Polyethylenimine-Based Electrochemical Sensor for the Determination of Caffeic Acid in Aromatic Herbs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Preparation of the PEI/CPE Sensor
2.3. Preparation of Samples
2.4. Instrumentation
3. Results and Discussion
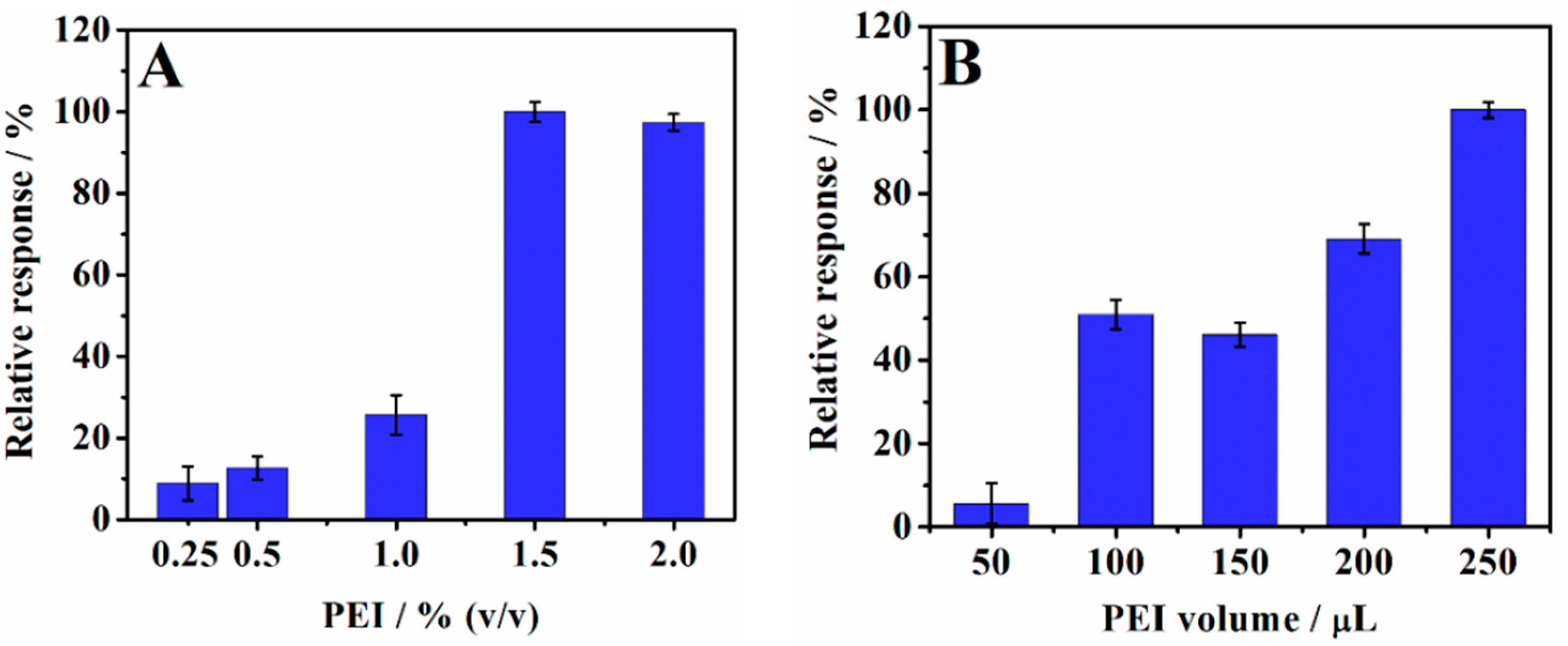
3.1. Optimization of the Preparation of PEI/CPE
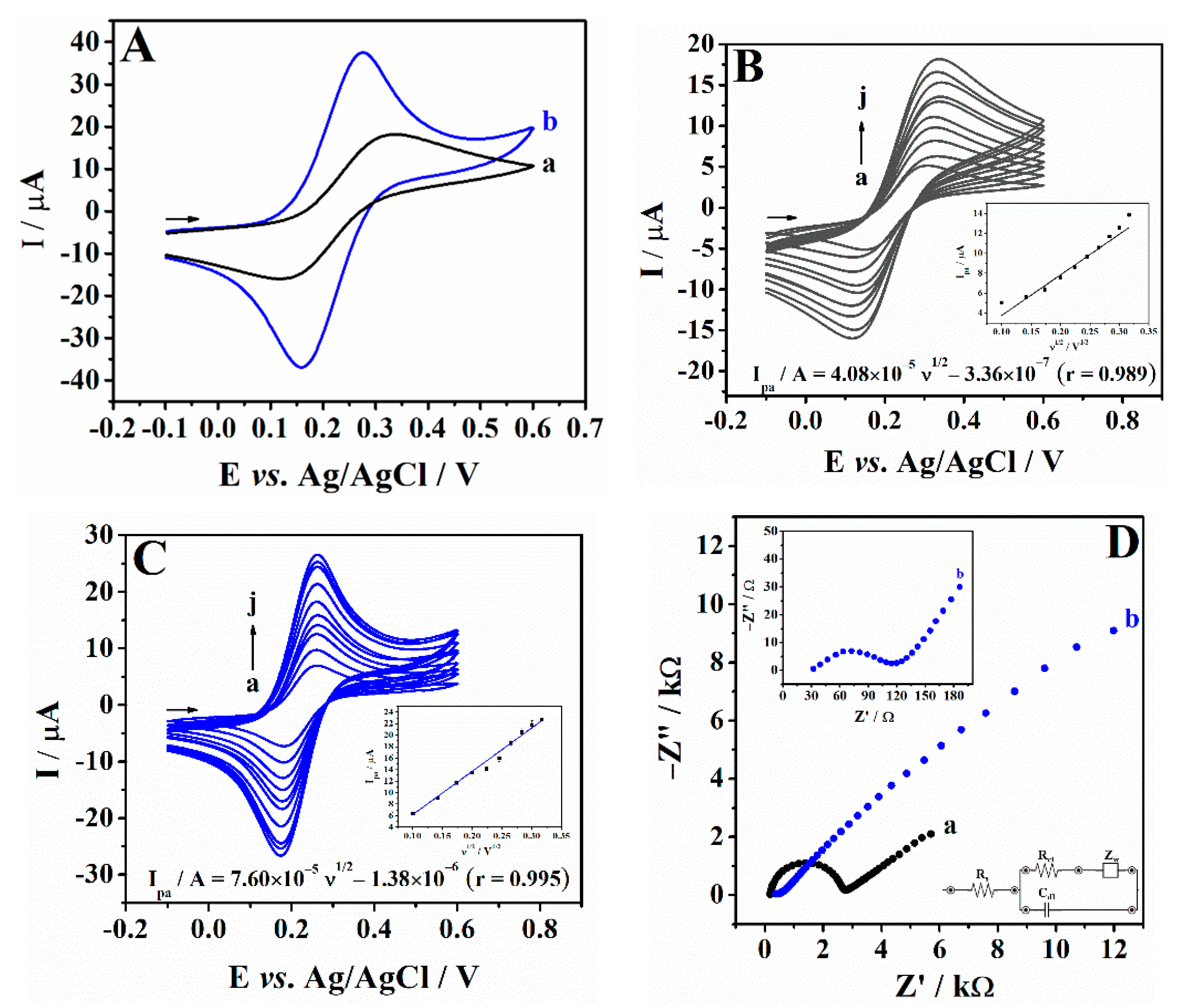
3.2. Electrochemical Characterization of PEI/CPE
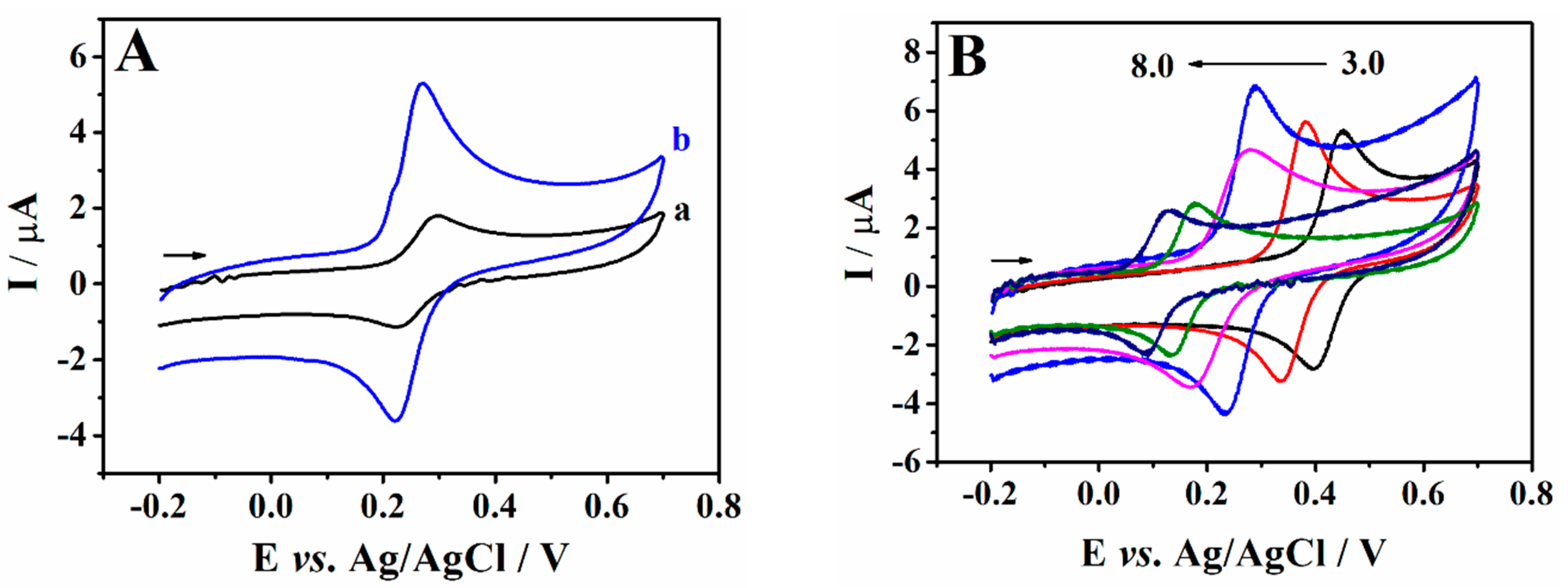
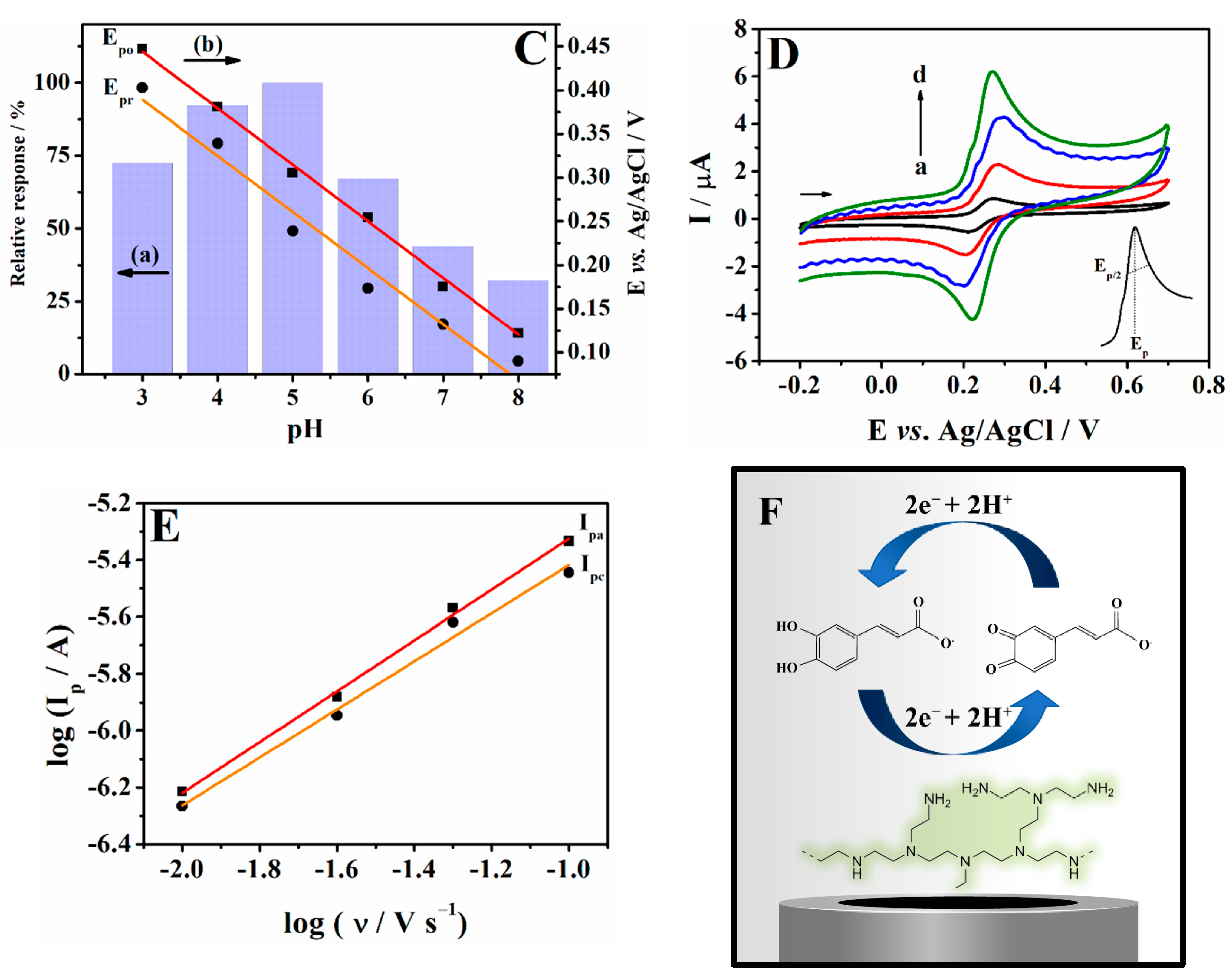
3.3. Electrochemical Behaviour of Caffeic Acid at the PEI/CPE
3.4. Optimization of SWV Parameters
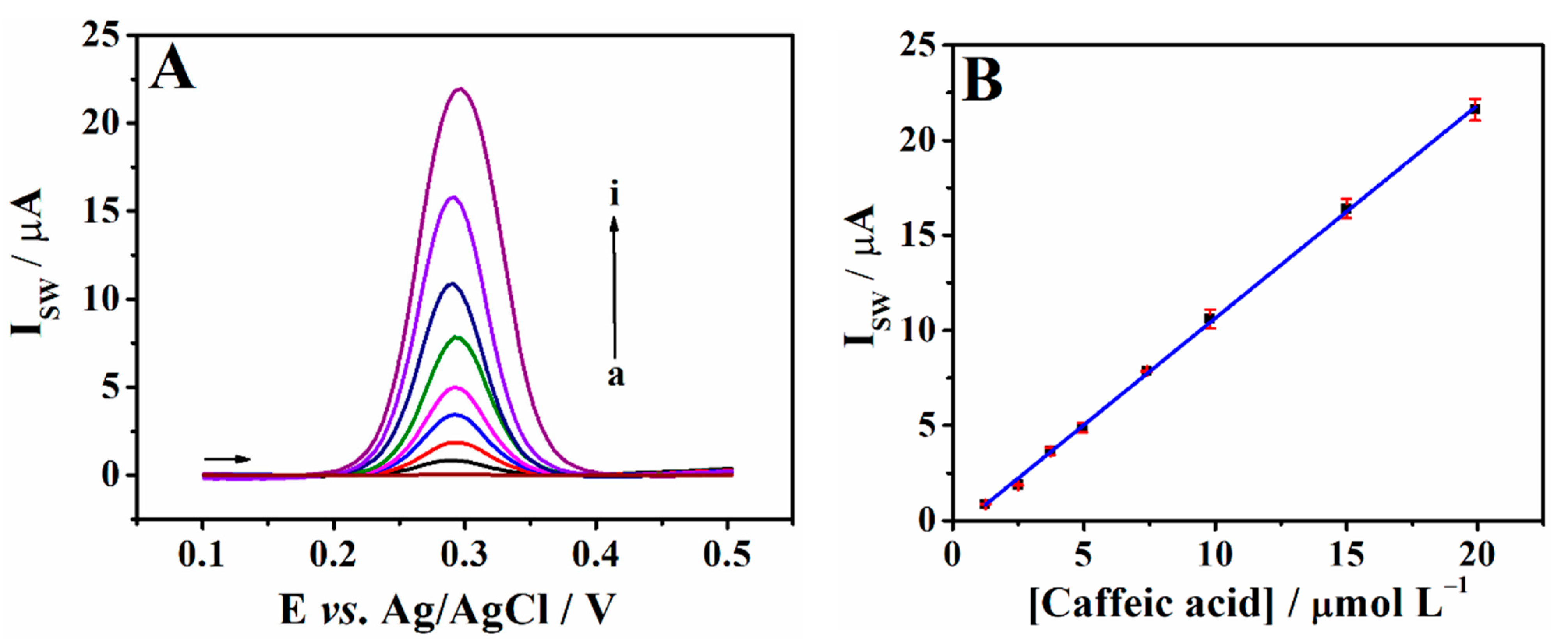
3.5. Calibration Plot for Caffeic Acid
3.6. Repeatability and Selectivity
3.7. Analytical Application
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ares, A.M.; Bernal, J.L.; Nozal, M.J.; Bernal, J. Analysis of Herbal Bioactives. In Aromatic Herbs in Food: Bioactive Compounds, Processing, and Applications; Elsevier Inc.: New York, NY, USA, 2021; pp. 201–232. ISBN 9780128227169. [Google Scholar]
- Chong, C.H.; Figiel, A.; Szummy, A.; Wojdyło, A.; Chua, B.L.; Khek, C.H.; Yuan, M.C. Herbs Drying. In Aromatic Herbs in Food: Bioactive Compounds, Processing, and Applications; Elsevier Inc.: New York, NY, USA, 2021; pp. 167–200. ISBN 978-0-12-822716-9. [Google Scholar]
- Guiné, R.P.F.; Gonçalves, F.J. Bioactive Compounds in Some Culinary Aromatic Herbs and Their Effects on Human Health. Mini-Rev. Med. Chem. 2016, 16, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Bounegru, A.V.; Apetrei, C. Voltamperometric Sensors and Biosensors Based on Carbon Nanomaterials Used for Detecting Caffeic Acid—A Review. Int. J. Mol. Sci. 2020, 21, 9275. [Google Scholar] [CrossRef] [PubMed]
