Development of Sonogel-Carbon Based Biosensors Using Sinusoidal Voltages and Currents Methods. Sens. Actuators B Chem. 2018, 255, 1525–1535. [Google Scholar] [CrossRef]
- Ribeiro, J.A.; Fernandes, P.M.V.; Pereira, C.M.; Silva, F. Electrochemical Sensors and Biosensors for Determination of Catecholamine Neurotransmitters: A Review. Talanta 2016, 160, 653–679. [Google Scholar] [CrossRef] [PubMed]
- Cubillana-Aguilera, L.M.; Franco-Romano, M.; Gil, M.L.A.; Naranjo-Rodríguez, I.; Hidalgo-Hidalgo De Cisneros, J.L.; Palacios-Santander, J.M. New, Fast and Green Procedure for the Synthesis of Gold Nanoparticles Based on Sonocatalysis. Ultrason. Sonochem. 2011, 18, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Bellido-Milla, D.; Cubillana-Aguilera, L.M.; El Kaoutit, M.; Hernández-Artiga, M.P.; Hidalgo-Hidalgo De Cisneros, J.L.; Naranjo-Rodríguez, I.; Palacios-Santander, J.M. Recent Advances in Graphite Powder-Based Electrodes. Anal. Bioanal. Chem. 2013, 405, 3525–3539. [Google Scholar] [CrossRef] [PubMed]
- Hilali, N.; Ghanam, A.; Mohammadi, H.; Amine, A.; García-Guzmán, J.J.; Cubillana-Aguilera, L.; Palacios-Santander, J.M. Comparison between Modified and Unmodified Carbon Paste Electrodes for Hexavalent Chromium Determination. Electroanalysis 2018, 30, 2750–2759. [Google Scholar] [CrossRef]
- Shrivastava, A. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Miller, J.N.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry, 6th ed.; Pearson Education Ltd.: Harlow, UK, 2010. [Google Scholar]
