Nanostructures for In Situ SERS Analysis of High-Temperature Processes
Abstract
:1. Introduction
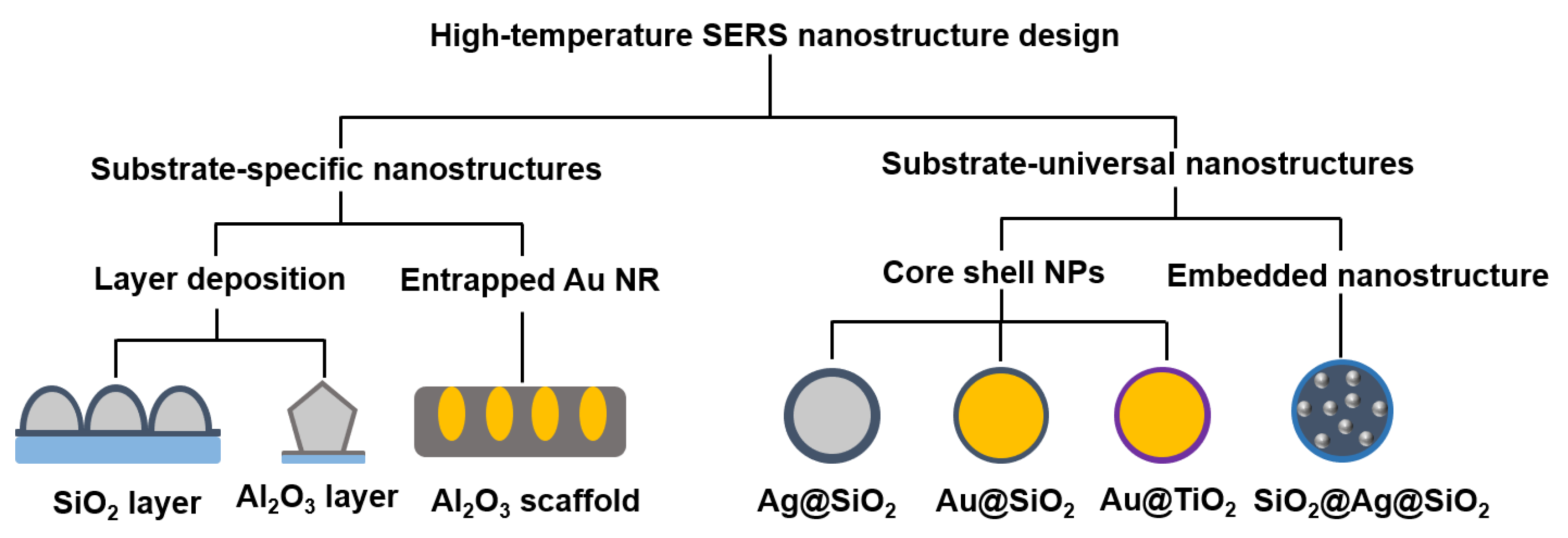
2. Design of Nanostructures for High-Temperature SERS
2.1. Substrate-Specific Nanostructures
2.1.1. Layer Deposition
2.1.2. Entrapped Nanorods
2.2. Substrate-Universal Nanostructures
2.2.1. Core–Shell Nanostructures
2.2.2. Embedded Core–Shell Nanostructure
3. Application of SERS Nanostructure in High-Temperature Processes
3.1. Structure Evolution
3.1.1. Thermal Crystallization
3.1.2. Interfacial Interaction
3.1.3. Lattice Dynamics
3.2. Heterogeneous Catalytic Reaction
3.2.1. Ethylene Epoxidation
3.2.2. NO Reduction
3.2.3. CO Oxidation
3.3. High-Temperature Electrochemistry
3.3.1. Molten Carbonate Electrolysis
3.3.2. Solid Oxide Fuel Cells
4. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Camden, J.P.; Dieringer, J.A.; Zhao, J.; Van Duyne, R.P. Controlled Plasmonic Nanostructures for Surface-Enhanced Spectroscopy and Sensing. Acc. Chem. Res. 2008, 41, 1653–1661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brus, L. Noble Metal Nanocrystals: Plasmon Electron Transfer Photochemistry and Single-Molecule Raman Spectroscopy. Acc. Chem. Res. 2008, 41, 1742–1749. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.T.; Tolia, A.A.; Chan, H.Y.H.; Takoudis, C.G.; Weaver, M.J. Surface-enhanced Raman spectroscopy as an in situ real-time probe of catalytic mechanisms at high gas pressures: The CO-NO reaction on platinum and palladium. J. Catal. 1996, 163, 63–76. [Google Scholar] [CrossRef]
- Williams, C.T.; Takoudis, C.G.; Weaver, M.J. Raman spectral evidence of reactive oxide formation during methanol oxidation on polycrystalline rhodium at high gas pressures. J. Catal. 1997, 170, 207–210. [Google Scholar] [CrossRef]
- Ren, B.; Li, X.Q.; She, C.X.; Wu, D.Y.; Tian, Z.Q. Surface Raman spectroscopy as a versatile technique to study methanol oxidation on rough Pt electrodes. Electrochim. Acta 2000, 46, 193–205. [Google Scholar] [CrossRef]
- Kneipp, K.; Wang, Y.; Kneipp, H.; Perelman, L.T.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Single molecule detection using surface-enhanced Raman scattering (SERS). Phys. Rev. Lett. 1997, 78, 1667. [Google Scholar] [CrossRef] [Green Version]
- Campion, A.; Kambhampati, P. Surface-enhanced Raman scattering. Chem. Soc. Rev. 1998, 27, 241–250. [Google Scholar] [CrossRef]
- Tian, Z.Q.; Ren, B.; Li, J.F.; Yang, Z.L. Expanding generality of surface-enhanced Raman spectroscopy with borrowing SERS activity strategy. Chem. Commun. 2007, 34, 3514–3534. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Luo, C.X.; Yu, H.M.; Kong, G.Z.; Hu, J.W. Surface sol-gel growth of ultrathin SiO2 films on roughened Au electrodes: Extending borrowed SERS to a SERS inactive material. Chem. Phys. Lett. 2014, 608, 35–39. [Google Scholar] [CrossRef]
- Van Duyne, R.P.; Haushalter, J.P. Surface-enhanced Raman spectroscopy of adsorbates on semiconductor electrode surfaces: Tris (bipyridine) ruthenium (II) adsorbed on silver-modified n-gallium arsenide (100). J. Phys. Chem. 1983, 87, 2999–3003. [Google Scholar] [CrossRef]
