Influences of Impurity Gases in Air on Room-Temperature Hydrogen-Sensitive Pt–SnO2 Composite Nanoceramics: A Case Study of H2S
Abstract
1. Introduction
2. Materials and Methods
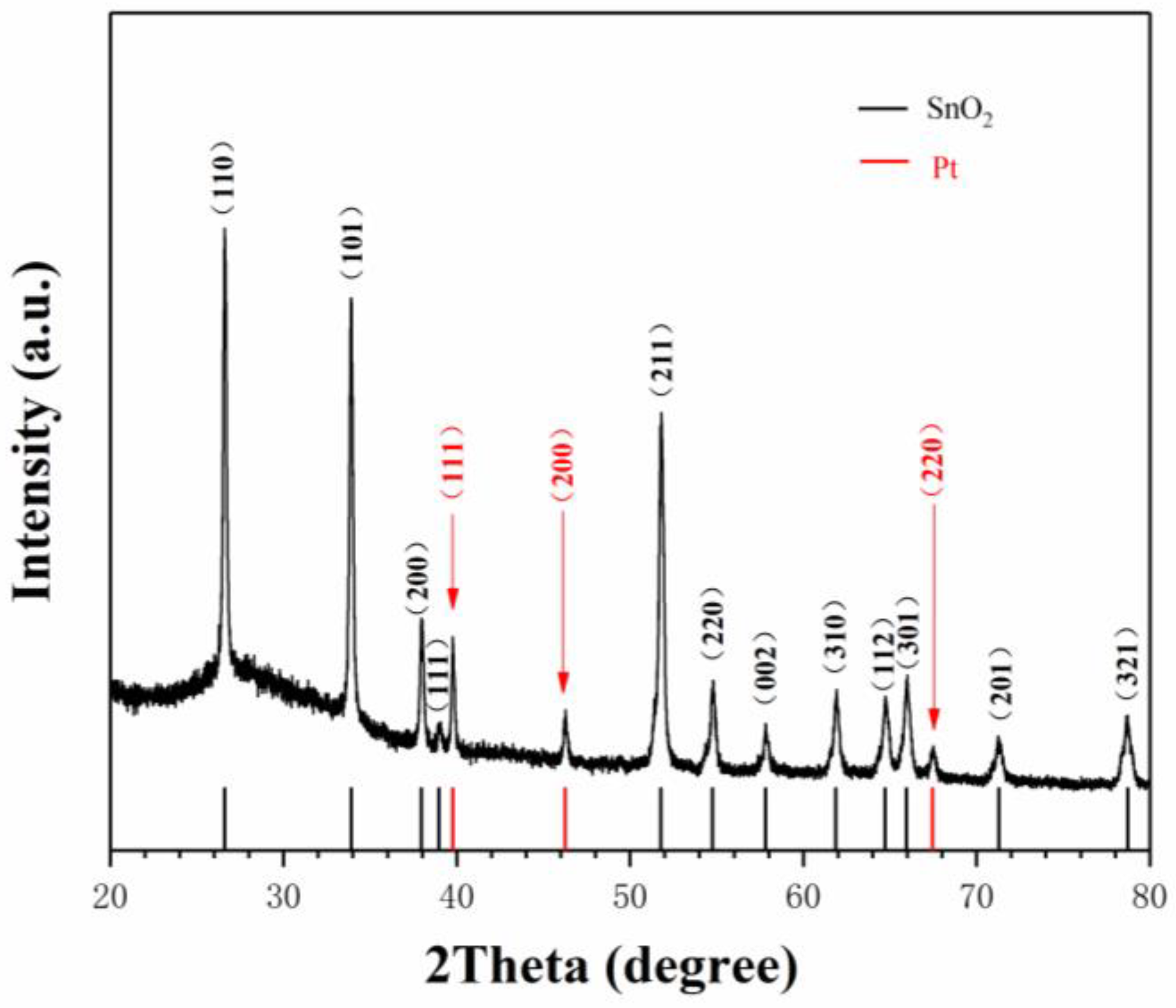
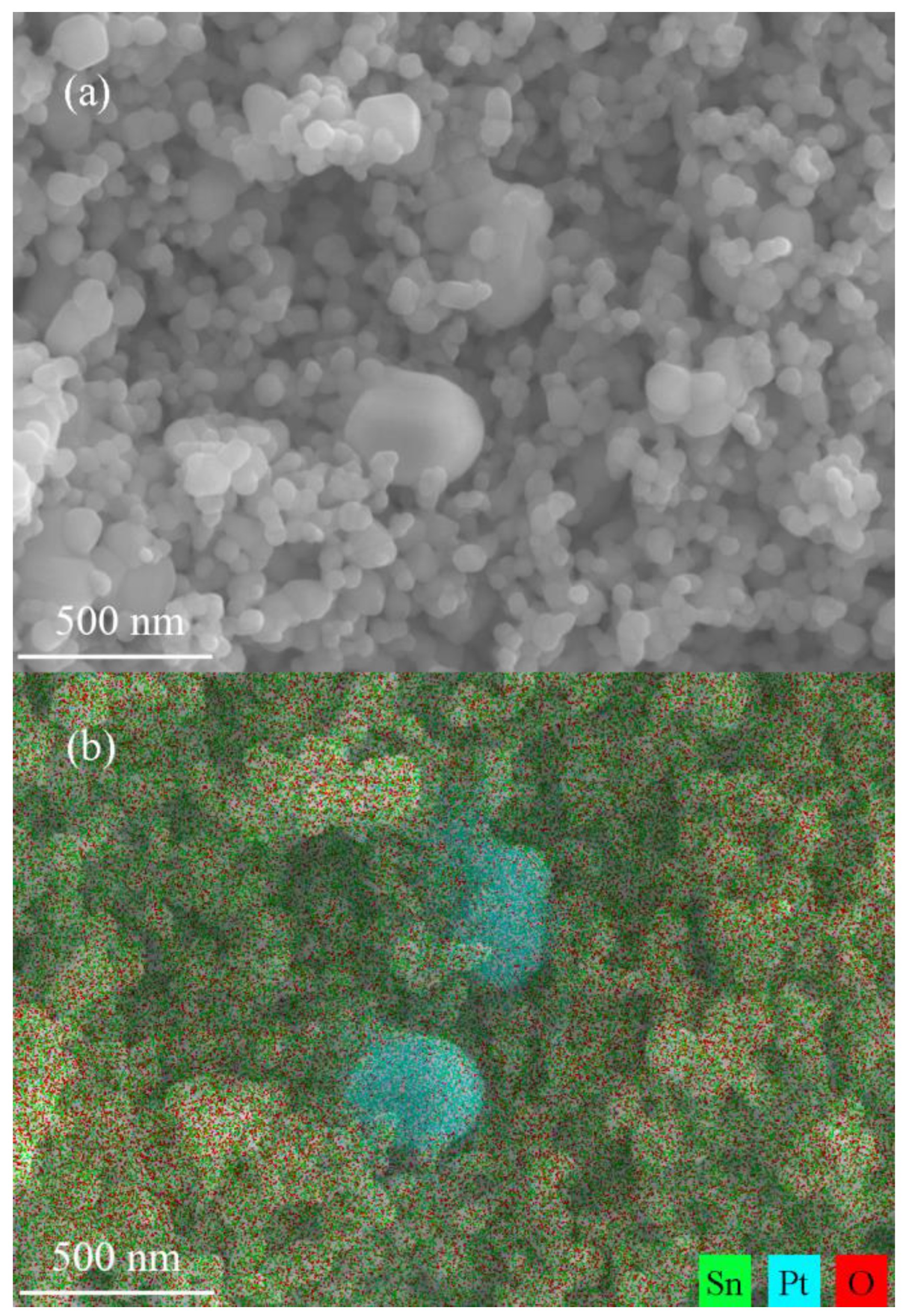
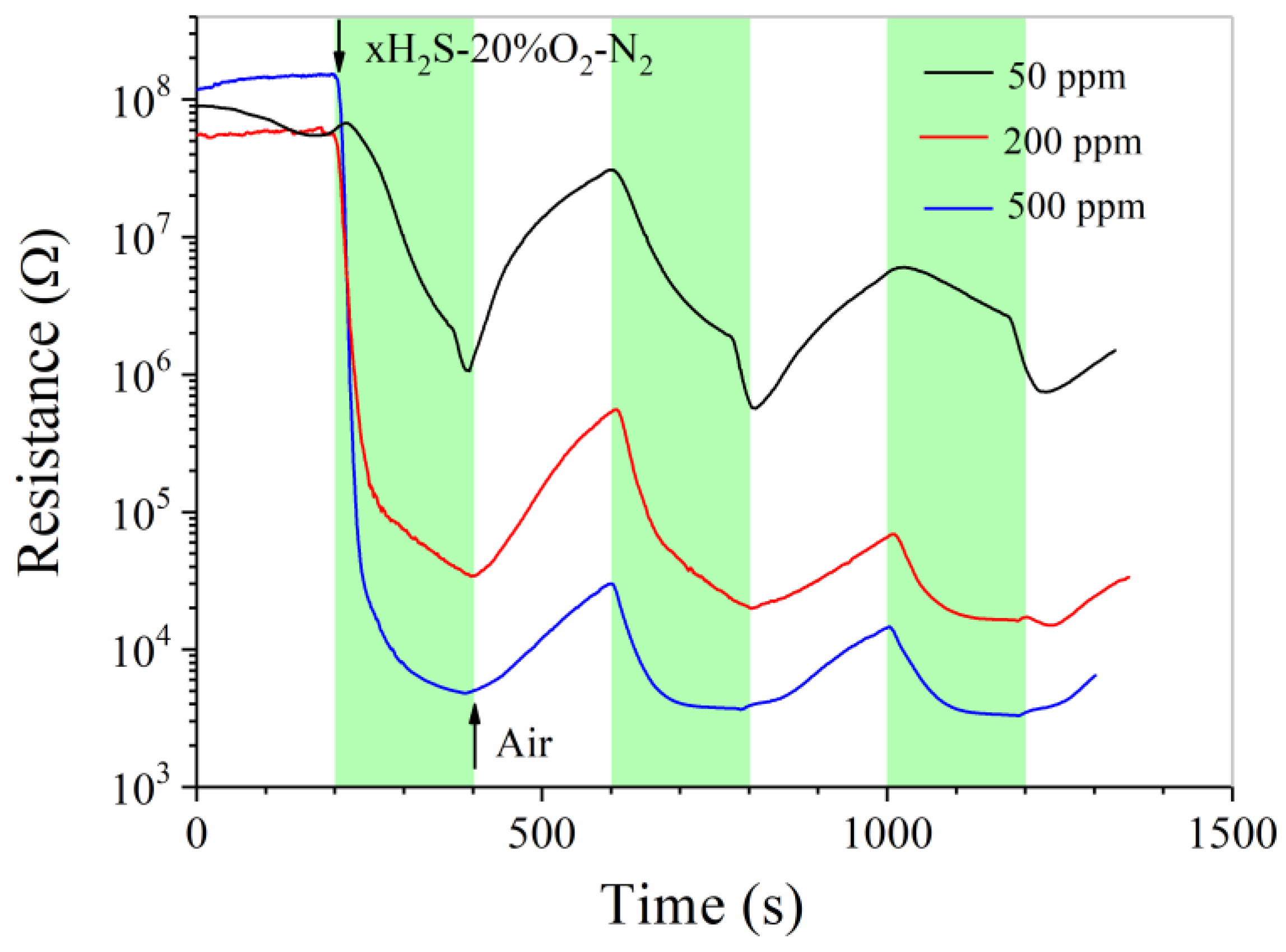
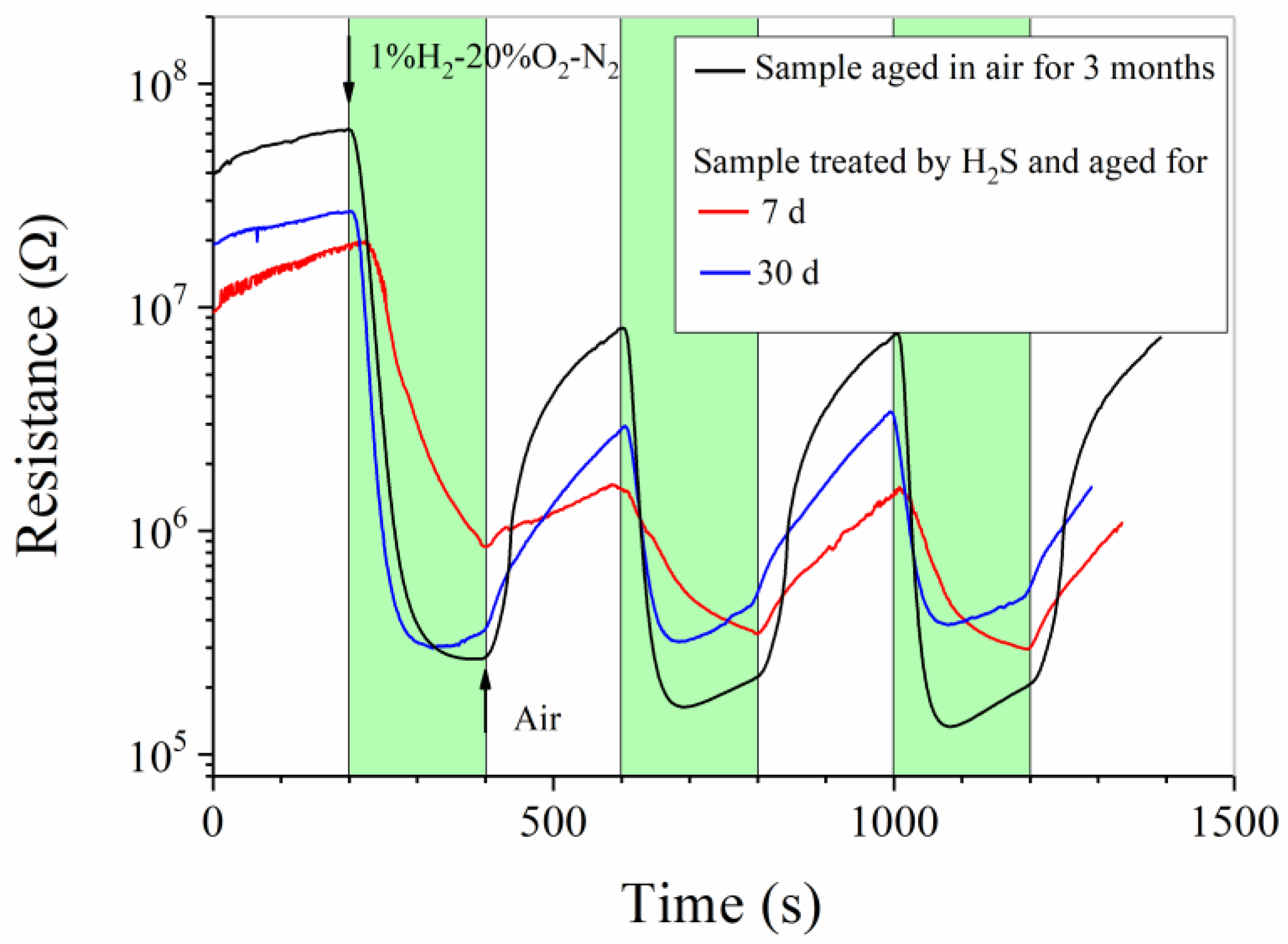
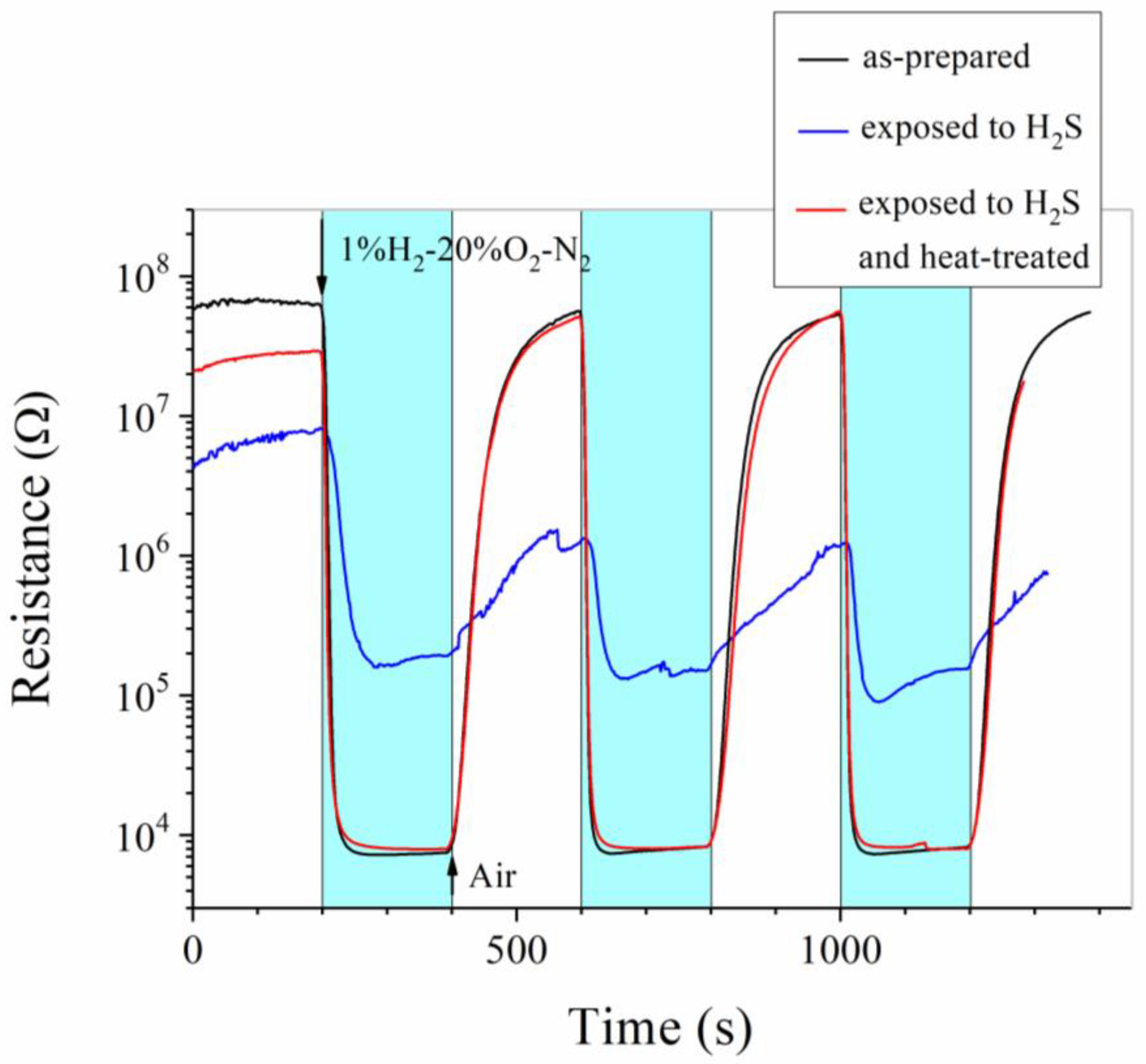
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kong, J.; Franklin, N.R.; Zhou, C.W.; Chapline, M.G.; Peng, S.; Cho, K.J.; Dai, H.J. Nanotube molecular wires as chemical sensors. Science 2000, 287, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.; Hong, S.; Jeong, Y.; Shin, W.; Park, J.; Kim, D.; Lee, J.-H. Highly Selective and Low-Power Carbon Monoxide Gas Sensor Based on the Chain Reaction of Oxygen and Carbon Monoxide to WO3. ACS Appl. Mater. Interfaces 2022, 14, 17950–17958. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, A.; Sharma, A.; Tomar, M.; Gupta, V. Carbon monoxide (CO) optical gas sensor based on ZnO thin films. Sens. Actuators B 2017, 250, 679–685. [Google Scholar] [CrossRef]
- Yamazoe, N. Toward innovations of gas sensor technology. Sens. Actuators B 2005, 108, 2–14. [Google Scholar] [CrossRef]
