An Economical and Portable Paper-Based Colorimetric Sensor for the Determination of Hydrogen Peroxide-Related Biomarkers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Instrumentation
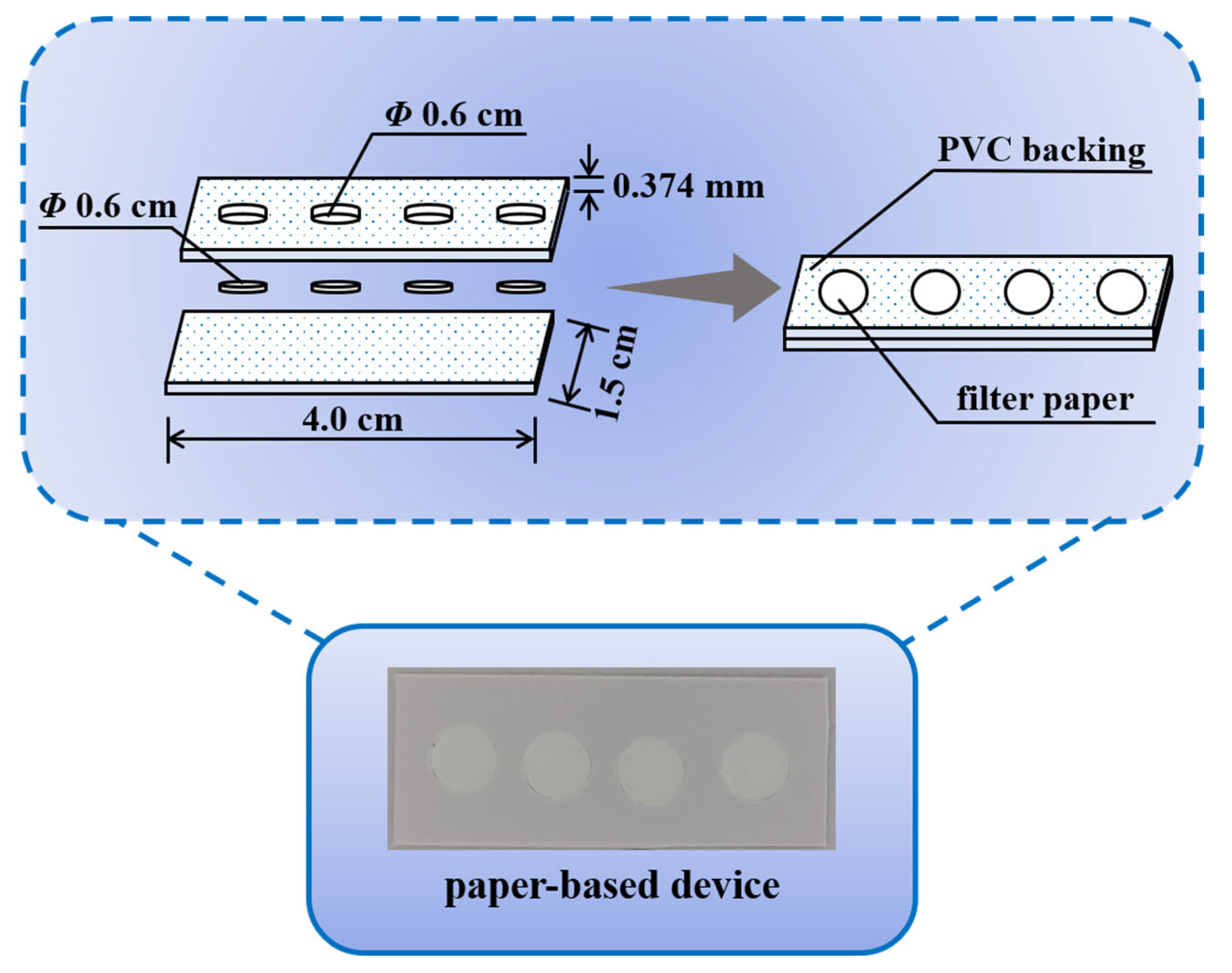
2.3. Fabrication of Paper-Based Device
2.4. Colorimetric Detection of H2O2 and Glucose
2.5. Glucose Assay of Practical Samples
3. Results
3.1. Design Principle of the Paper-Based Biosensor
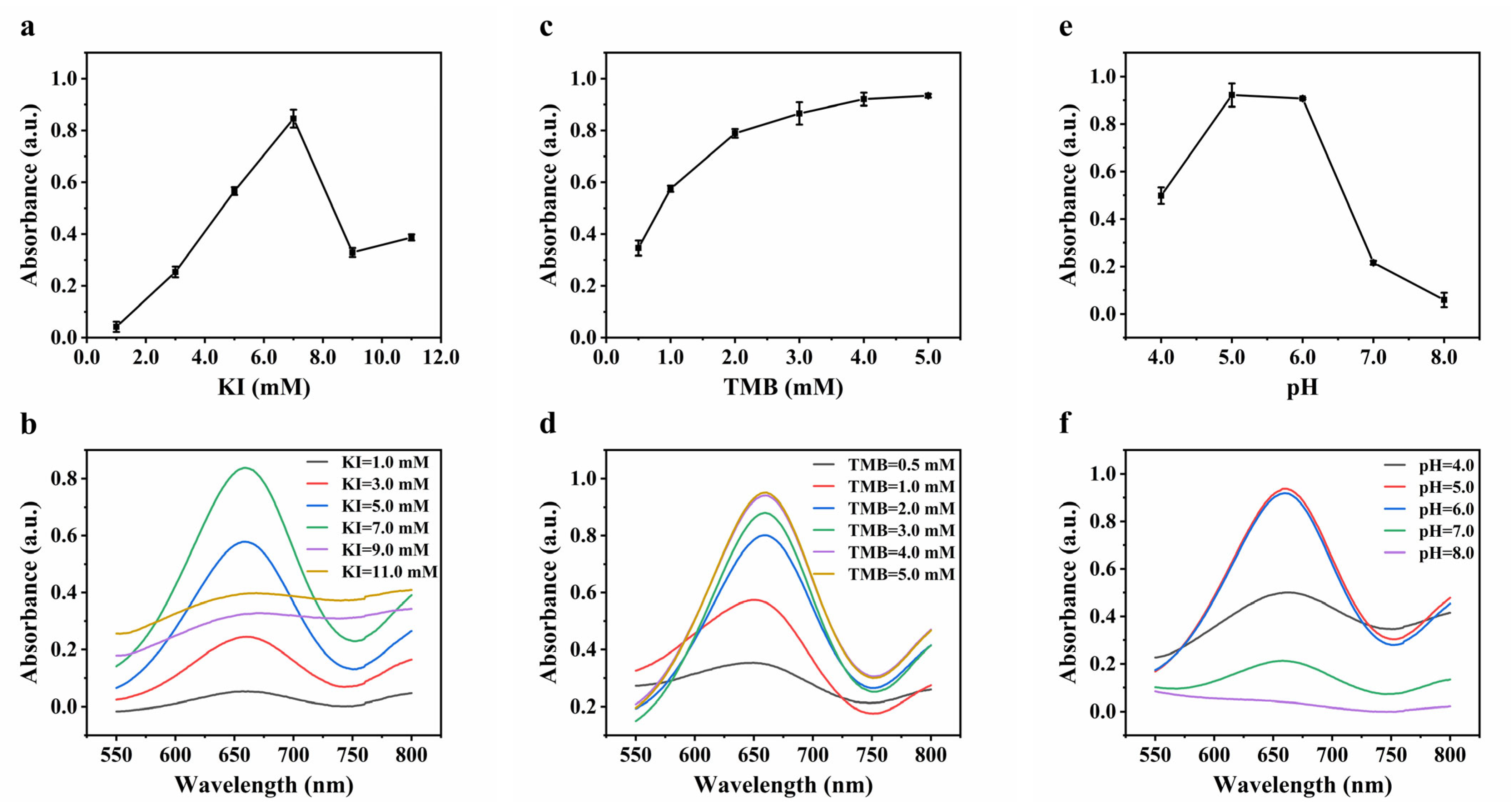
3.2. Optimization of Experimental Conditions
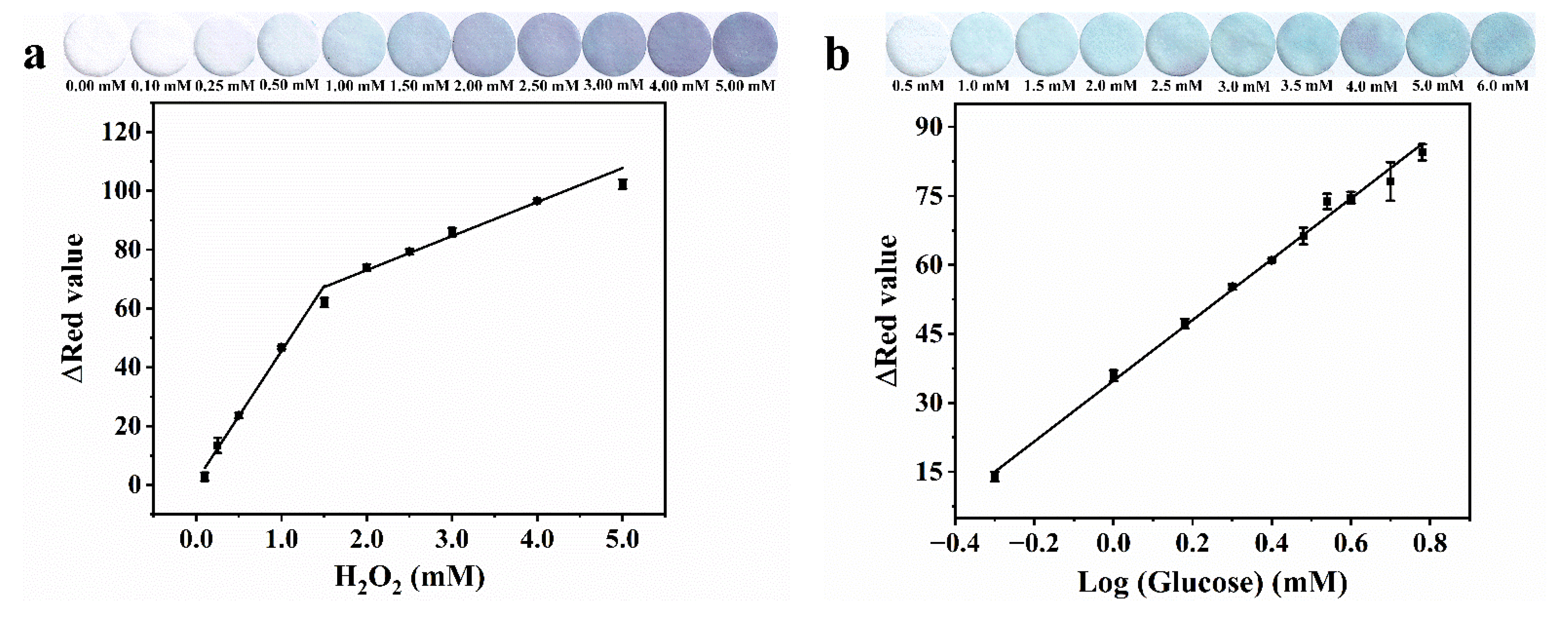
3.3. Analytical Performance
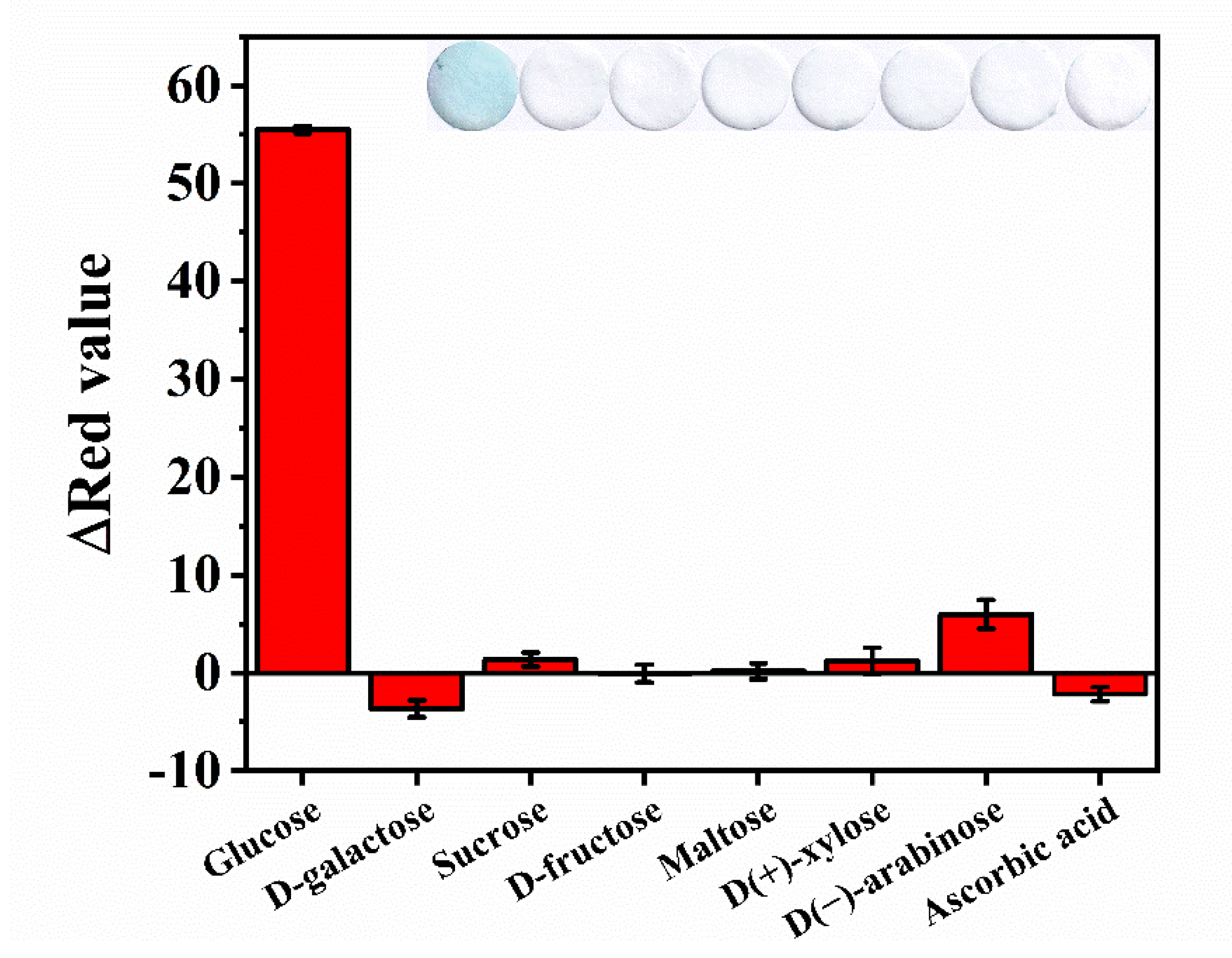
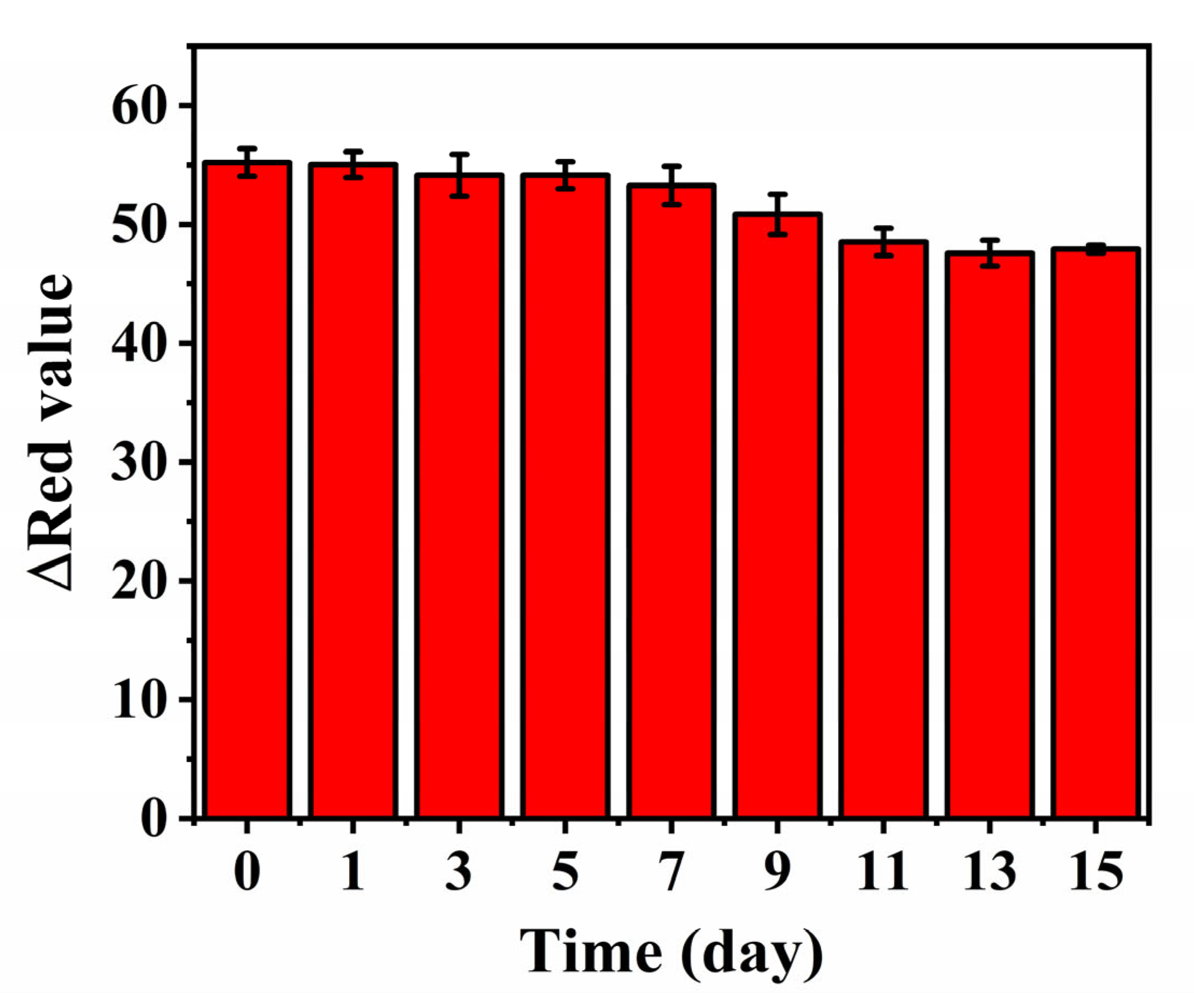
3.4. Selectivity and Stability of the Colorimetric Assay
3.5. Measurements of Glucose in Practical Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turner Anthony, P.F. Biosensors–Sense and Sensitivity. Science 2000, 290, 1315–1317. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, J.; Wu, L.; Huang, Y.; Zhang, Y.; Zhu, M.; Wang, Y.; Zhu, Z.; Yang, C. Trends in miniaturized biosensors for point-of-care testing. Trac-Trends Anal. Chem. 2020, 122, 115701. [Google Scholar] [CrossRef]
- Zhang, J.; Lan, T.; Lu, Y. Translating in vitro diagnostics from centralized laboratories to point-of-care locations using commercially-available handheld meters. Trac-Trends Anal. Chem. 2020, 124, 115782. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, Y.; Li, X.; Li, M.; Wu, Q.; Song, Y.; Zhu, Z.; Yang, C. Integrated microfluidic devices for in vitro diagnostics at point of care. Aggregate 2022, e184. [Google Scholar] [CrossRef]
