Luminescent Metal-Organic Framework with 2,1,3-Benzothiadiazole Units for Highly Sensitive Gossypol Sensing
Abstract
:1. Introduction
2. Materials and Methods
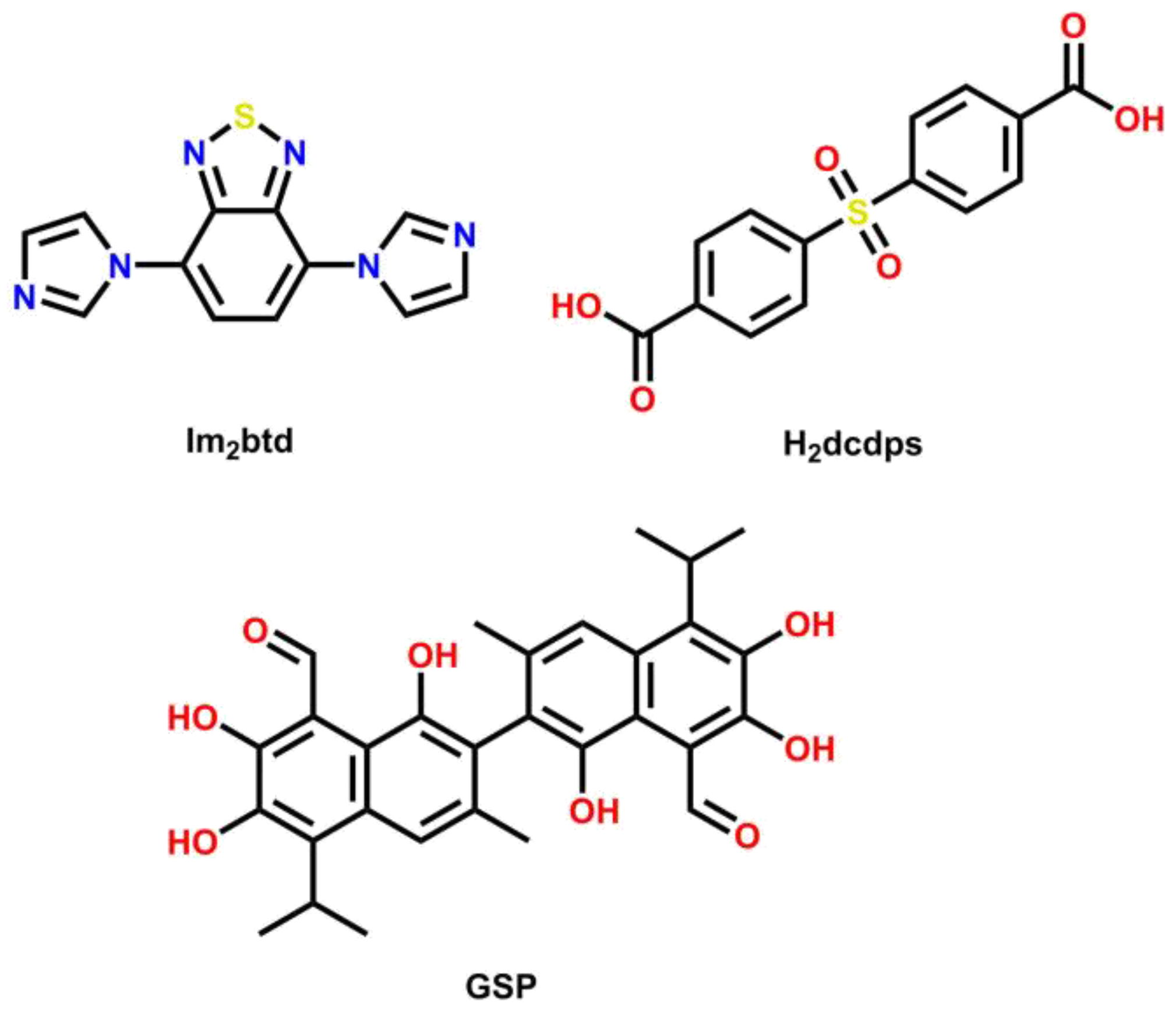
2.1. Starting Materials and Synthetic Procedures
Synthesis of {[Cd(dcdps)(im2btd)]∙DMA}n (MOF 1)
2.2. Physical Methods of Analysis
2.2.1. Luminescence Measurements
2.2.2. Luminescence Sensing Experiments
2.3. Single Crystal X-ray Analysis
Crystal Data for MOF 1
2.4. Computational Chemistry Details
3. Results and Discussion
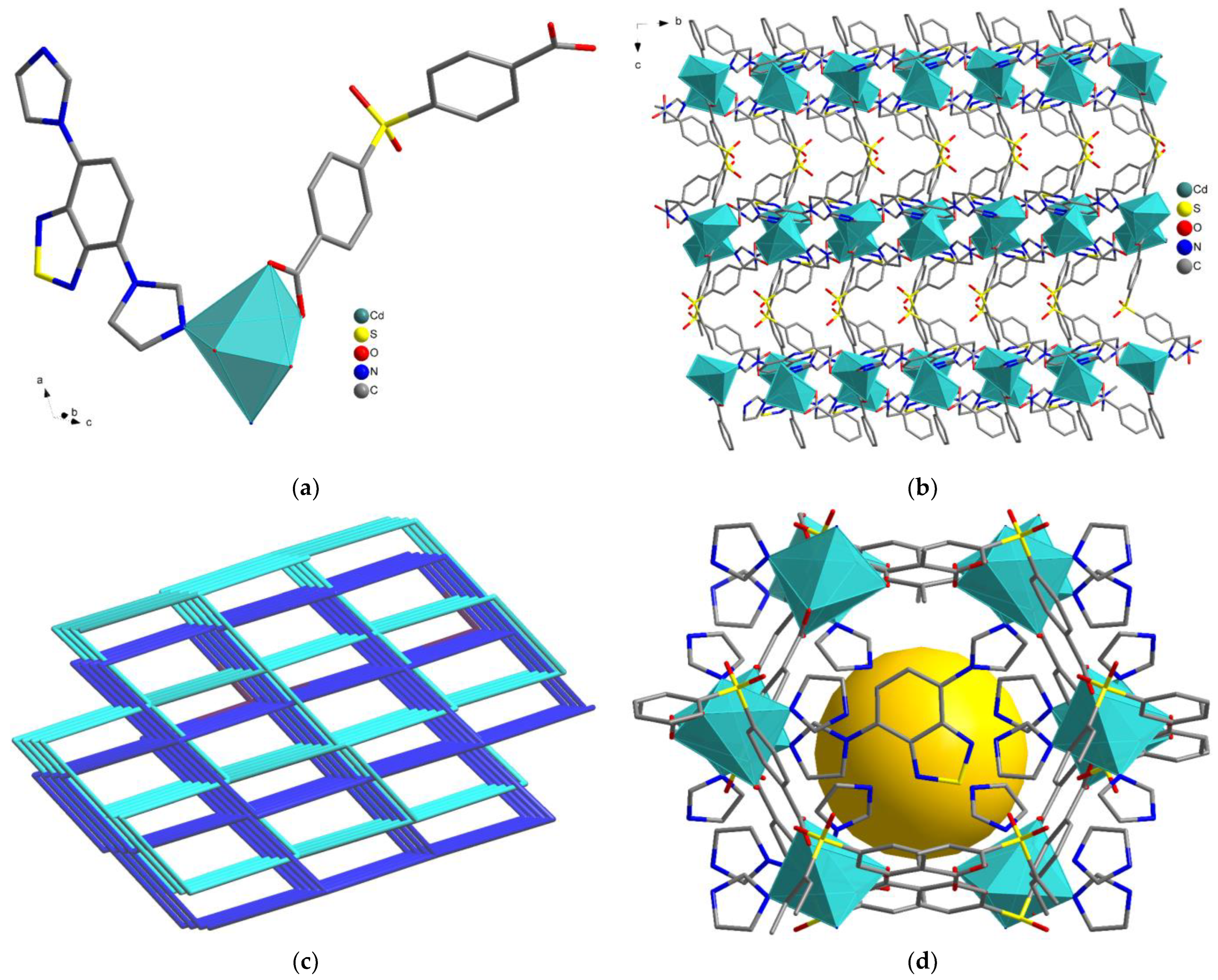
3.1. Synthesis and Crystal Structures of MOF
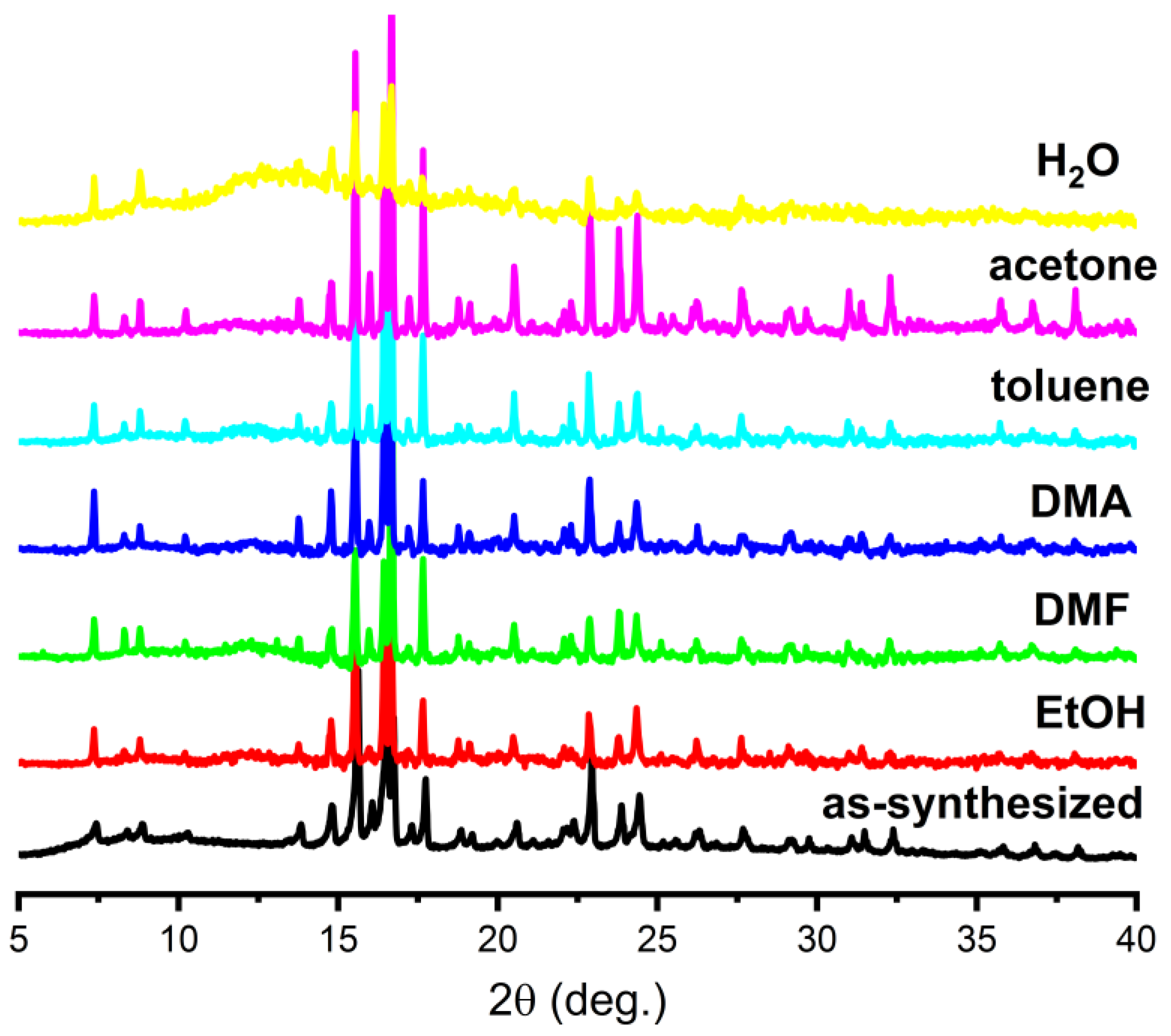
3.2. PXRD Analysis and Thermal Behaviour
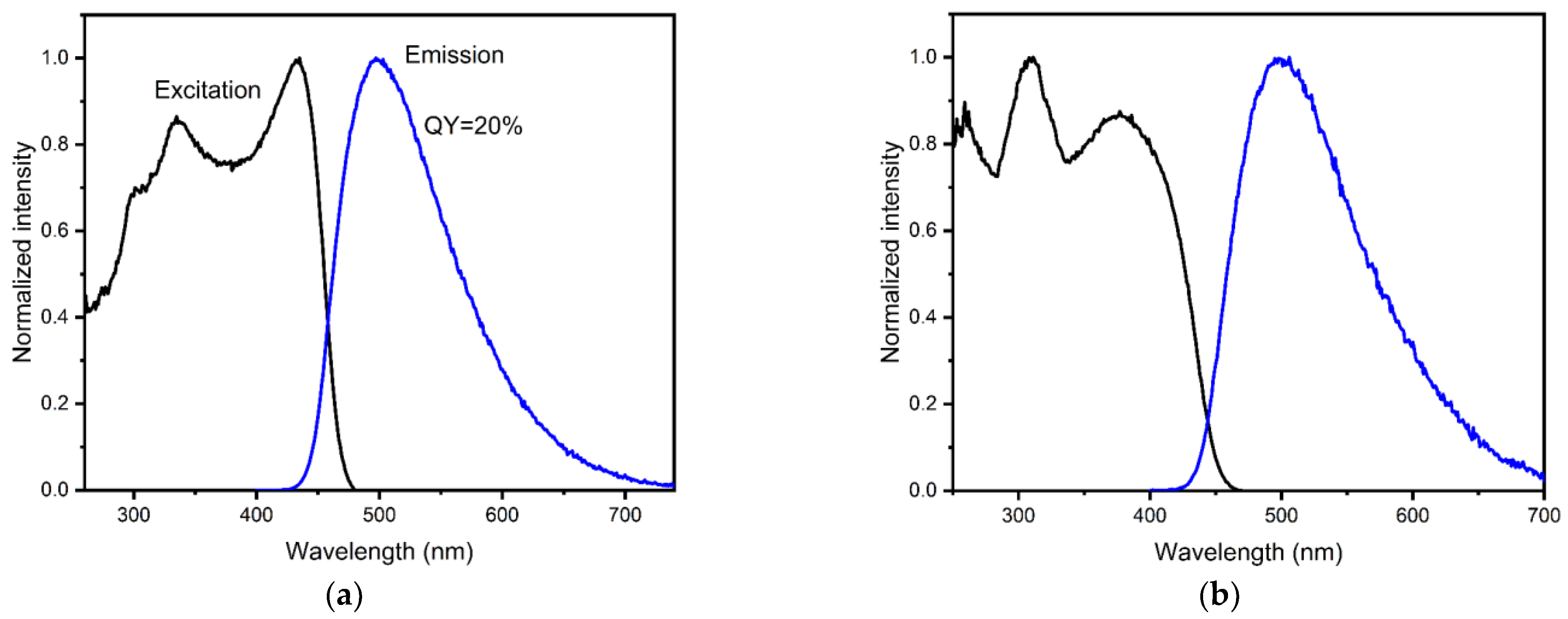
3.3. Luminescent Properties of MOFs
3.3.1. Metal Ion Detection
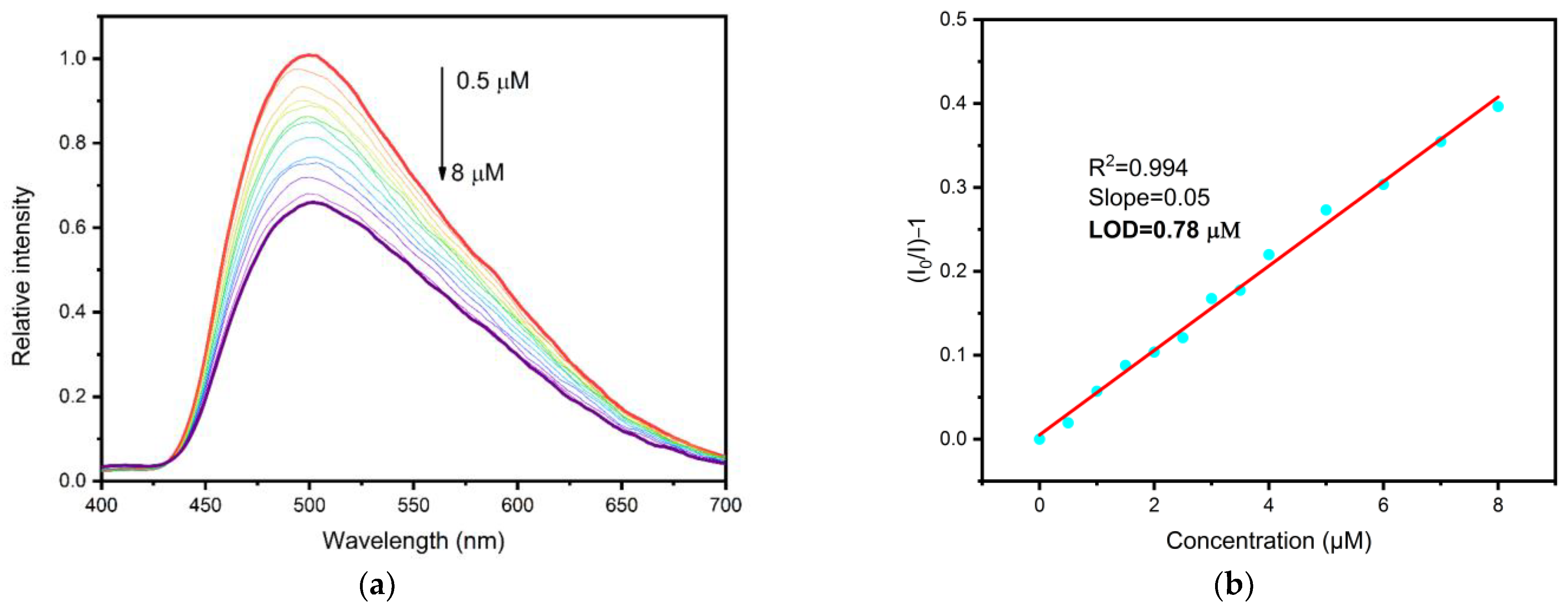
3.3.2. Gossypol and Antibiotic Detection