- Peltola, E.; Sainio, S.; Holt, K.B.; Palomäki, T.; Koskinen, J.; Laurila, T. Electrochemical Fouling of Dopamine and Recovery of Carbon Electrodes. Anal. Chem. 2018, 90, 1408–1416. [Google Scholar] [CrossRef] [Green Version]
- Duvall, S.H.; McCreery, R.L. Self-Catalysis by Catechols and Quinones during Heterogeneous Electron Transfer at Carbon Electrodes. J. Am. Chem. Soc. 2000, 122, 6759–6764. [Google Scholar] [CrossRef]
- Jackowska, K.; Krysinski, P. New Trends in the Electrochemical Sensing of Dopamine. Anal. Bioanal. Chem. 2013, 405, 3753–3771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Pillier, F.; Cachet, H.; Debiemme-Chouvy, C. One-Pot Electrosynthesis of Ultrathin Overoxidized Poly(3,4-Ethylenedioxythiophene) Films. Electrochim. Acta 2022, 401, 139472. [Google Scholar] [CrossRef]
- Pingarrón, J.M.; Yáñez-Sedeño, P.; González-Cortés, A. Gold Nanoparticle-Based Electrochemical Biosensors. Electrochim. Acta 2008, 53, 5848–5866. [Google Scholar] [CrossRef]
- Lupu, S.; Lete, C.; Balaure, P.; Caval, D.; Mihailciuc, C.; Lakard, B.; Hihn, J.; Javier del Campo, F. Development of Amperometric Biosensors Based on Nanostructured Tyrosinase-Conducting Polymer Composite Electrodes. Sensors 2013, 13, 6759–6774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anshori, I.; Kepakisa, K.; Rizalputri, L.; Althof, R.; Nugroho, A.; Siburian, R. Facile synthesis of graphene oxide/Fe3O4 nanocomposite for electrochemical sensing on determination of dopamine. Nanocomposites 2022, 8, 155–166. [Google Scholar] [CrossRef]