- Bounegru, A.V.; Apetrei, C. Voltammetric Sensors Based on Nanomaterials for Detection of Caffeic Acid in Food Supplements. Chemosensors 2020, 8, 41. [Google Scholar] [CrossRef]
- Venkadesh, A.; Mathiyarasu, J.; Radhakrishnan, S. MOF Mediated Synthesis of Porous Copper Oxide and Their Electrochemical Sensing of Caffeic Acid in Caffeinated Drinks. Inorg. Chem. Commun. 2021, 128, 108573. [Google Scholar] [CrossRef]
- Khan, F.; Bamunuarachchi, N.I.; Tabassum, N.; Kim, Y.M. Caffeic Acid and Its Derivatives: Antimicrobial Drugs toward Microbial Pathogens. J. Agric. Food Chem. 2021, 69, 2979–3004. [Google Scholar] [CrossRef]
- Adem, Ş.; Eyupoglu, V.; Sarfraz, I.; Rasul, A.; Zahoor, A.F.; Ali, M.; Abdalla, M.; Ibrahim, I.M.; Elfiky, A.A. Caffeic Acid Derivatives (CAFDs) as Inhibitors of SARS-CoV-2: CAFDs-Based Functional Foods as a Potential Alternative Approach to Combat COVID-19. Phytomedicine 2021, 85, 153310. [Google Scholar] [CrossRef]
- Tartaglia, A.; Romasco, T.; D’Ovidio, C.; Rosato, E.; Ulusoy, H.I.; Furton, K.G.; Kabir, A.; Locatelli, M. Determination of Phenolic Compounds in Human Saliva after Oral Administration of Red Wine by High Performance Liquid Chromatography. J. Pharm. Biomed. Anal. 2022, 209, 114486. [Google Scholar] [CrossRef]
- Mao, H.; Zhang, Y.; Chen, G. Determination of Three Phenolic Acids in Cimicifugae Rhizoma by Capillary Electrophoresis with a Graphene-Phenolic Resin Composite Electrode. Anal. Methods 2019, 11, 303–308. [Google Scholar] [CrossRef]
- Santana, E.R.; Martins, E.C.; Spinelli, A. Electrode Modified with Nitrogen-Doped Graphene Quantum Dots Supported in Chitosan for Triclocarban Monitoring. Microchem. J. 2021, 167, 106297. [Google Scholar] [CrossRef]