3.4. Luminescene Quenching Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rathore, K.S.; Pandeya, D.; Campbell, L.A.M.; Wedegaertner, T.C.; Puckhaber, L.; Stipanovic, R.D.; Thenell, J.S.; Hague, S.; Hake, K. Ultra-low gossypol cottonseed: Selective gene silencing opens up a vast resource of plant-based protein to improve human nutrition. CRC Crit. Rev. Plant Sci. 2020, 39, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Tomar, M.; Punia, S.; Grasso, S.; Arrutia, F.; Choudhary, J.; Singh, S.; Verma, P.; Mahapatra, A.; Patil, S.; et al. Cottonseed: A sustainable contributor to global protein requirements. Trends Food Sci. Technol. 2021, 111, 100–113. [Google Scholar] [CrossRef]
- Soares Neto, C.B.; Conceição, A.A.; Gomes, T.G.; de Aquino Ribeiro, J.A.; Campanha, R.B.; Barroso, P.A.V.; Machado, A.E.V.; Mendonça, S.; De Siqueira, F.G.; Miller, R.N.G. A comparison of physical, chemical, biological and combined treatments for detoxification of free gossypol in crushed whole cottonseed. Waste Biomass Valoriz. 2021, 12, 3965–3975. [Google Scholar] [CrossRef]