- Roychoudhury, A.; Basu, S.; Kumar Jha, S. Dopamine biosensor based on surface functionalized nanostructured nickel oxide platform. Biosens. Bioelectron. 2016, 84, 72–81. [Google Scholar] [CrossRef]
- Wasserstein, R.L.; Lazar, N.A. The ASA’s Statement on p-Values: Context, Process, and Purpose. Am. Stat. 2016, 70, 129–133. [Google Scholar] [CrossRef] [Green Version]
- Morgan, E. Chemometrics: Experimental Design, 1st ed.; John Wiley & Sons: Chichester, UK, 1995. [Google Scholar]
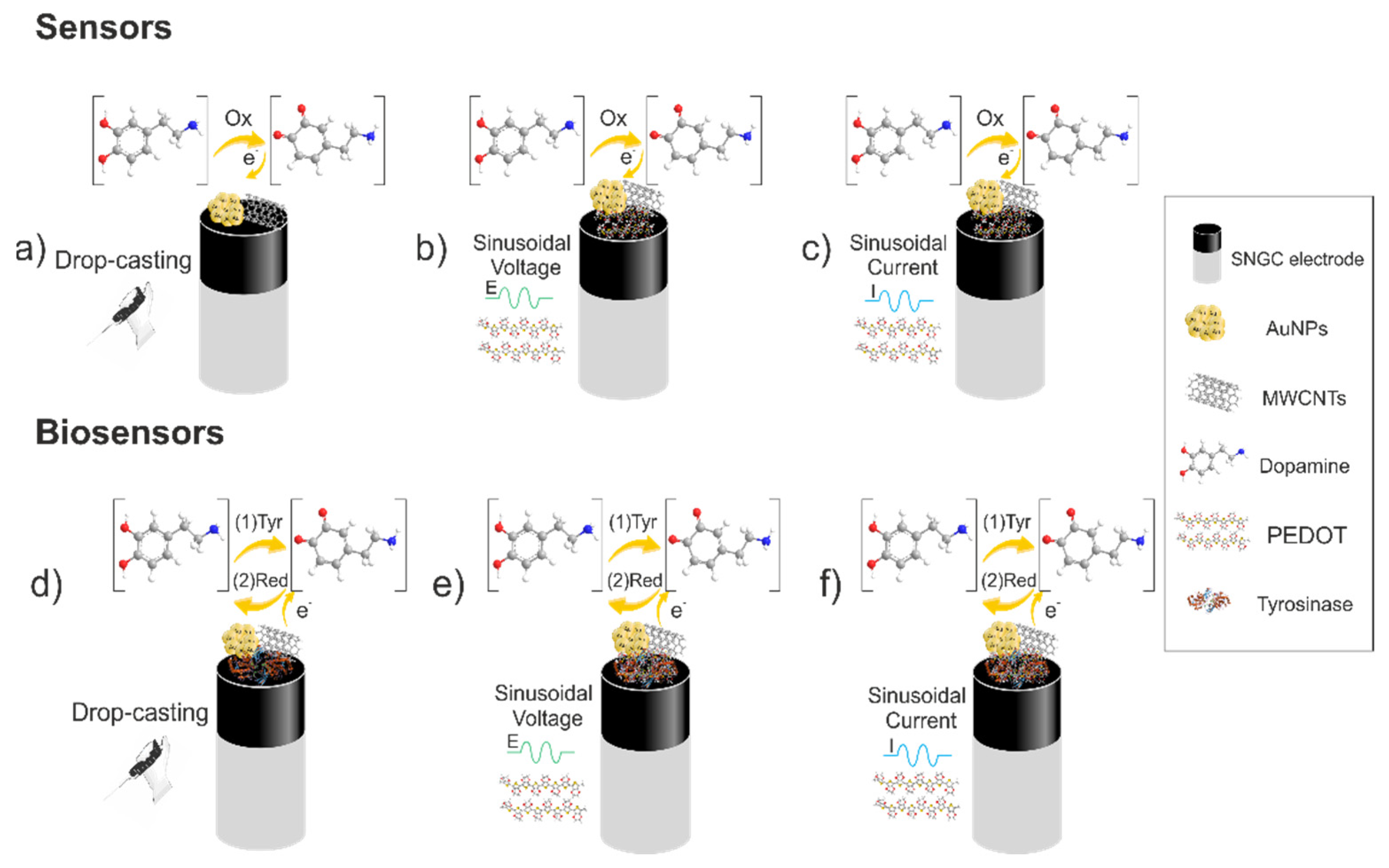
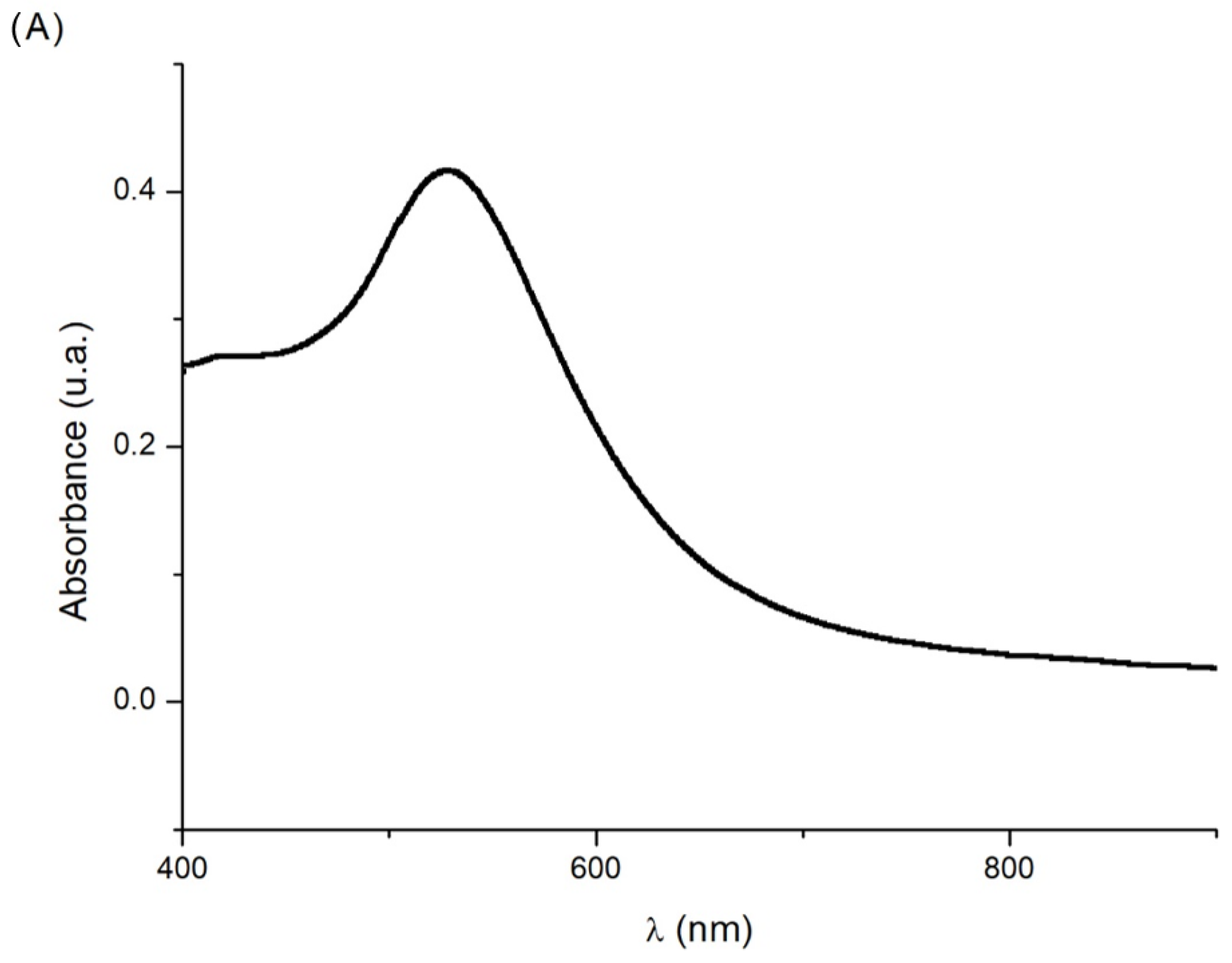

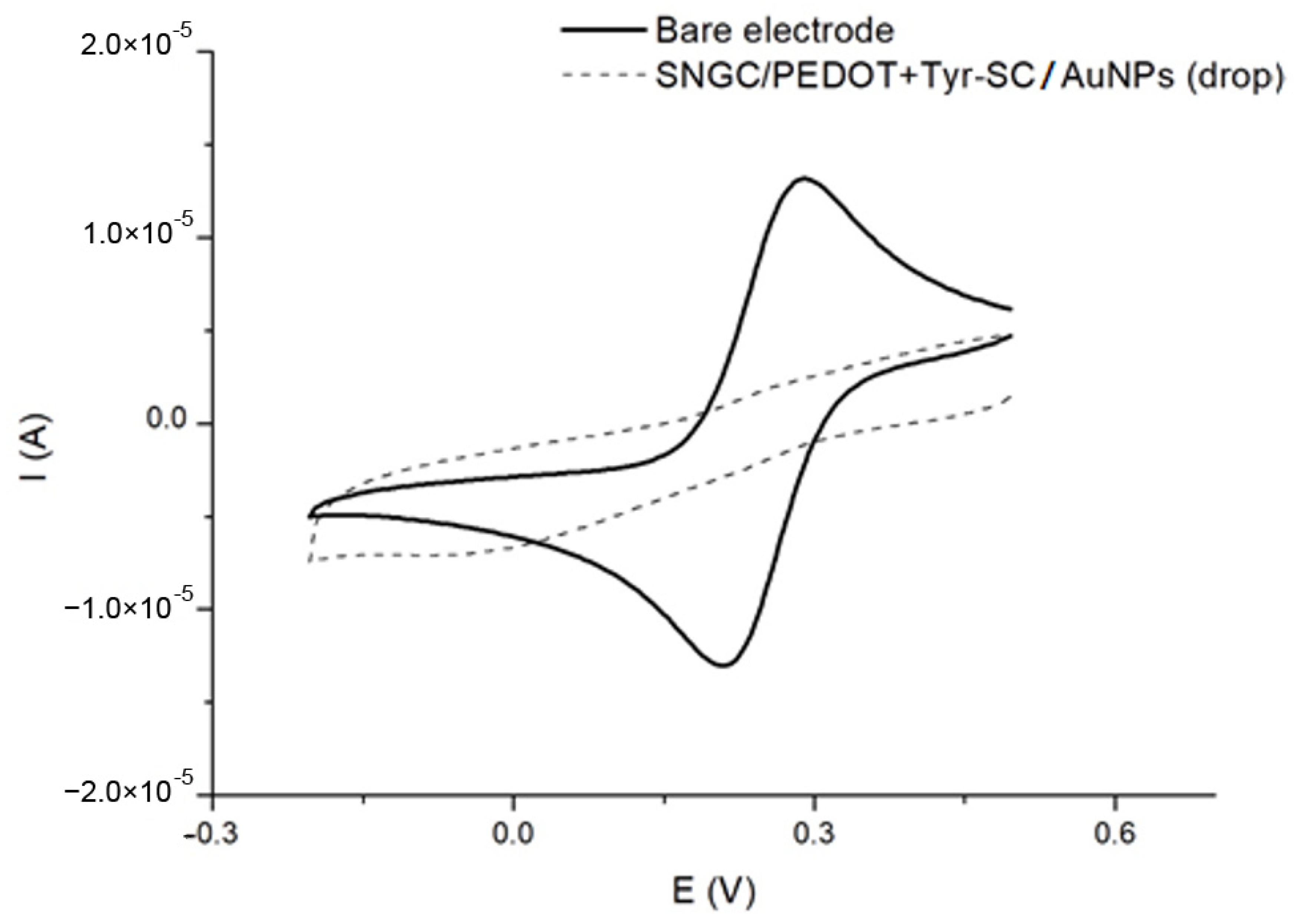
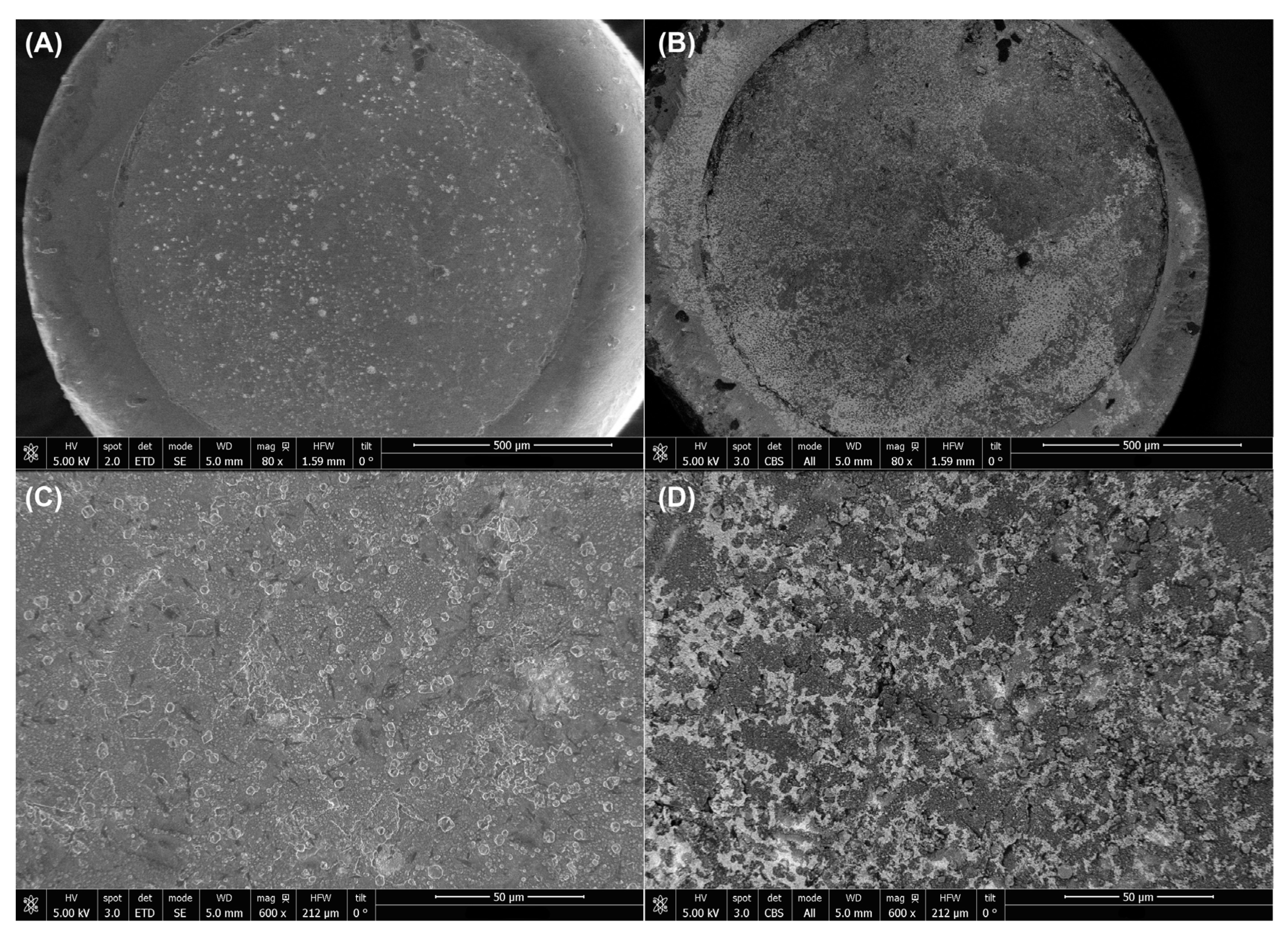


| Configuration | Type of Modification | Procedure |
|---|---|---|
| C1 | SNGC/AuNPs (drop) | 4.1 µL of freshly synthesized AuNPs were drop-casted onto the surface of a pre-treated SNGC electrode. The electrode was allowed to dry overnight in a dark chamber at room temperature. |
| Sinusoidal Voltage based sensors | ||
| C2 | SNGC/PEDOT-SV | The electrodeposition of PEDOT layer was performed from an aqueous solution containing the optimum concentrations of 0.01 M EDOT, and 0.05 M phosphate buffer solution (PBS) at pH 7. |
| C3 | SNGC/PEDOT+Tyr-SV | The electrodeposition of PEDOT-Tyr layers was done from an aqueous solution containing the optimum concentrations of 0.01 M EDOT, 2 mg/mL of Tyr, and 0.05 M phosphate buffer solution (PBS) at pH 7 [33]. |