- Van Duyne, R.P.; Haushalter, J.P.; Janik-Czachor, M.; Levinger, N. Surface-enhanced resonance Raman spectroscopy of adsorbates on semiconductor electrode surfaces. 2. In situ studies of transition metal (iron and ruthenium) complexes on silver/gallium arsenide and silver/silicon. J. Phys. Chem. 1985, 89, 4055–4061. [Google Scholar] [CrossRef]
- Rubim, J.C.; Kannen, G.; Schumacher, D.; Dunnwald, J.; Otto, A. Raman-spectra of silver coated graphite and glassy-carbon electrodes. Appl. Surf. Sci. 1989, 37, 233–243. [Google Scholar] [CrossRef]
- Mengoli, G.; Musiani, M.; Fleischman, M.; Mao, B.; Tian, Z. Enhanced Raman scattering from iron electrodes. Electrochim. Acta 1987, 32, 1239–1245. [Google Scholar] [CrossRef]
- Fleischmann, M.; Tian, Z.Q.; Li, L.J. Raman-spectroscopy of adsorbates on thin-film electrodes deposited on silver substrates. J. Electroanal. Chem. 1987, 217, 397–410. [Google Scholar] [CrossRef]
- Aravind, P.K.; Nitzan, A.; Metiu, H. The interaction between electromagnetic resonances and its role in spectroscopic studies of molecules adsorbed on colloidal particles or metal spheres. Surf. Sci. 1981, 110, 189–204. [Google Scholar] [CrossRef]
- Leung, L.W.H.; Weaver, M.J. Adsorption and electrooxidation of carbon monoxide on rhodium-and ruthenium-coated gold electrodes as probed by surface-enhanced Raman spectroscopy. Langmuir ACS J. Surf. Colloids 1988, 4, 1076–1083. [Google Scholar] [CrossRef]
- Leung, L.W.H.; Weaver, M.J. Extending surface-enhanced Raman spectroscopy to transition-metal surfaces: Carbon monoxide adsorption and electrooxidation on platinum-and palladium-coated gold electrodes. J. Am. Chem. Soc. 1987, 109, 5113–5119. [Google Scholar] [CrossRef]
- Weaver, M.J.; Zou, S.; Chan, H.Y.H. Peer reviewed: The new interfacial ubiquity of surface-enhanced Raman spectroscopy. Anal. Chem. 2000, 72, 38A–47A. [Google Scholar] [CrossRef] [Green Version]
- Zou, S.Z.; Williams, C.T.; Chen, E.K.Y.; Weaver, M.J. Probing molecular vibrations at catalytically significant interfaces: A new ubiquity of surface-enhanced Raman scattering. J. Am. Chem. Soc. 1998, 120, 3811–3812. [Google Scholar] [CrossRef]
- Ren, B.; Picardi, G.; Pettinger, B.; Schuster, R.; Ertl, G. Tip-enhanced Raman spectroscopy of benzenethiol adsorbed on Au and Pt single-crystal surfaces. Angew. Chem.-Int. Edit. 2005, 44, 139–142. [Google Scholar] [CrossRef]
- Stöckle, R.M.; Suh, Y.D.; Deckert, V.; Zenobi, R. Nanoscale chemical analysis by tip-enhanced Raman spectroscopy. Chem. Phys. Lett. 2000, 318, 131–136. [Google Scholar] [CrossRef]
- Pettinger, B.; Ren, B.; Picardi, G.; Schuster, R.; Ertl, G. Nanoscale probing of adsorbed species by tip-enhanced Raman spectroscopy. Phys. Rev. Lett. 2004, 92, 096101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Liu, Z.; Zhuang, M.-D.; Zhang, H.-M.; Wang, X.; Xie, Z.-X.; Wu, D.-Y.; Ren, B.; Tian, Z.-Q. Tip-enhanced Raman spectroscopy for investigating adsorbed species on a single-crystal surface using electrochemically prepared Au tips. Appl. Phys. Lett. 2007, 91, 101105. [Google Scholar] [CrossRef] [Green Version]
