Electrochemiluminescence Detection and Imaging of Biomolecules at the Single-Cell Level
Abstract
:1. Introduction
| Method | Advantages | Disadvantages |
|---|---|---|
| ECL | High sensitivity Low background emission Restricted information on the cell-based membrane | Restricted on the electrode surface Weak luminescence intensity Low spatial–temporal resolution |
| Fluorescence | Strong fluorescence emission High spatial–temporal resolution Excellent biocompatibility | High background noise Fluorescence quenching |
2. ECL Measurement of Biomolecules at the Single-Cell Level
3. ECL Imaging of Biomolecules at the Single-Cell Level
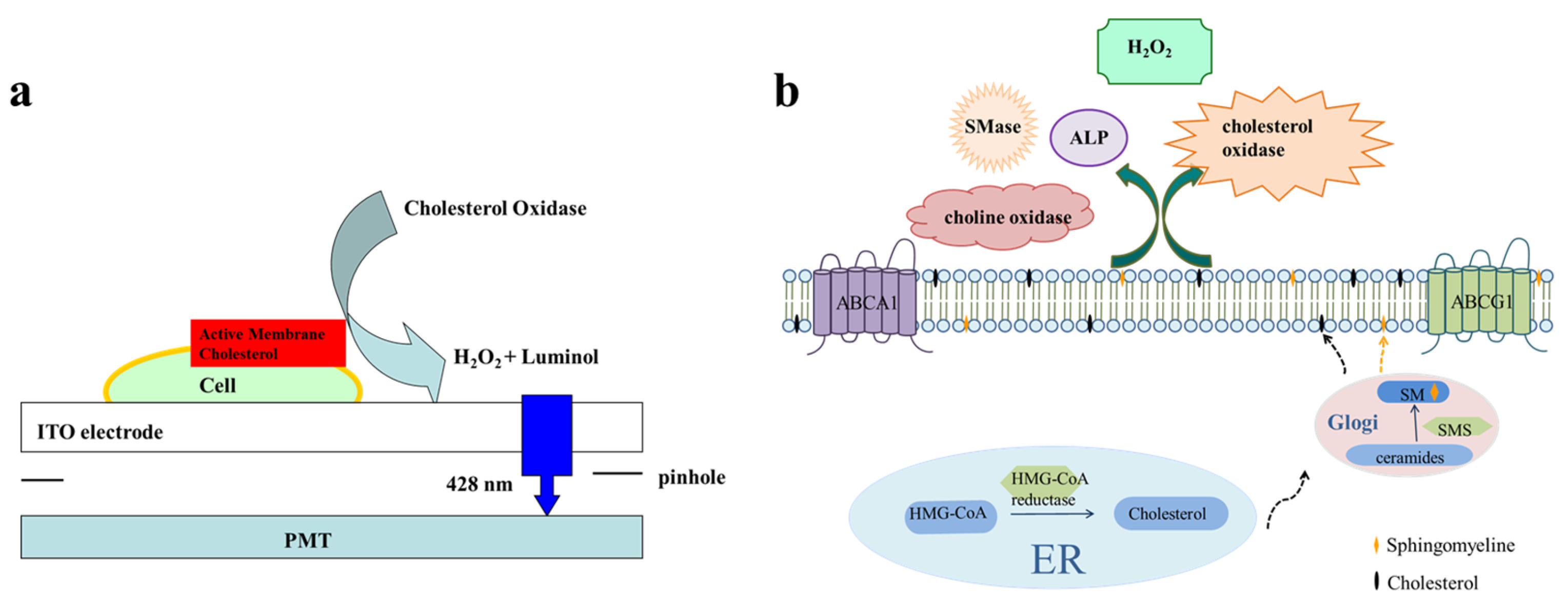
3.1. Imaging of Small Molecules at the Single-Cell Level

3.2. ECL Imaging of Membrane Proteins
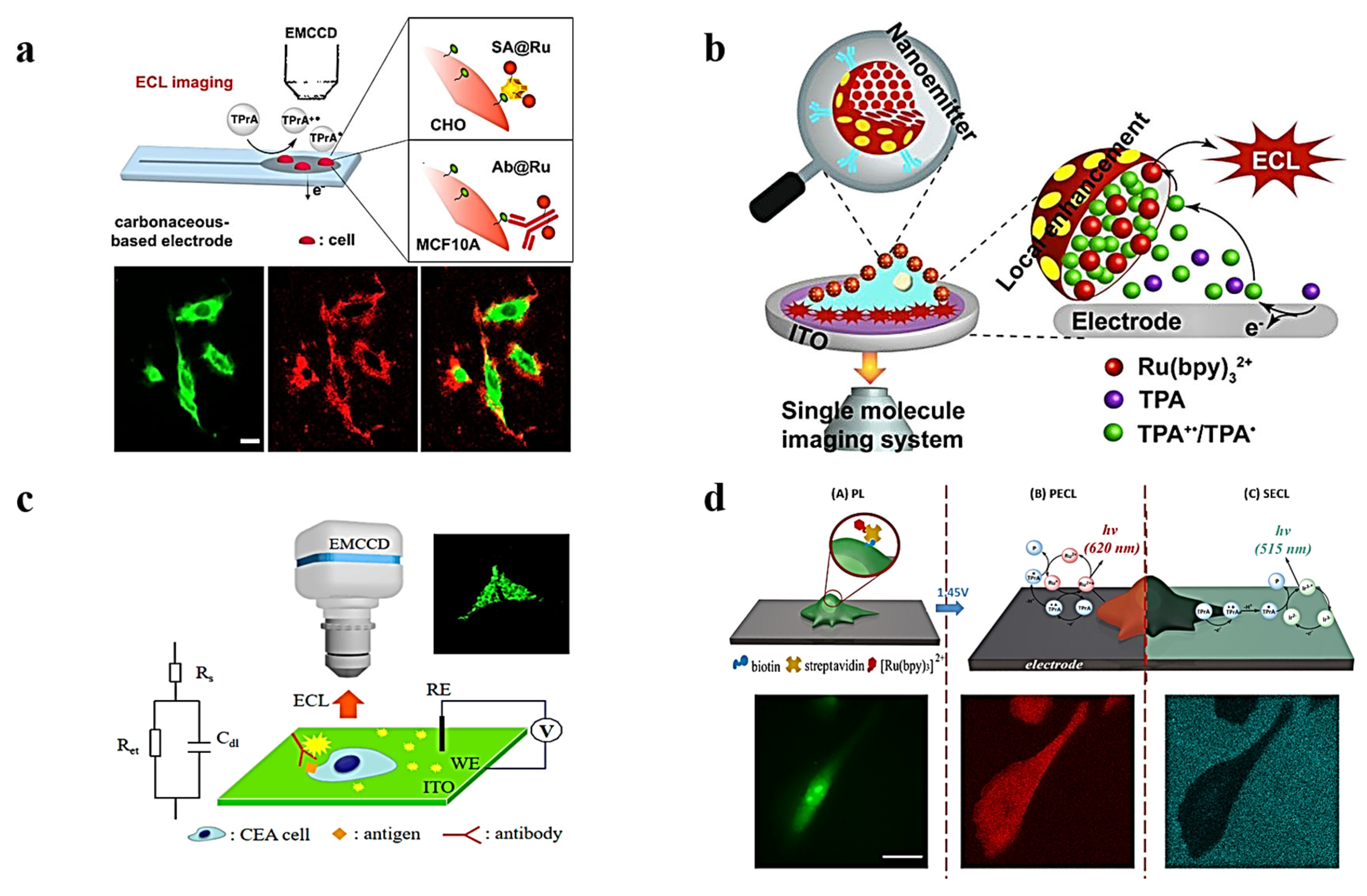
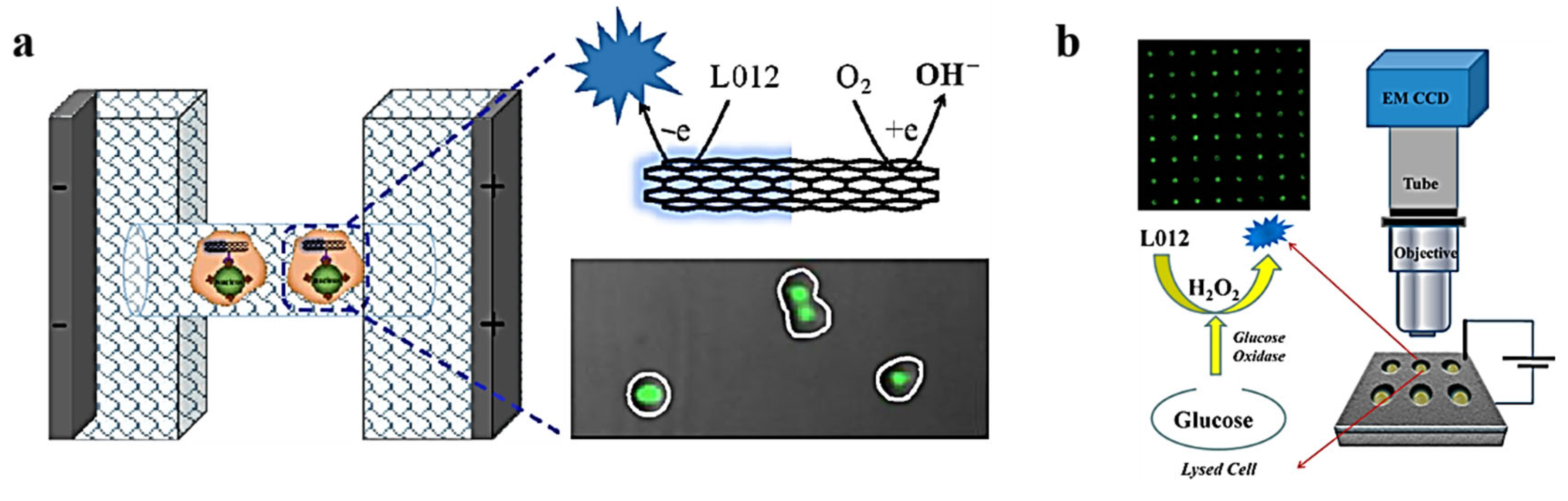
3.3. ECL Imaging of Intracellular Molecules
| Single-Cell ECL Method | Category | Luminophore | Cell Type | Target | References |