| C4 | SNGC/AuNPs (drop)/PEDOT+Tyr-SV | A SNGC/AuNPs (drop-casted) sensor was modified employing the procedure described in C3. |
| C5 | SNGC/PEDOT+Tyr-SV/AuNPs (drop) | A SNGC/PEDOT-Tyr-SV biosensor was modified employing the procedure of SNGC/AuNPs(drop-casted) previously used in C1. |
| C6 | SNGC/AuNPs (Electrogen)/PEDOT+Tyr-SV | A bare SNGC electrode was immersed in a solution of 5 mL containing 1.5 mM KAuCl4, 0.1 M KCl and 0.1 M H2SO4. A chronoamperometry technique was used applying 0.18 V for 200 s. After that, the electrode was polarized in a solution of 0.05 M H2SO4 by cyclic voltammetry with a potential step from 0.0 to 1.5 V and scan rate of 50 mV/s for 5 cycles. Then, the electrodeposition of PEDOT-enzyme layer was performed using the same previous protocol abovementioned in C3 [37]. |
| C7 | SNGC/AuNPs (Electrochem)/PEDOT+Tyr-SV | A SNGC electrode was immersed in 2.4 mL of a solution containing 200 µL of AuNPs, 0.01 M EDOT, 2 mg/mL of Tyr, and 0.05 M phosphate buffer solution (PBS) at pH 7. After that, the Sinusoidal Voltage procedure was applied. |
| C8 | SNGC/PEDOT-SV/AuNPs (drop) | A SNGC/PEDOT-SV sensor was modified employing the procedure of SNGC/AuNPs (drop-cast) previously mentioned in C1. |