- Li, J.F.; Huang, Y.F.; Ding, Y.; Yang, Z.L.; Li, S.B.; Zhou, X.S.; Fan, F.R.; Zhang, W.; Zhou, Z.Y.; Wu, D.Y.; et al. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 2010, 464, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Zheng, S.S.; Yang, W.M.; Zhou, R.Y.; He, Q.F.; Radjenovic, P.; Dong, J.C.; Li, S.N.; Zheng, J.X.; Yang, Z.L.; et al. In situ Raman spectroscopy reveals the structure and dissociation of interfacial water. Nature 2021, 600, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, X.-G.; Dong, J.-C.; Radjenovic, P.M.; Young, D.J.; Yao, J.-L.; Yuan, Y.-X.; Tian, Z.-Q.; Li, J.-F. Real-Time Monitoring of Surface Effects on the Oxygen Reduction Reaction Mechanism for Aprotic Na–O2 Batteries. J. Am. Chem. Soc. 2021, 143, 20049–20054. [Google Scholar] [CrossRef]
- Pettinger, B.; Schambach, P.; Villagomez, C.J.; Scott, N. Tip-Enhanced Raman Spectroscopy: Near-Fields Acting on a Few Molecules. Annu. Rev. Phys. Chem. 2012, 63, 379–399. [Google Scholar] [CrossRef] [Green Version]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.; Boisen, A.; Brolo, A.G. Present and future of surface-enhanced Raman scattering. ACS Nano 2019, 14, 28–117. [Google Scholar] [CrossRef] [Green Version]
- Nie, S.; Emory, S.R. Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Yan, B.; Chen, L.X. SERS Tags: Novel Optical Nanoprobes for Bioanalysis. Chem. Rev. 2013, 113, 1391–1428. [Google Scholar] [CrossRef]
- Hakonen, A.; Rindzevicius, T.; Schmidt, M.S.; Andersson, P.O.; Juhlin, L.; Svedendahl, M.; Boisen, A.; Kall, M. Detection of nerve gases using surface-enhanced Raman scattering substrates with high droplet adhesion. Nanoscale 2016, 8, 1305–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, P.; Dhara, S. Single molecule detection using SERS study in PVP functionalized Ag nanoparticles. In Proceedings of the 57th DAE Solid State Physics Symposium, Indian Institute of Technology, Bombay, India, 3–7 December 2012; pp. 206–207. [Google Scholar]
- Li, J.F.; Zhang, Y.J.; Rudnev, A.V.; Anema, J.R.; Li, S.B.; Hong, W.J.; Rajapandiyan, P.; Lipkowski, J.; Wandlowski, T.; Tian, Z.Q. Electrochemical Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy: Correlating Structural Information and Adsorption Processes of Pyridine at the Au(hkl) Single Crystal/Solution Interface. J. Am. Chem. Soc. 2015, 137, 2400–2408. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.Y.; Ren, B.; Jiang, Y.X.; Xu, X.; Tian, Z.Q. Density functional study and normal-mode analysis of the bindings and vibrational frequency shifts of the pyridine-M (M = Cu, Ag, Au, Cu+, Ag+, Au+, and Pt) complexes. J. Phys. Chem. A 2002, 106, 9042–9052. [Google Scholar] [CrossRef]