- Kim, J.H.; Mun, S.; Ko, H.U.; Yun, G.Y.; Kim, J. Disposable chemical sensors and biosensors made on cellulose paper. Nanotechnology 2014, 25, 092001. [Google Scholar] [CrossRef] [PubMed]
- Kamel, S.; Khattab, T.A. Recent Advances in Cellulose-Based Biosensors for Medical Diagnosis. Biosensors 2020, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Busa, L.S.A.; Mohammadi, S.; Maeki, M.; Ishida, A.; Tani, H.; Tokeshi, M. Advances in Microfluidic Paper-Based Analytical Devices for Food and Water Analysis. Micromachines 2016, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, X.; Qian, Z.M.; Wang, S.; Yang, F.Q. Glucose oxidase-mediated sodium alginate gelation: Equipment-Free detection of glucose in fruit samples. Enzym. Microb. Technol. 2021, 148, 109805. [Google Scholar] [CrossRef] [PubMed]
- Mooltongchun, M.; Teepoo, S. A Simple and Cost-effective Microfluidic Paper-Based Biosensor Analytical Device and its Application for Hypoxanthine Detection in Meat Samples. Food Anal. Meth. 2019, 12, 2690–2698. [Google Scholar] [CrossRef]
- Liu, M.M.; Lian, X.; Liu, H.; Guo, Z.Z.; Huang, H.H.; Lei, Y.; Peng, H.P.; Chen, W.; Lin, X.H.; Liu, A.L.; et al. A colorimetric assay for sensitive detection of hydrogen peroxide and glucose in microfluidic paper-based analytical devices integrated with starch-iodide-gelatin system. Talanta 2019, 200, 511–517. [Google Scholar] [CrossRef]
- Moreira, F.T.C.; Correia, B.P.; Sousa, M.P.; Sales, G.F. Colorimetric cellulose-based test-strip for rapid detection of amyloid β-42. Mikrochim. Acta 2021, 188, 334. [Google Scholar] [CrossRef]
- Shariati, S.; Khayatian, G. Microfluidic nanopaper based analytical device for colorimetric and naked eye determination of cholesterol using the color change of triangular silver nanoprisms. New J. Chem. 2021, 45, 21788–21794. [Google Scholar] [CrossRef]
- Gong, X.; Shao, J.; Guo, S.; Pan, J.; Fan, X. Determination of inhibitory activity of Salvia miltiorrhiza extracts on xanthine oxidase with a paper-based analytical device. J. Pharm. Anal. 2021, 11, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.; Collier, C.M.; Singh, A. A Paper-Based Colorimetric Aptasensor for the Detection of Gentamicin. Biosensors-Basel 2021, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Deng, X.L.; Zhao, Y.L.; Zhang, X.Y.; Bai, Y.P. Paper-Based Enzymatic Colorimetric Assay for Rapid Malathion Detection. Appl. Biochem. Biotechnol. 2021, 193, 2534–2546. [Google Scholar] [CrossRef]