| C9 | SNGC/PEDOT-SV/[CNTsAuNPs] | 2 mL of a solution containing 1.5 mL of AuNPs, and 1 mg·mL−1 CNTs in 0.05 M PBS pH 7 was placed in a vial. The mixture was stirred by vortex for 20 min. After centrifugation (2 min, 10,000 rpm at 20 °C) the solid phase was added into a 0.01 M EDOT and 0.05 M PBS pH 7 solution. Finally, a SNGC electrode was immersed in the solution and electrodeposited following the procedure for SNGC/PEDOT-SV abovementioned in C2. |
| C10 | SNGC/PEDOT-SV/CNTs-AuNPs | A SNGC electrode was immersed in a 5 mL solution containing 0.01 M EDOT, 1 mg·mL−1 CNTs and 200 µL of AuNPs in 0.05 M PBS pH 7; this solution was previously sonicated for 2 min. Later, the electrodeposition was performed using C2 procedure. |
| C11 | SNGC/PEDOT-SV/CNTs | The modification procedure was similar to the one described for C10 but without AuNPs in the initial mixture. |
| C12 | SNGC/PEDOT+Tyr-SV/CNTs | The procedure of this modification is similar to the one explained in C11 but with the addition of 2 mg·mL−1 of Tyr in the initial solution. |
| C13 | SNGC/PEDOT+Tyr-SV/CNTs-AuNPs | The modification procedure in this case follows the same steps than the one performed in C12. Nevertheless, 200 µL of AuNPs were added to the mixture before the electrodeposition. |
| C14 | SNGC/PEDOT-SV/[CNTsAuNPs] (drop) | Following the procedure to attach AuNPs in CNTs described in C9, the CNTs-AuNPs nanocomposite solution is obtained. 4.1 µL are drop-casted onto the surface of a SNGC/PEDOT-SV. The electrode was allowed to dry overnight in a dark chamber at room temperature. |