- Winiarski, J.P.; Rampanelli, R.; Bassani, J.C.; Mezalira, D.Z.; Jost, C.L. Multi-Walled Carbon Nanotubes/Nickel Hydroxide Composite Applied as Electrochemical Sensor for Folic Acid (Vitamin B9) in Food Samples. J. Food Compos. Anal. 2020, 92, 103511. [Google Scholar] [CrossRef]
- Piovesan, J.V.; Santana, E.R.; Spinelli, A. A Carbon Paste Electrode Improved with Poly(Ethylene Glycol) for Tannic Acid Surveillance in Beer Samples. Food Chem. 2020, 326, 127055. [Google Scholar] [CrossRef] [PubMed]
- Antoniazzi, C.; Lima, C.A.; Marangoni, R.; Castro, E.G.; Santana, E.R.; Spinelli, A. Molybdenum Trioxide Incorporated in a Carbon Paste as a Sensitive Device for Bisphenol A Monitoring. Microchem. J. 2020, 159, 105528. [Google Scholar] [CrossRef]
- Moreira, F.; Santana, E.R.; Spinelli, A. Ionic Liquid-Supported Magnetite Nanoparticles as Electrode Modifier Materials for Estrogens Sensing. Sci. Rep. 2020, 10, 1955. [Google Scholar] [CrossRef]
- Ulbrich, K.F.; Winiarski, J.P.; Jost, C.L.; de Campos, C.E.M. Green and Facile Solvent-Free Synthesis of NiTe2 Nanocrystalline Material Applied to Voltammetric Determination of Antioxidant Morin. Mater. Today Commun. 2020, 25, 101251. [Google Scholar] [CrossRef]
- Wegmann, F.; Gartlan, K.H.; Harandi, A.M.; Brinckmann, S.A.; Coccia, M.; Hillson, W.R.; Kok, W.L.; Cole, S.; Ho, L.P.; Lambe, T.; et al. Polyethyleneimine Is a Potent Mucosal Adjuvant for Viral Glycoprotein Antigens. Nat. Biotechnol. 2012, 30, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zeng, J.; Zhang, Z.B.; Zhang, Z.J.; Ma, S.; Tang, C.M.; Xu, J.Q. Preparation and Application of Polyethyleneimine-Modified Corncob Magnetic Gel for Removal of Pb(II) and Cu(II) Ions from Aqueous Solution. RSC Adv. 2022, 12, 1950–1960. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, L.Y.; Wang, K.Y.; Li, J.; Feng, L.; Song, L.; Liu, X.; He, J.H.; Sakthivel, R.; Chung, R.J. Streptavidin-Functionalized-Polyethyleneimine/Chitosan/HfO2-Pr6O11 Nanocomposite Using Label-Free Electrochemical Immunosensor for Detecting the Hunger Hormone Ghrelin. Compos. Part B Eng. 2021, 224, 109231. [Google Scholar] [CrossRef]
- Riva, L.; Fiorati, A.; Punta, C. Synthesis and Application of Cellulose-Polyethyleneimine Composites and Nanocomposites: A Concise Review. Materials 2021, 14, 473. [Google Scholar] [CrossRef]
- Usman, M.A.; Khan, A.Y. Selective Adsorption of Anionic Dye from Wastewater Using Polyethyleneimine Based Macroporous Sponge: Batch and Continuous Studies. J. Hazard. Mater. 2022, 428, 128238. [Google Scholar] [CrossRef]
- Luo, J.H.; Li, B.L.; Li, N.B.; Luo, H.Q. Sensitive Detection of Gallic Acid Based on Polyethyleneimine- Functionalized Graphene Modified Glassy Carbon Electrode. Sens. Actuators B Chem. 2013, 186, 84–89. [Google Scholar] [CrossRef]
- Arman, A.; Saǧlam, Ş.; Üzer, A.; Apak, R. Direct Electrochemical Determination of Peroxide-Type Explosives Using Well-Dispersed Multi-Walled Carbon Nanotubes/Polyethyleneimine-Modified Glassy Carbon Electrodes. Anal. Chem. 2021, 93, 11451–11460. [Google Scholar] [CrossRef] [PubMed]