- Tran, T.D.; Nguyen, P.T.; Le, T.N.; Kim, M.I. DNA-copper hybrid nanoflowers as efficient laccase mimics for colorimetric detection of phenolic compounds in paper microfluidic devices. Biosens. Bioelectron. 2021, 182, 113187. [Google Scholar] [CrossRef]
- Attaallah, R.; Amine, A. Highly selective and sensitive detection of cadmium ions by horseradish peroxidase enzyme inhibition using a colorimetric microplate reader and smartphone paper-based analytical device. Microchem. J. 2022, 172, 106940. [Google Scholar] [CrossRef]
- Liu, J.; Xing, Y.; Xue, B.; Zhou, X. Nanozyme enhanced paper-based biochip with a smartphone readout system for rapid detection of cyanotoxins in water. Biosens. Bioelectron. 2022, 205, 114099. [Google Scholar] [CrossRef]
- Rhee Sue, G. H2O2, a Necessary Evil for Cell Signaling. Science 2006, 312, 1882–1883. [Google Scholar] [CrossRef]
- Evans, E.; Gabriel, E.F.; Benavidez, T.E.; Tomazelli Coltro, W.K.; Garcia, C.D. Modification of microfluidic paper-based devices with silica nanoparticles. Analyst 2014, 139, 5560–5567. [Google Scholar] [CrossRef]
- Feng, L.X.; Tang, C.; Han, X.X.; Zhang, H.C.; Guo, F.N.; Yang, T.; Wang, J.H. Simultaneous and sensitive detection of multiple small biological molecules by microfluidic paper-based analytical device integrated with zinc oxide nanorods. Talanta 2021, 232, 122499. [Google Scholar] [CrossRef] [PubMed]