| Sinusoidal Current based sensors | ||
| C15 | SNGC/PEDOT+Tyr-SC | The electrodeposition of PEDOT-Tyr layers was performed from an aqueous solution containing the optimum concentrations of 0.01 M EDOT, 2 mg·mL−1 of Tyr, and 0.05 M phosphate buffer solution (PBS) at pH 7, using the SC procedure [33]. |
| C16 | SNGC/PEDOT-SC/CNTs | A SNGC electrode was immersed in a 5 mL solution containing 0.01 M EDOT, 1 mg·mL−1 CNTs in 0.05 M PBS pH 7; this solution was previously sonicated for 2 min. Later, the electrodeposition was carried out by using C15 procedure |
| C17 | SNGC/PEDOT-SC/AuNPs (drop) | A SNGC/PEDOT-SC sensor was modified employing the procedure for C1. |
| C18 | SNGC/AuNPs (drop)/PEDOT+Tyr-SC | In this case, C1 electrode was modified by using the procedure described in C15. |
| C19 | SNGC/PEDOT+Tyr-SC/AuNPs (drop) | 4.1 µL of freshly synthesized AuNPs were drop-casted onto the surface of the SNGC/PEDOT+Tyr-SC biosensor as explained in C1. |
| Configuration | Type of Modification | R2 | Sensitivity (A·µM−1) | RSDsensitivity (%) | LOD (µM) | RSDLOD (%) | LOQ (µM) |
|---|---|---|---|---|---|---|---|
| C1 | SNGC/AuNPs (drop) | 0.999 | −2.65 × 10−9 | 19.60 | 2.05 | 49.22 | 6.84 |
| Sinusoidal Voltage based sensors | |||||||
| C2 | SNGC/PEDOT-SV | 0.996 | −7.39 × 10−9 | 14.57 | 10.52 | 7.15 | 35.07 |
| C3 | SNGC/PEDOT+Tyr-SV | 0.999 | −3.96 × 10−9 | 14.09 | 4.28 | 31.34 | 14.28 |
| C4 | SNGC/AuNPs (drop)/PEDOT+Tyr-SV | 0.998 | −6.75 × 10−9 | 21.88 | 6.01 | 11.61 | 20.03 |