- Song, C.Y.; Sun, Y.Z.; Li, J.X.; Dong, C.; Zhang, J.J.; Jiang, X.Y.; Wang, L.H. Silver-mediated temperature-controlled selective deposition of Pt on hexoctahedral Au nanoparticles and the high performance of Au@AgPt NPs in catalysis and SERS. Nanoscale 2019, 11, 18881–18893. [Google Scholar] [CrossRef]
- Liu, J.B.; Long, L.; Zhang, Y.S.; Wang, Y.P.; Liu, F.S.; Xu, W.Y.; Zong, M.J.; Ma, L.; Liu, W.Q.; Zhang, H.; et al. Optical properties of Au-core Pt-shell nanorods studied using FDTD simulations. Front. Phys. 2016, 11, 118501. [Google Scholar] [CrossRef]
- Li, X.X.; Blinn, K.; Chen, D.C.; Liu, M.L. In situ and surface-enhanced Raman spectroscopy study of electrode materials in solid oxide fuel cells. Electrochem. Energy Rev. 2018, 1, 433–459. [Google Scholar] [CrossRef]
- Alarcon, A.; Guilera, J.; Soto, R.; Andreu, T. Higher tolerance to sulfur poisoning in CO2 methanation by the presence of CeO2. Appl. Catal. B-Environ. 2020, 263, 118346. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, C.; Li, Q.; Ren, Y.; Neuefeind, J.C.; Gu, L.; Liu, H.B.; Fan, L.L.; Chen, J.; Deng, J.X.; et al. Charge transfer drives anomalous phase transition in ceria. Nat. Commun. 2018, 9, 5063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alarifi, H.; Hu, A.M.; Yavuz, M.; Zhou, Y.N. Silver Nanoparticle Paste for Low-Temperature Bonding of Copper. J. Electron. Mater. 2011, 40, 1394–1402. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, S.H.; Li, J.C. Grain size-dependent diffusion activation energy in nanomaterials. Solid State Commun. 2004, 130, 581–584. [Google Scholar] [CrossRef]
- Bao, L.L.; Mahurin, S.M.; Dai, S. Controlled layer-by-layer formation of ultrathin TiO2 on silver island films via a surface sol-gel method for surface-enhanced Raman scattering measurement. Anal. Chem. 2004, 76, 4531–4536. [Google Scholar] [CrossRef] [PubMed]
- Li, X.X.; Lee, J.P.; Blinn, K.S.; Chen, D.C.; Yoo, S.; Kang, B.; Bottomley, L.A.; El-Sayed, M.A.; Park, S.; Liu, M.L. High-temperature surface enhanced Raman spectroscopy for in situ study of solid oxide fuel cell materials. Energy Environ. Sci. 2014, 7, 306–310. [Google Scholar] [CrossRef] [Green Version]
- Pettinger, B.; Bao, X.H.; Wilcock, I.; Muhler, M.; Schlogl, R.; Ertl, G. Thermal-decomposition of silver-oxide monitored by raman-spectroscopy—From ago units to oxygen-atoms chemisorbed on the silver surface. Angew. Chem. Int. Ed. Engl. 1994, 33, 85–86. [Google Scholar] [CrossRef]
- Mahurin, S.M.; John, J.; Sepaniak, M.J.; Dai, S. A Reusable Surface-Enhanced Raman Scattering (SERS) Substrate Prepared by Atomic Layer Deposition of Alumina on a Multi-Layer Gold and Silver Film. Appl. Spectrosc. 2011, 65, 417–422. [Google Scholar] [CrossRef] [PubMed]
- John, J.F.; Mahurin, S.; Dai, S.; Sepaniak, M.J. Use of atomic layer deposition to improve the stability of silver substrates for in situ, high temperature SERS measurements. J. Raman Spectrosc. 2010, 41, 4–11. [Google Scholar] [CrossRef]
- Formo, E.V.; Mahurin, S.M.; Dai, S. Robust SERS Substrates Generated by Coupling a Bottom-Up Approach and Atomic Layer Deposition. Acs Appl. Mater. Interfaces 2010, 2, 1987–1991. [Google Scholar] [CrossRef]