- Gadelha, I.C.N.; Fonseca, N.B.S.; Oloris, S.C.S.; Melo, M.M.; Soto-Blanco, B. Gossypol toxicity from cottonseed products. Sci. World J. 2014, 2014, 231635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abou-Donia, S.A.; Lasker, J.M.; Abou-Donia, M.B. High-performance liquid chromatographic analysis of gossypol. J. Chromatogr. A 1981, 206, 606–610. [Google Scholar] [CrossRef]
- Mirghani, M.E.S.; Che Man, Y.B. A new method for determining gossypol in cottonseed oil by FTIR spectroscopy. JAOCS J. Am. Oil Chem. Soc. 2003, 80, 625–628. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, D. Rapid detection assay for the molecular imprinting of gossypol using a two-layer PMAA/SiO2 bulk structure with a piezoelectric imprinting sensor. Sens. Actuators B Chem. 2013, 181, 104–113. [Google Scholar] [CrossRef]
- Li, C.; Zhao, T.; Li, C.; Mei, L.; Yu, E.; Dong, Y.; Chen, J.; Zhu, S. Determination of gossypol content in cottonseeds by near infrared spectroscopy based on Monte Carlo uninformative variable elimination and nonlinear calibration methods. Food Chem. 2017, 221, 990–996. [Google Scholar] [CrossRef]