- Moy, R. Liability and the hydrogen economy. Science 2003, 301, 47. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, Y.; Maeng, B.; Jung, D.G.; Lee, J.; Kim, Y.; Kwon, J.; An, H.K.; Jung, D. Annealing Effects on SnO2 Thin Film for H2 Gas Sensing. Nanomaterials 2022, 12, 3277. [Google Scholar] [CrossRef]
- Cho, S.H.; Suh, J.M.; Jeong, B.; Lee, T.H.; Choi, K.S.; Eom, T.H.; Kim, T.; Jang, H.W. Fast responding and highly reversible gasochromic H2 sensor using Pd-decorated amorphous WO3 thin films. Chem. Eng. J. 2022, 446, 136862. [Google Scholar]
- Palla-Papavlu, A.; Voicu, S.I.; Dinescu, M. Sensitive Materials and Coating Technologies for Surface Acoustic Wave Sensors. Chemosensors 2021, 9, 105. [Google Scholar] [CrossRef]
- Brunet, E.; Maier, T.; Mutinati, G.C.; Steinhauer, S.; Koeck, A.; Gspan, C.; Grogger, W. Comparison of the gas sensing performance of SnO2 thin film and SnO2 nanowire sensors. Sens. Actuators B 2012, 165, 110–118. [Google Scholar] [CrossRef]
- Korotcenkov, G. Handbook of Gas Sensor Materials: Properties, Advantages and Shortcomings for Applications; Springer: New York, NY, USA, 2013. [Google Scholar]