- Liu, M.; Xiang, R.; Lee, Y.; Otsuka, K.; Ho, Y.L.; Inoue, T.; Chiashi, S.; Delaunay, J.J.; Maruyama, S. Fabrication, characterization, and high temperature surface enhanced Raman spectroscopic performance of SiO2 coated silver particles. Nanoscale 2018, 10, 5449–5456. [Google Scholar] [CrossRef]
- Liu, K.; Ohodnicki, P.R.; Kong, X.Q.; Lee, S.S.; Du, H. Plasmonic Au nanorods stabilized within anodic aluminum oxide pore channels against high-temperature treatment. Nanotechnology 2019, 30, 405704. [Google Scholar] [CrossRef]
- Petrova, H.; Juste, J.P.; Pastoriza-Santos, I.; Hartland, G.V.; Liz-Marzan, L.M.; Mulvaney, P. On the temperature stability of gold nanorods: Comparison between thermal and ultrafast laser-induced heating. Phys. Chem. Chem. Phys. 2006, 8, 814–821. [Google Scholar] [CrossRef]
- Hartman, T.; Weckhuysen, B.M. Thermally Stable TiO2- and SiO2-Shell-Isolated Au Nanoparticles for In Situ Plasmon-Enhanced Raman Spectroscopy of Hydrogenation Catalysts. Chem. -A Eur. J. 2018, 24, 3733–3741. [Google Scholar] [CrossRef]
- Allen, G.; Bayles, R.; Gile, W.; Jesser, W. Small particle melting of pure metals. Thin Solid Film. 1986, 144, 297–308. [Google Scholar] [CrossRef]
- Sun, C.Q.; Wang, Y.; Tay, B.K.; Li, S.; Huang, H.; Zhang, Y.B. Correlation between the melting point of a nanosolid and the cohesive energy of a surface atom. J. Phys. Chem. B 2002, 106, 10701–10705. [Google Scholar] [CrossRef]
- Kim, E.H.; Lee, B.J. Size dependency of melting point of crystalline nano particles and nano wires: A thermodynamic modeling. Met. Mater. Int. 2009, 15, 531–537. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, J.M.; Tao, Y.K.; Li, N.; Yang, M.T.; Shao, J. Ag-Embedded Silica Core-Shell Nanospheres for Operando Surface Enhanced Raman Spectroscopy of High-Temperature Processes. Anal. Chem. 2020, 92, 9566–9573. [Google Scholar] [CrossRef] [PubMed]
- Sobol, A.; Shukshin, V.; Lukanin, V.; Moiseeva, L. High-temperature Raman scattering study of the Li2O–B2O3–MoO3 system: I. Detailed comparison of the structures of molten lithium and potassium molybdates. Inorg. Mater. 2018, 54, 984–989. [Google Scholar] [CrossRef]
- Qiu, H.; Wang, A.; You, J.; Liu, X.; Chen, H.; Yin, S. High temperature Raman spectra and structure character study of BSO crystal. Guang Pu Xue Yu Guang Pu Fen Xi= Guang Pu 2005, 25, 222–225. [Google Scholar] [PubMed]
- Wang, L.-Y.; Chen, L.-L.; Huang, X.-L.; Wang, M.; Zhu, X.-H.; Zou, J.-H. 24.4: A Linear High-Tg Arylphosphine Oxide-Triazine Conjugate as a Doped Electron-Transport Material for Stable Phosphorescent OLEDs. Proc. SID Symp. Dig. Tech. Pap. 2021, 52, 321–324. [Google Scholar] [CrossRef]
- Tang, C.W.; VanSlyke, S.A. Organic electroluminescent diodes. Appl. Phys. Lett. 1987, 51, 913–915. [Google Scholar] [CrossRef]
- Muraki, N. In Situ Monitoring of Thermal Crystallization of Ultrathin Tris(8-Hydroxyquinoline) Aluminum Films Using Surface-Enhanced Raman Scattering. Appl. Spectrosc. 2014, 68, 39–43. [Google Scholar] [CrossRef]