- Luo, T.Y.; Das, P.; White, D.L.; Liu, C.; Star, A.; Rosi, N.L. Luminescence “turn-on” detection of gossypol using Ln3+-based metal-organic frameworks and Ln3+ salts. J. Am. Chem. Soc. 2020, 142, 2897–2904. [Google Scholar] [CrossRef]
- SK, M.; Biswas, S. A thiadiazole-functionalized Zr(IV)-based metal-organic framework as highly fluorescent probe for the selective detection of picric acid. Cryst. Eng. Comm. 2016, 18, 3104–3113. [Google Scholar] [CrossRef]
- Jana, A.K.; Natarajan, S. Fluorescent metal-organic frameworks for selective sensing of toxic cations (Tl3+, Hg2+) and highly oxidizing anions ((CrO4)2−, (Cr2O7)2−, (MnO4)−). Chempluschem 2017, 82, 1153–1163. [Google Scholar] [CrossRef]
- Mallick, A.; El-Zohry, A.M.; Shekhah, O.; Yin, J.; Jia, J.; Aggarwal, H.; Emwas, A.-H.; Mohammed, O.F.; Eddaoudi, M. Unprecedented ultralow detection limit of amines using a thiadiazole-functionalized Zr(IV)-based metal–organic framework. J. Am. Chem. Soc. 2019, 141, 7245–7249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlov, D.I.; Sukhikh, T.S.; Ryadun, A.A.; Matveevskaya, V.V.; Kovalenko, K.A.; Benassi, E.; Fedin, V.P.; Potapov, A.S.A. Luminescent 2,1,3-benzoxadiazole-decorated zirconium-organic framework as an exceptionally sensitive turn-on sensor for ammonia and aliphatic amines in water. J. Mater. Chem. C 2022, 10, 5567–5575. [Google Scholar] [CrossRef]
- Fan, W.; Cheng, Y.; Zhao, H.; Yang, S.; Wang, L.; Zheng, L.; Cao, Q.; Fan, W.; Cheng, Y.; Zhao, H.; et al. A turn-on NIR fluorescence sensor for gossypol based on Yb-based metal-organic framework. Talanta 2022, 238, 123030. [Google Scholar] [CrossRef] [PubMed]
- Zongsu, H.; Kunyu, W.; Yinlin, C.; Jiangnan, L.; Teat, S.J.; Sihai, Y.; Wei, S.; Peng, C. A multicenter metal–organic framework for quantitative detection of multicomponent organic mixtures. CCS Chem. 2022, 4, 3238–3245. [Google Scholar]