- Arman, A.; Sağlam, Ş.; Üzer, A.; Apak, R. Electrochemical Determination of Nitroaromatic Explosives Using Glassy Carbon/Multi Walled Carbon Nanotube/Polyethyleneimine Electrode Coated with Gold Nanoparticles. Talanta 2022, 238, 122990. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Park, H.; Lee, J.Y.; Park, D.J.; Lee, J.Y.; Myung, N.V.; Lee, K.H. Hierarchically Palladium Nanoparticles Embedded Polyethyleneimine-Reduced Graphene Oxide Aerogel (RGA-PEI-Pd) Porous Electrodes for Electrochemical Detection of Bisphenol A and H2O2. Chem. Eng. J. 2022, 431, 134250. [Google Scholar] [CrossRef]
- Duan, Y.; Yang, Y.; Wang, J.; Li, H.; Liu, K. A Facile One-Pot Synthesis of Polyethyleneimine Functionalized Graphene for the Highly-Sensitive and Selective Electrochemical Impedance Aptasensing of Kanamycin in Serum. Anal. Methods 2020, 12, 132–140. [Google Scholar] [CrossRef]
- Zanello, P. Inorganic Electrochemistry: Theory, Practice and Application; Royal Society of Chemistry Publishing: Cambridge, UK, 2003; ISBN 0854046615. [Google Scholar]
- Klingler, R.J.; Kochi, J.K. Electron-Transfer Kinetics from Cyclic Voltammetry. Quantitative Description of Electrochemical Reversibility. J. Phys. Chem. 1981, 85, 1731–1741. [Google Scholar] [CrossRef]
- Konopka, S.J.; McDuffie, B. Diffusion Coefficients of Ferri- and Ferrocyanide Ions in Aqueous Media Using Twin-Electrode Thin-Layer Electrochemistry. Anal. Chem. 1970, 42, 1741–1746. [Google Scholar] [CrossRef]
- Luo, Y.; Li, Z. A Sensitive Electrochemical Sensor Manufactured from Multi-Wall Carbon Nanotubes-Polyethylenimine Nanocomposite for Malachite Green Detection. J. Alloys Compd. 2022, 897, 163216. [Google Scholar] [CrossRef]
- Ferrari, A.G.M.; Foster, C.W.; Kelly, P.J.; Brownson, D.A.C.; Banks, C.E. Determination of the Electrochemical Area of Screen-Printed Electrochemical Sensing Platforms. Biosensors 2018, 8, 53. [Google Scholar] [CrossRef]