- Behrouzifar, F.; Shahidi, S.-A.; Chekin, F.; Hosseini, S.; Ghorbani-HasanSaraei, A. Colorimetric assay based on horseradish peroxidase/reduced graphene oxide hybrid for sensitive detection of hydrogen peroxide in beverages. Spectroc. Acta Pt. A-Molec. Biomolec. Spectr. 2021, 257, 119761. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-Y.; Zhang, H.; Yang, F.-Q. Enhanced peroxidase-like activity of copper phosphate modified by hydrophilic phytic-acid and its application in colorimetric detection of hydrogen peroxide. Microchem. J. 2021, 168, 106489. [Google Scholar] [CrossRef]
- Lin, J.; Ni, P.; Sun, Y.; Wang, Y.; Wang, L.; Li, Z. Highly sensitive colorimetric determination of biothiols based on I−-H2O2-3,3’,5,5’-tetramethylbenzidine system. Sens. Actuator B Chem. 2018, 255, 3472–3478. [Google Scholar] [CrossRef]
- Dogan, V.; Yuzer, E.; Kilic, V.; Sen, M. Non-enzymatic colorimetric detection of hydrogen peroxide using a μPAD coupled with a machine learning-based smartphone app. Analyst 2021, 146, 7336–7344. [Google Scholar] [CrossRef]
- Ye, X.; Shi, H.; He, X.; Wang, K.; He, D.; Yan, L.a.; Xu, F.; Lei, Y.; Tang, J.; Yu, Y. Iodide-Responsive Cu–Au Nanoparticle-Based Colorimetric Platform for Ultrasensitive Detection of Target Cancer Cells. Anal. Chem. 2015, 87, 7141–7147. [Google Scholar] [CrossRef]
- Yao, L.; Kong, F.Y.; Wang, Z.X.; Li, H.Y.; Zhang, R.; Fang, H.L.; Wang, W. UV-assisted one-pot synthesis of bimetallic Ag-Pt decorated reduced graphene oxide for colorimetric determination of hydrogen peroxide. Mikrochim. Acta 2020, 187, 410. [Google Scholar] [CrossRef]