- Jia, Z.; Han, Z.; Wang, K.; Zhou, T.; Min, H.; Sun, T.; Liao, Y.; Wang, L.; Cheng, P.; Shi, W. An efficient, multiplexed strategy for instant detection of bacterial biomarker by a lanthanide–organic material. Inorg. Chem. 2022, 61, 14313–14321. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Matveevskaya, V.; Pavlov, D.; Yakunenkov, A.; Potapov, A. Coordination polymers based on highly emissive ligands: Synthesis and functional properties. Materials 2020, 13, 2699. [Google Scholar] [CrossRef]
- Tian, X.M.; Yao, S.L.; Qiu, C.Q.; Zheng, T.F.; Chen, Y.Q.; Huang, H.; Chen, J.L.; Liu, S.J.; Wen, H.R. Turn-on luminescent sensor toward Fe3+, Cr3+, and Al3+ based on a Co(II) metal-organic framework with open functional sites. Inorg. Chem. 2020, 59, 2803–2810. [Google Scholar] [CrossRef]
- Committee, A.M. Recommendations for the definition, estimation and use of the detection limit. Analyst 1987, 112, 199–204. [Google Scholar]
- CrysAlisPro; Version 1.171.38.46; Rigaku Oxford Diffraction: The Woodlands, TX, USA, 2015.