- Xie, A.; Wang, H.; Zhu, J.; Chang, J.; Gu, L.; Liu, C.; Yang, Y.; Ren, Y.; Luo, S. A Caffeic Acid Sensor Based on CuZnOx/MWCNTs Composite Modified Electrode. Microchem. J. 2021, 161, 105786. [Google Scholar] [CrossRef]
- İlyasoğlu, H.; Nadzieja, M.; Guo, Z. Caffeic Acid Grafted Chitosan as a Novel Dual-Functional Stabilizer for Food-Grade Emulsions and Additive Antioxidant Property. Food Hydrocoll. 2019, 95, 168–176. [Google Scholar] [CrossRef]
- Mintis, D.G.; Alexiou, T.S.; Mavrantzas, V.G. Effect of PH and Molecular Length on the Structure and Dynamics of Linear and Short-Chain Branched Poly(Ethylene Imine) in Dilute Solution: Scaling Laws from Detailed Molecular Dynamics Simulations. J. Phys. Chem. B 2020, 124, 6154–6169. [Google Scholar] [CrossRef] [PubMed]
- Gumustas, M.; Ozkan, S.A. The Role of and the Place of Method Validation in Drug Analysis Using Electroanalytical Techniques. Open Anal. Chem. J. 2011, 5, 1–21. [Google Scholar] [CrossRef]
- Araújo, D.A.G.; Camargo, J.R.; Pradela-Filho, L.A.; Lima, A.P.; Muñoz, R.A.A.; Takeuchi, R.M.; Janegitz, B.C.; Santos, A.L. A Lab-Made Screen-Printed Electrode as a Platform to Study the Effect of the Size and Functionalization of Carbon Nanotubes on the Voltammetric Determination of Caffeic Acid. Microchem. J. 2020, 158, 105297. [Google Scholar] [CrossRef]
- Shi, Y.; Xu, H.; Gu, Z.; Wang, C.; Du, Y. Sensitive Detection of Caffeic Acid with Trifurcate PtCu Nanocrystals Modified Glassy Carbon Electrode. Colloids Surf. A Physicochem. Eng. Asp. 2019, 567, 27–31. [Google Scholar] [CrossRef]
- Wang, J.; Yang, B.; Gao, F.; Song, P.; Li, L.; Zhang, Y.; Lu, C.; Goh, M.C.; Du, Y. Ultra-Stable Electrochemical Sensor for Detection of Caffeic Acid Based on Platinum and Nickel Jagged-like Nanowires. Nanoscale Res. Lett. 2019, 14, 11. [Google Scholar] [CrossRef]