- Boon-Brett, L.; Bousek, J.; Black, G.; Moretto, P.; Castello, P.; Huebert, T.; Banach, U. Identifying performance gaps in hydrogen safety sensor technology for automotive and stationary applications. Int. J. Hydrogen Energy 2010, 35, 373–384. [Google Scholar] [CrossRef]
- Nelli, P.; Faglia, G.; Sberveglieri, G.; Cereda, E.; Gabetta, G.; Dieguez, A.; Romano-Rodriguez, A.; Morante, J.R. The aging effect on SnO2-Au thin film sensors: Electrical and structural characterization. Thin Solid Films 2000, 371, 249–253. [Google Scholar] [CrossRef]
- Choi, K.J.; Jang, H.W. One-Dimensional Oxide Nanostructures as Gas-Sensing Materials: Review and Issues. Sensors 2010, 10, 4083–4099. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; You, K.; Hu, Y.; Yang, S.; Pan, X.; Wang, Z.; Chen, W.; Gu, H. Rapid hydrogen sensing response and aging of a-MoO3 nanowires paper sensor. Int. J. Hydrogen Energy 2017, 42, 8399–8405. [Google Scholar] [CrossRef]
- Wang, M.-Y.; Sun, B.; Jiang, Z.; Liu, Y.; Wang, X.; Tang, Z.; Wang, Y.; Chen, W.-P. Preparation and Extraordinary Room-Temperature CO Sensing Capabilities of Pd-SnO2 Composite Nanoceramics. J. Nanosci. Nanotechnol. 2018, 18, 4176–4181. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, P.-C.; Huang, Y.; Cheng, L.; Hu, Y.-M.; Tang, Z.; Chen, W.-P. Room-Temperature Hydrogen-Sensing Capabilities of Pt-SnO2 and Pt-ZnO Composite Nanoceramics Occur via Two Different Mechanisms. Nanomaterials 2021, 11, 504. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Liu, Y.; Wu, G.; Fei, L.; Zhang, S.; Hu, Y.-M.; Yan, Z.; Wang, Y.; Gu, H.; Chen, W.-P. Mechanism study on extraordinary room-temperature CO sensing capabilities of Pd-SnO2 composite nanoceramics. Sens. Actuators B 2019, 285, 49–55. [Google Scholar] [CrossRef]
- Zhu, S.; Li, P.-C.; Wu, G.; Li, Z.; Wu, P.; Hu, Y.-M.; Gu, H.; Chen, W.-P. Extraordinary room-temperature hydrogen sensing capabilities with high humidity tolerance of Pt-SnO2 composite nanoceramics prepared using SnO2 agglomerate powder. Int. J. Hydrogen Energy 2018, 43, 21177–21185. [Google Scholar] [CrossRef]
- Huang, Y.; Li, P.; Xu, L.; Yu, Y.; Chen, W. Ultrahigh humidity tolerance of room-temperature hydrogen sensitive Pt-WO3 porous composite ceramics with ultra-large WO3 grains. Appl. Phys. A 2021, 127, 952. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, F.; Meng, L.; Hu, Y.-M.; Chen, W.-P. Aging and activation of room temperature hydrogen sensitive Pt-SnO2 composite nanoceramics. J. Mater. Sci. 2022, 57, 15267–15275. [Google Scholar] [CrossRef]
- Wu, M.-H.; Gui, F.-B.; Lu, X.-L.; Yan, Z.; Chen, F.; Jiang, Y.; Luo, X.; Chen, W.-P. Achieving a high long-term stability for room temperature CO-sensitive Pt-SnO2 composite nanoceramics through two strategies. Mater. Sci. Eng. B 2022, 286, 116070. [Google Scholar] [CrossRef]
- Ali, F.I.M.; Awwad, F.; Greish, Y.E.; Mahmoud, S.T. Hydrogen Sulfide (H2S) Gas Sensor: A Review. IEEE Sens. J. 2019, 19, 2394–2407. [Google Scholar] [CrossRef]
- Wang, R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sokolovskij, R.; Chen, G.; Zhu, Y.; Qi, Y.; Lin, X.; Li, W.; Zhang, G.Q.; Jiang, Y.-L.; Yu, H. Impact of high temperature H2 pre-treatment on Pt-AlGaN/GaN HEMT sensor for H2S detection. Sens. Actuators B 2019, 280, 138–143. [Google Scholar] [CrossRef]
- Abu Haija, M.; Abu-Hani, A.F.S.; Hamdan, N.; Stephen, S.; Ayesh, A.I. Characterization of H2S gas sensor based on CuFe2O4 nanoparticles. J. Alloys Compd. 2017, 690, 461–468. [Google Scholar] [CrossRef]