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spek, A.L. Platon squeeze: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C 2015, 71, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Rev. D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: A critical analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef] [Green Version]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z = 11–18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Clark, T.; Chandrasekhar, J.; Spitznagel, G.W.; Schleyer, P.V.R. Efficient diffuse function-augmented basis sets for anion calculations. III. The 3-21+G basis set for first-row elements, Li–F. J. Comput. Chem. 1983, 4, 294–301. [Google Scholar] [CrossRef]
- Frisch, M.J.; Pople, J.A.; Binkley, J.S. Self-consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J. Chem. Phys. 1984, 80, 3265–3269. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Barthel, S.; Alexandrov, E.V.; Proserpio, D.M.; Smit, B. Distinguishing metal–organic frameworks. Cryst. Growth Des. 2018, 18, 1738–1747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlov, D.I.; Ryadun, A.A.; Potapov, A.S. A Zn(II)-based sql type 2D coordination polymer as a highly sensitive and selective turn-on fluorescent probe for Al3+. Molecules 2021, 26, 7392. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Xia, H.-L.; Zhou, K.; Wu, S.; Liu, X.-Y.; Wang, X.; Li, J. Tuning and directing energy transfer in the whole visible spectrum through linker installation in metal–organic frameworks. Angew. Chem. Int. Ed. 2021, 60, 25048–25054. [Google Scholar] [CrossRef]
- Hu, X.-T.; Yin, Z.; Luo, X.-P.; Shen, C.-H.; Zeng, M.-H. Acid and alkalinity stable pillared-layer and fluorescent zinc(II) metal–organic framework for selective sensing of Fe3+ ions in aqueous solution. Inorg. Chem. Commun. 2021, 129, 108664. [Google Scholar] [CrossRef]
- Zhao, D.; Yue, D.; Jiang, K.; Cui, Y.; Zhang, Q.; Yang, Y.; Qian, G. Ratiometric dual-emitting MOF⊃dye thermometers with a tunable operating range and sensitivity. J. Mater. Chem. C 2017, 5, 1607–1613. [Google Scholar] [CrossRef]
- Jin, J.K.; Wu, K.; Liu, X.Y.; Huang, G.Q.; Huang, Y.L.; Luo, D.; Xie, M.; Zhao, Y.; Lu, W.; Zhou, X.P.; et al. Building a pyrazole–benzothiadiazole–pyrazole photosensitizer into metal–organic frameworks for photocatalytic aerobic oxidation. J. Am. Chem. Soc. 2021, 143, 21340–21349. [Google Scholar] [CrossRef]
- Chai, B.-L.; Yao, S.-L.; Xie, X.; Xu, H.; Zheng, T.-F.; Li, J.-Y.; Chen, J.-L.; Liu, S.-J.; Wen, H.-R. Luminescent metal–organic framework-based fluorescence turn-on and red-shift sensor toward Al3+ and Ga3+: Experimental study and DFT calculation. Cryst. Growth Des. 2022, 22, 277–284. [Google Scholar] [CrossRef]
- Li, Y.; Chai, B.-L.; Xu, H.; Zheng, T.-F.; Chen, J.-L.; Liu, S.-J.; Wen, H.-R. Temperature- and solvent-induced reversible single-crystal-to-single-crystal transformations of TbIII-based MOFs with excellent stabilities and fluorescence sensing properties toward drug molecules. Inorg. Chem. Front. 2022, 9, 1504–1513. [Google Scholar] [CrossRef]
- Yao, S.L.; Xiong, Y.C.; Tian, X.M.; Liu, S.J.; Xu, H.; Zheng, T.F.; Chen, J.L.; Wen, H.R. A multifunctional benzothiadiazole-based fluorescence sensor for Al3+, Cr3+ and Fe3+. CrystEngComm 2021, 23, 1898–1905. [Google Scholar] [CrossRef]