- Zarif, F.; Rauf, S.; Khurshid, S.; Muhammad, N.; Hayat, A.; Rahim, A.; Shah, N.S.; Yang, C.P. Effect of pyridinium based ionic liquid on the sensing property of Ni0 nanoparticle for the colorimetric detection of hydrogen peroxide. J. Mol. Struct. 2020, 1219, 128620. [Google Scholar] [CrossRef]
- Lima, L.S.; Rossini, E.L.; Pezza, L.; Pezza, H.R. Bioactive paper platform for detection of hydrogen peroxide in milk. Spectroc. Acta Pt. A-Molec. Biomolec. Spectr. 2020, 227, 117774. [Google Scholar] [CrossRef]
- Sharma, L.; Gouraj, S.; Raut, P.; Tagad, C. Development of a surface-modified paper-based colorimetric sensor using synthesized Ag NPs-alginate composite. Environ. Technol. 2021, 42, 3441–3450. [Google Scholar] [CrossRef]
- Khazaei, S.; Mozaffari, S.A.; Ebrahimi, F. Polyvinyl alcohol as a crucial omissible polymer to fabricate an impedimetric glucose biosensor based on hierarchical 3D-NPZnO/chitosan. Carbohydr. Polym. 2021, 266, 118105. [Google Scholar] [CrossRef] [PubMed]
- Fatoni, A.; Aziz, A.N.; Anggraeni, M.D. Low-cost and real-time color detector developments for glucose biosensor. Sens. Biosensing. Res. 2020, 28, 100325. [Google Scholar] [CrossRef]
- Luo, X.; Xia, J.; Jiang, X.; Yang, M.; Liu, S. Cellulose-Based Strips Designed Based on a Sensitive Enzyme Colorimetric Assay for the Low Concentration of Glucose Detection. Anal. Chem. 2019, 91, 15461–15468. [Google Scholar] [CrossRef] [PubMed]