| C5 | SNGC/PEDOT+Tyr-SV/AuNPs (drop) | 0.999 | −5.02 × 10−9 | 26.12 | 3.59 | 34.78 | 11.97 |
| C6 | SNGC/AuNPs (Electrogen)/PEDOT+Tyr-SV | 0.999 | −3.58 × 10−9 | 19.68 | 5.74 | 12.06 | 19.15 |
| C7 | SNGC/AuNPs (Electrochem)/PEDOT+Tyr-SV | 0.999 | −2.92 × 10−9 | 18.52 | 5.12 | 27.70 | 17.05 |
| C8 | SNGC/PEDOT-SV/AuNPs (drop) | 0.999 | −7.04 × 10−9 | 1.27 | 4.67 | 11.49 | 15.58 |
| C9 | SNGC/PEDOT-SV/[CNTs-AuNPs] | 0.999 | −5.96 × 10−9 | 20.97 | 5.03 | 11.90 | 16.76 |
| C10 | SNGC/PEDOT-SV/CNTs-AuNPs | 0.999 | −4.89 × 10−9 | 5.23 | 6.92 | 3.09 | 23.06 |
| C11 | SNGC/PEDOT-SV/CNTs | 0.999 | −8.20 × 10−9 | 45.79 | 2.36 | 3.76 | 7.87 |
| C12 | SNGC/PEDOT+Tyr-SV/CNTs | 0.998 | −4.72 × 10−9 | 10.70 | 7.97 | 18.90 | 26.56 |
| C13 | SNGC/PEDOT+Tyr-SV/CNTs-AuNPs | 0.998 | −4.76 × 10−9 | 11.46 | 8.98 | 10.47 | 29.93 |
| C14 | SNGC/PEDOT-SV /[CNTsAuNPs] (drop) | 0.997 | −5.32 × 10−9 | 3.66 | 10.93 | 9.61 | 36.4 |
| Sinusoidal Current based sensors | |||||||
| C15 | SNGC/PEDOT+Tyr-SC | 0.998 | −5.35 × 10−9 | 6.18 | 6.50 | 5.96 | 21.66 |
| C16 | SNGC/PEDOT-SC/CNTs | 0.999 | −6.10 × 10−9 | 4.83 | 6.09 | 8.32 | 20.30 |
| C17 | SNGC/PEDOT-SC/AuNPs (drop) | 0.997 | −7.02 × 10−9 | 9.95 | 8.03 | 8.03 | 26.77 |
| C18 | SNGC/AuNPs (drop)/PEDOT+Tyr-SC | 0.997 | −7.31 × 10−9 | 4.16 | 8.79 | 9.22 | 29.30 |
| C19 | SNGC/PEDOT+Tyr-SC /AuNPs (drop) | 0.999 | −4.92 × 10−9 | 1.60 | 5.56 | 6.10 | 18.53 |
| Electrode Material | LOD (µM) | Reference |