- Li, L.Q.; Yao, S.L.; Tian, X.M.; Zheng, T.F.; Li, J.Y.; Liu, S.J.; Chen, J.L.; Yu, M.H.; Wen, H.R. A fluorescence red-shift and turn-on sensor for acetylacetone derived from ZnII-based metal-organic framework with new topology. CrystEngComm 2021, 23, 2532–2537. [Google Scholar] [CrossRef]
- Li, J.; Yao, S.-L.; Zheng, T.-F.; Xu, H.; Li, J.-Y.; Peng, Y.; Chen, J.-L.; Liu, S.-J.; Wen, H.-R. Turn-on and blue-shift fluorescence sensor toward l-histidine based on stable CdII metal-organic framework with tetranuclear cluster units. Dalton Trans. 2022, 51, 5983–5988. [Google Scholar] [CrossRef]
- He, Q.Q.; Yao, S.L.; Zheng, T.F.; Xu, H.; Liu, S.J.; Chen, J.L.; Li, N.; Wen, H.R. A multi-responsive luminescent sensor based on a stable Eu(iii) metal-organic framework for sensing Fe3+, MnO4−, and Cr2O72− in aqueous solutions. CrystEngComm 2022, 24, 1041–1048. [Google Scholar] [CrossRef]
- Wang, Y.-M.; Liu, C.; Zhi, H.; Zhang, X.; Xu, Y.; Wang, Y.; Yang, R.; Yin, X.-B. Thiadiazole-functionalized metal-organic frameworks multifunction-architectonics for dual-target sensing of ethylamine and gossypol. Chem. Eng. J. 2022, 441, 136049. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, J.; Fan, R.; Zhou, X.; Zhu, K.; Yang, Y. Multiple interpenetrating metal-organic frameworks with channel-size-dependent behavior for selective gossypol detection and perovskite quantum dot encapsulation. ACS Appl. Mater. Interfaces 2022, 14, 49945–49956. [Google Scholar] [CrossRef]
- Van De Weert, M.; Stella, L. Fluorescence quenching and ligand binding: A critical discussion of a popular methodology. J. Mol. Struct. 2011, 998, 144–150. [Google Scholar] [CrossRef]
- Keizer, J. Nonlinear fluorescence quenching and the origin of positive curvature in Stern-Volmer plots. J. Am. Chem. Soc. 1983, 105, 1494–1498. [Google Scholar] [CrossRef]
- Santra, A.; Francis, M.; Parshamoni, S.; Konar, S. Nanoporous Cu(I) metal–organic framework: Selective adsorption of benzene and luminescence sensing of nitroaromatics. ChemistrySelect 2017, 2, 3200–3206. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlov, D.I.; Yu, X.; Ryadun, A.A.; Fedin, V.P.; Potapov, A.S. Luminescent Metal-Organic Framework with 2,1,3-Benzothiadiazole Units for Highly Sensitive Gossypol Sensing. Chemosensors 2023, 11, 52. https://doi.org/10.3390/chemosensors11010052
Pavlov DI, Yu X, Ryadun AA, Fedin VP, Potapov AS. Luminescent Metal-Organic Framework with 2,1,3-Benzothiadiazole Units for Highly Sensitive Gossypol Sensing. Chemosensors. 2023; 11(1):52. https://doi.org/10.3390/chemosensors11010052
Chicago/Turabian StylePavlov, Dmitry I., Xiaolin Yu, Alexey A. Ryadun, Vladimir P. Fedin, and Andrei S. Potapov. 2023. "Luminescent Metal-Organic Framework with 2,1,3-Benzothiadiazole Units for Highly Sensitive Gossypol Sensing" Chemosensors 11, no. 1: 52. https://doi.org/10.3390/chemosensors11010052
APA StylePavlov, D. I., Yu, X., Ryadun, A. A., Fedin, V. P., & Potapov, A. S. (2023). Luminescent Metal-Organic Framework with 2,1,3-Benzothiadiazole Units for Highly Sensitive Gossypol Sensing. Chemosensors, 11(1), 52. https://doi.org/10.3390/chemosensors11010052