| Detection Methods | Material | Linear Range | LOD | Ref. |
|---|---|---|---|---|
| UV–visible spectrophotometer | Solution | 0.1–5.0 mM | 0.079 mM | [23] |
| UV–visible spectrophotometer | Solution | 0.4–4.0 mM | 0.12 mM | [28] |
| Colorimetric analysis with a scanner | Paper | 0.5–6.0 mM | 0.05 mM | [16] |
| Colorimetric analysis with a smartphone | Paper | 1.25–15.00 mM | 0.354 mM | [29] |
| Colorimetric analysis with a smartphone | Paper | 0.1–1.5 and 2−10 mM | 0.1 mM | [30] |
| Colorimetric analysis with a scanner | Paper | 0.1–5.0 mM | 0.03 mM | This work |
| Detection Methods | Material | Linear Range | LOD/LOQ (mM) | Ref. |
|---|---|---|---|---|
| Electrical feature analysis with two electrodes cell | Electrode | 1–18 mM | 0.2/- | [31] |
| Colorimetric analysis with color sensor TCS230 | Solution | 0.1–2.5 mM | 0.14/0.58 | [32] |
| Diffusion diameter analysis with a Vernier caliper | Paper | 1.4–7.0 mM | -/1.4 | [3] |
| Colorimetric analysis with a scanner | Paper | 0.5–5.0 mM | 0.1/- | [16] |
| Colorimetric analysis with a camera | Paper | 1–11 mM | 0.45/- | [33] |
| Colorimetric analysis with a scanner | Paper | 0.5–6.0 mM | -/0.5 | This work |
| Samples | Initially Detected Concentration (mM) | Added (mM) | Total Found ± SD (mM) | Recovery (%) |