- Mahrye; Anwar, F.; Mehmood, T.; Qadir, R.; Riaz, M. Phenolics Profiling and Biological Activities of Different Solvent Extracts from Aerial Parts of Wild Thyme (Thymus vulgaris L.). J. Food Meas. Charact. 2022, 16, 610–618. [Google Scholar] [CrossRef]
- Gavarić, N.; Kladar, N.; Mišan, A.; Nikolić, A.; Samojlik, I.; Mimica-Dukić, N.; Božin, B. Postdistillation Waste Material of Thyme (Thymus vulgaris L., Lamiaceae) as a Potential Source of Biologically Active Compounds. Ind. Crops Prod. 2015, 74, 457–464. [Google Scholar] [CrossRef]
- Alharbi, Y.M.; Sakr, S.S.; Albarrak, S.M.; Almundarij, T.I.; Barakat, H.; Hassan, M.F.Y. Antioxidative, Antidiabetic, and Hypolipidemic Properties of Probiotic-Enriched Fermented Camel Milk Combined with Salvia Officinalis Leaves Hydroalcoholic Extract in Streptozotocin-Induced Diabetes in Rats. Antioxidants 2022, 11, 668. [Google Scholar] [CrossRef]
- Milevskaya, V.V.; Temerdashev, Z.A.; Butyl’skaya, T.S.; Kiseleva, N.V. Determination of Phenolic Compounds in Medicinal Plants from the Lamiaceae Family. J. Anal. Chem. 2017, 72, 342–348. [Google Scholar] [CrossRef]
| Electrodes | Technique | pH | Linear range /µmol L−1 | LOD/µmol L−1 | Reference |
|---|---|---|---|---|---|
| Porous CuO/GCE | AMP a | Phosphate buffer (pH 7.0) | 0.05–229.5 | 0.04 | [6] |
| MWCNTs b/SPCE c | CV | Phosphate buffer (pH 3.6) | 0.1–40.0 | 0.06 | [5] |
| SPCE | CV | Phosphate buffer (pH 3.6) | 0.1–40.0 | 0.13 | [5] |
| CuZnOx/MWCNTs/GCE | AMP | Phosphate buffer (pH 7.0) | 1.0–100 | 0.15 | [32] |