|---|---|---|
| SNGC/PEDOT-SV/CNTs (C11) | 2.4 | This work |
| SNGC/PEDOT+Tyr-SC/AuNPs (drop)(C19) | 5.6 | This work |
| Au-disk/PEDOT-tyrosinase | 4.2 | [45] |
| Graphene oxide/Fe3O4 | 0.48 | [46] |
| Tyrosinase/NiO/ITO | 1.04 | [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.T.; López-Iglesias, D.; Sierra-Padilla, A.; García-Guzmán, J.J.; Cubillana-Aguilera, L.M.; Bellido-Milla, D.; Palacios-Santander, J.M. How Meaningful Are Minor Details in the Generation of Nanomodified Electrochemical Enzyme Biosensors? Exploring the Scenario with Sinusoidal Approaches. Chemosensors 2022, 10, 316. https://doi.org/10.3390/chemosensors10080316
Rahman MT, López-Iglesias D, Sierra-Padilla A, García-Guzmán JJ, Cubillana-Aguilera LM, Bellido-Milla D, Palacios-Santander JM. How Meaningful Are Minor Details in the Generation of Nanomodified Electrochemical Enzyme Biosensors? Exploring the Scenario with Sinusoidal Approaches. Chemosensors. 2022; 10(8):316. https://doi.org/10.3390/chemosensors10080316
Chicago/Turabian StyleRahman, Md. Towhidur, David López-Iglesias, Alfonso Sierra-Padilla, Juan José García-Guzmán, Laura M. Cubillana-Aguilera, Dolores Bellido-Milla, and José María Palacios-Santander. 2022. "How Meaningful Are Minor Details in the Generation of Nanomodified Electrochemical Enzyme Biosensors? Exploring the Scenario with Sinusoidal Approaches" Chemosensors 10, no. 8: 316. https://doi.org/10.3390/chemosensors10080316