|---|---|---|---|---|
| Apple | 1.54 ± 0.17 | 1.0 | 2.50 ± 0.19 | 95.5 |
| 2.0 | 3.58 ± 0.04 | 101.8 | ||
| 3.0 | 4.47 ± 0.15 | 97.6 | ||
| Pear | 2.10 ± 0.10 | 1.0 | 3.14 ± 0.06 | 104.5 |
| 2.0 | 4.09 ± 0.24 | 99.5 | ||
| 3.0 | 4.96 ± 0.35 | 95.4 | ||
| Coconut | 2.43 ± 0.32 | 1.0 | 3.48 ± 0.09 | 105.3 |
| 2.0 | 4.55 ± 0.29 | 106.1 | ||
| 3.0 | 5.55 ± 0.18 | 104.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.-Y.; Zhang, H.; Yang, F.-Q. An Economical and Portable Paper-Based Colorimetric Sensor for the Determination of Hydrogen Peroxide-Related Biomarkers. Chemosensors 2022, 10, 335. https://doi.org/10.3390/chemosensors10080335
Zhang W-Y, Zhang H, Yang F-Q. An Economical and Portable Paper-Based Colorimetric Sensor for the Determination of Hydrogen Peroxide-Related Biomarkers. Chemosensors. 2022; 10(8):335. https://doi.org/10.3390/chemosensors10080335
Chicago/Turabian StyleZhang, Wei-Yi, Hao Zhang, and Feng-Qing Yang. 2022. "An Economical and Portable Paper-Based Colorimetric Sensor for the Determination of Hydrogen Peroxide-Related Biomarkers" Chemosensors 10, no. 8: 335. https://doi.org/10.3390/chemosensors10080335
APA StyleZhang, W.-Y., Zhang, H., & Yang, F.-Q. (2022). An Economical and Portable Paper-Based Colorimetric Sensor for the Determination of Hydrogen Peroxide-Related Biomarkers. Chemosensors, 10(8), 335. https://doi.org/10.3390/chemosensors10080335