| MWCNTs/SPCE | DPV | Perchloric acid solution | 2.0–50.0 | 0.20 | [36] |
| PtCu nanocrystals/GCE | DPV | Phosphate buffer (pH 2.0) | 1.2–357.9 | 0.30 | [37] |
| PtNi-C d/GCE | DPV | B-R buffer (pH 2.0) | 0.75–591 | 0.50 | [38] |
| PEI/CPE | SWV | Acetate buffer (pH 5.0) | 1.25 to 19.9 | 0.13 | This study |
| Sample | Determined a | Added/µmol L−1 | Found a/µmol L−1 | Recovery/% | |
|---|---|---|---|---|---|
| mg g−1 | µmol L−1 | ||||
| Thyme (Thymus vulgaris) | 0.37 ± 0.05 | 3.17 ± 0.43 | 1.00 | 4.20 | 95–102 |
| Sage (Salvia officinalis) | 0.45 ± 0.18 | 3.87 ± 0.77 | 1.00 | 4.90 | 93–105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamarchi, F.; Silva, T.R.; Winiarski, J.P.; Santana, E.R.; Vieira, I.C. Polyethylenimine-Based Electrochemical Sensor for the Determination of Caffeic Acid in Aromatic Herbs. Chemosensors 2022, 10, 357. https://doi.org/10.3390/chemosensors10090357
Zamarchi F, Silva TR, Winiarski JP, Santana ER, Vieira IC. Polyethylenimine-Based Electrochemical Sensor for the Determination of Caffeic Acid in Aromatic Herbs. Chemosensors. 2022; 10(9):357. https://doi.org/10.3390/chemosensors10090357
Chicago/Turabian StyleZamarchi, Felipe, Tânia Regina Silva, João Paulo Winiarski, Edson Roberto Santana, and Iolanda Cruz Vieira. 2022. "Polyethylenimine-Based Electrochemical Sensor for the Determination of Caffeic Acid in Aromatic Herbs" Chemosensors 10, no. 9: 357. https://doi.org/10.3390/chemosensors10090357
APA StyleZamarchi, F., Silva, T. R., Winiarski, J. P., Santana, E. R., & Vieira, I. C. (2022). Polyethylenimine-Based Electrochemical Sensor for the Determination of Caffeic Acid in Aromatic Herbs. Chemosensors, 10(9), 357. https://doi.org/10.3390/chemosensors10090357