- Formo, E.V.; Wu, Z.L.; Mahurin, S.M.; Dai, S. In Situ High Temperature Surface Enhanced Raman Spectroscopy for the Study of Interface Phenomena: Probing a Solid Acid on Alumina. J. Phys. Chem. C 2011, 115, 9068–9073. [Google Scholar] [CrossRef]
- Schilling, C.; Hofmann, A.; Hess, C.; Ganduglia-Pirovano, M.V. Raman Spectra of Polycrystalline CeO2: A Density Functional Theory Study. J. Phys. Chem. C 2017, 121, 20834–20849. [Google Scholar] [CrossRef]
- Kondarides, D.I.; Papatheodorou, G.N.; Vayenas, C.G.; Verykios, X.E. In-situ high-temperature sers study of oxygen adsorbed on AG-support and electrochemical promotion effects. Ber. Der Bunsen-Ges. -Phys. Chem. Chem. Phys. 1993, 97, 709–720. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, C.; Sun, H.L.; Fu, G.; Chen, S.; Zhang, Y.J.; Chen, B.H.; Anema, J.R.; Yang, Z.L.; Li, J.F.; et al. In situ dynamic tracking of heterogeneous nanocatalytic processes by shell-isolated nanoparticle-enhanced Raman spectroscopy. Nat. Commun. 2017, 8, 15447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.F.; Tian, X.D.; Li, S.B.; Anema, J.R.; Yang, Z.L.; Ding, Y.; Wu, Y.F.; Zeng, Y.M.; Chen, Q.Z.; Ren, B. Surface analysis using shell-isolated nanoparticle-enhanced Raman spectroscopy. Nat. Protoc. 2013, 8, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Adeel, S.; Ilyas, M.; Shahzad, M.A.; Usman, M.; Ehsan-ul, H.; Hamayun, M. Oxidative degradation of Methyl Orange catalyzed by lab prepared nickel hydroxide in aqueous medium. Desalination Water Treat. 2016, 57, 12804–12813. [Google Scholar] [CrossRef]
- Wei, D.-Y.; Yue, M.-F.; Qin, S.-N.; Zhang, S.; Wu, Y.-F.; Xu, G.-Y.; Zhang, H.; Tian, Z.-Q.; Li, J.-F. In situ Raman observation of oxygen activation and reaction at platinum–ceria interfaces during CO oxidation. J. Am. Chem. Soc. 2021, 143, 15635–15643. [Google Scholar] [CrossRef]
- Hu, X.W.; Deng, W.T.; Shi, Z.N.; Wang, Z.X.; Gao, B.L.; Wang, Z.W. Solubility of CO2 in Molten Li2O-LiCl System: A Raman Spectroscopy Study. J. Chem. Eng. Data 2019, 64, 202–210. [Google Scholar] [CrossRef]
- Yu, J.Y.; Liu, C.Y.; Hu, X.W.; Yuan, T.; Zhang, Y.F.; Wang, Z.W.; Ji, W.R. Preparation of a Au SERS substrate and its application in the in-situ Raman spectroelectrochemistry study of Li2CO3-K2CO3 molten salt. Int. J. Electrochem. Sci. 2021, 16, 211131. [Google Scholar] [CrossRef]
- Appleby, A.; Nicholson, S. Reduction of oxygen in alkali carbonate melts. J. Electroanal. Chem. Interfacial Electrochem. 1977, 83, 309–328. [Google Scholar] [CrossRef]
- Itoh, T.; Maeda, T.; Kasuya, A. In situ surface-enhanced Raman scattering spectroelectrochemistry of oxygen species. Faraday Discuss. 2006, 132, 95–109. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Hossain, S.; Azad, A.T.; Petra, P.M.I.; Begum, F.; Eriksson, S.G.; Azad, A.K. Nanomaterials for solid oxide fuel cells: A review. Renew. Sustain. Energy Rev. 2018, 82, 353–368. [Google Scholar] [CrossRef]