- Liu, M.; Wang, C.; Li, P.; Cheng, L.; Hu, Y.; Xiong, Y.; Guo, S.; Gu, H.; Chen, W. Transforming Pt-SnO2 Nanoparticles into Pt-SnO2 Composite Nanoceramics for Room-Temperature Hydrogen-Sensing Applications. Materials 2021, 14, 2123. [Google Scholar] [CrossRef]
- He, F.; Muliane, U.; Weon, S.; Choi, W. Substrate-specific mineralization and deactivation behaviors of TiO2 as an air-cleaning photocatalyst. Appl. Catal. B 2020, 275, 119145. [Google Scholar] [CrossRef]
- Maki-Arvela, P.; Martin, G.; Simakova, I.; Tokarev, A.; Warna, J.; Hemming, J.; Holmbom, B.; Salmi, T.; Murzin, D.Y. Kinetics, catalyst deactivation and modeling in the hydrogenation of beta-sitosterol to beta-sitostanol over microporous and mesoporous carbon supported Pd catalysts. Chem. Eng. J. 2009, 154, 45–51. [Google Scholar] [CrossRef]
- Ravanchi, M.T.; Sahebdelfar, S.; Fard, M.R.; Fadaeerayeni, S.; Bigdeli, P. Pd-Ag/-Al2O3 Catalyst Deactivation in Acetylene Selective Hydrogenation Process. Chem. Eng. Technol. 2016, 39, 301–310. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Wu, M.; Huang, Y.; Song, J.; Liu, Y.; Yan, Z.; Chen, F.; Zhao, J.; Chen, W. Influences of Impurity Gases in Air on Room-Temperature Hydrogen-Sensitive Pt–SnO2 Composite Nanoceramics: A Case Study of H2S. Chemosensors 2023, 11, 31. https://doi.org/10.3390/chemosensors11010031
Lu X, Wu M, Huang Y, Song J, Liu Y, Yan Z, Chen F, Zhao J, Chen W. Influences of Impurity Gases in Air on Room-Temperature Hydrogen-Sensitive Pt–SnO2 Composite Nanoceramics: A Case Study of H2S. Chemosensors. 2023; 11(1):31. https://doi.org/10.3390/chemosensors11010031
Chicago/Turabian StyleLu, Xilai, Menghan Wu, Yong Huang, Jiannan Song, Yong Liu, Zhiqiao Yan, Feng Chen, Jieting Zhao, and Wanping Chen. 2023. "Influences of Impurity Gases in Air on Room-Temperature Hydrogen-Sensitive Pt–SnO2 Composite Nanoceramics: A Case Study of H2S" Chemosensors 11, no. 1: 31. https://doi.org/10.3390/chemosensors11010031
APA StyleLu, X., Wu, M., Huang, Y., Song, J., Liu, Y., Yan, Z., Chen, F., Zhao, J., & Chen, W. (2023). Influences of Impurity Gases in Air on Room-Temperature Hydrogen-Sensitive Pt–SnO2 Composite Nanoceramics: A Case Study of H2S. Chemosensors, 11(1), 31. https://doi.org/10.3390/chemosensors11010031