- Fan, L.D.; Zhu, B.; Su, P.C.; He, C.X. Nanomaterials and technologies for low temperature solid oxide fuel cells: Recent advances, challenges and opportunities. Nano Energy 2018, 45, 148–176. [Google Scholar] [CrossRef]
- Hossain, S.; Abdalla, A.M.; Jamain, S.N.B.; Zaini, J.H.; Azad, A.K. A review on proton conducting electrolytes for clean energy and intermediate temperature-solid oxide fuel cells. Renew. Sustain. Energy Rev. 2017, 79, 750–764. [Google Scholar] [CrossRef]
- Bao, C.; Wang, Y.; Feng, D.L.; Jiang, Z.Y.; Zhang, X.X. Macroscopic modeling of solid oxide fuel cell (SOFC) and model-based control of SOFC and gas turbine hybrid system. Prog. Energy Combust. Sci. 2018, 66, 83–140. [Google Scholar] [CrossRef]
- Li, X.X.; Liu, M.F.; Lee, J.P.; Ding, D.; Bottomley, L.A.; Park, S.; Liu, M.L. An operando surface enhanced Raman spectroscopy (SERS) study of carbon deposition on SOFC anodes. Phys. Chem. Chem. Phys. 2015, 17, 21112–21119. [Google Scholar] [CrossRef]
- Chen, Y.; Yoo, S.; Choi, Y.; Kim, J.H.; Ding, Y.; Pei, K.; Murphy, R.; Zhang, Y.; Zhao, B.; Zhang, W. A highly active, CO2-tolerant electrode for the oxygen reduction reaction. Energy Environ. Sci. 2018, 11, 2458–2466. [Google Scholar] [CrossRef]
- Cheng, Z.; Wang, J.H.; Choi, Y.M.; Yang, L.; Lin, M.C.; Liu, M.L. From Ni-YSZ to sulfur-tolerant anode materials for SOFCs: Electrochemical behavior, in situ characterization, modeling, and future perspectives. Energy Environ. Sci. 2011, 4, 4380–4409. [Google Scholar] [CrossRef]
- Chen, H.L.; Wang, F.; Wang, W.; Chen, D.F.; Li, S.D.; Shao, Z.P. H2S poisoning effect and ways to improve sulfur tolerance of nickel cermet anodes operating on carbonaceous fuels. Appl. Energy 2016, 179, 765–777. [Google Scholar] [CrossRef]
- Kim, J.H.; Liu, M.F.; Chen, Y.; Murphy, R.; Choi, Y.; Liu, Y.; Liu, M.L. Understanding the Impact of Sulfur Poisoning on the Methane-Reforming Activity of a Solid Oxide Fuel Cell Anode. ACS Catal. 2021, 11, 13556–13566. [Google Scholar] [CrossRef]
- Da, Y.L.; Xiao, Y.; Zhong, Z.; Pan, Z.H.; Jiao, Z.J. Predictions on conductivity and mechanical property evolutions of yttria-stabilized zirconia in solid oxide fuel cells based on phase-field modeling of cubic-tetragonal phase transformation. J. Eur. Ceram. Soc. 2022, 42, 3489–3499. [Google Scholar] [CrossRef]












Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Wu, J.; Shao, J.; Tao, Y. Nanostructures for In Situ SERS Analysis of High-Temperature Processes. Chemosensors 2023, 11, 21. https://doi.org/10.3390/chemosensors11010021
Huang J, Wu J, Shao J, Tao Y. Nanostructures for In Situ SERS Analysis of High-Temperature Processes. Chemosensors. 2023; 11(1):21. https://doi.org/10.3390/chemosensors11010021
Chicago/Turabian StyleHuang, Jingying, Jiahao Wu, Jing Shao, and Youkun Tao. 2023. "Nanostructures for In Situ SERS Analysis of High-Temperature Processes" Chemosensors 11, no. 1: 21. https://doi.org/10.3390/chemosensors11010021