|---|---|---|---|---|---|
| ECL Measurement | small molecules | Luminol | Raw264.7 | Cholesterol | [43] |
| macromolecule | ZnCQDs | MCF-7, MDA-MB-231 | CD44 | [45] | |
| Au@Cu-PbCQD | MCF-7 | CD44 | [46] | ||
| ECL Imaging | small molecules | L-012 | Hela | Cholesterol | [70,77] |
| (Ru(bpy)32+ | PC12 | Dopamine | [71,72] | ||
| Pdots | PC12 | Dopamine | [78] | ||
| L-012 | MCF-7, Hela | H2O2 | [73,74,75] | ||
| L-012 | HMSC-BM, MCF-7 | O2 | [76] | ||
| membrane proteins | (Ru(bpy)32+ | CHO-K1 | EGFR | [83] | |
| (Ru(bpy)32+ | L-02, MCF-7 | CK19 | [85] | ||
| (Ru(bpy)32+ | MCF-7 | EpCAM | [86] | ||
| (Ru(bpy)32+ | Hela, Ramos, CCRF-CEM | PTK7 | [87] | ||
| (Ru(bpy)32+ | MCF-7 | CEA | [89] | ||
| L-012 | MCF-7 | CEA | [91] | ||
| TEA-Pdots | SK-BR-3 | HER2 | [90] | ||
| L-012 | MCF-7 | EGFR, PS | [97] | ||
| (Ru(bpy)32+ | apoptotic | PS | [98] | ||
| intracellular molecules | Luminol | Hela | H2O2 | [100] | |
| L-012 | Hela | Glucose, H2O2 | [101] | ||
| Luminol | Hela | miRNA-21 | [102] | ||
| L-012 | MCF-7 | KDM1/LSD1 | [104] | ||
| L-012 | Hela | Glucose | [105] |
4. Summary and Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miao, W. Electrogenerated chemiluminescence and its biorelated applications. Chem. Rev. 2008, 108, 2506–2553. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Xu, G. Applications and trends in electrochemiluminescence. Chem. Soc. Rev. 2010, 39, 3275–3304. [Google Scholar] [CrossRef] [PubMed]
- Abdussalam, A.; Xu, G. Recent advances in electrochemiluminescence luminophores. Anal. Bioanal. Chem. 2022, 414, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Zanut, A.; Fiorani, A.; Canola, S.; Saito, T.; Ziebart, N.; Rapino, S.; Rebeccani, S.; Barbon, A.; Irie, T.; Josel, H.P.; et al. Insights into the mechanism of coreactant electrochemiluminescence facilitating enhanced bioanalytical performance. Nat. Commun. 2020, 11, 2668. [Google Scholar] [CrossRef]
- Dufford, R.T.; Nightingale, D.; Gaddum, L.W. Luminescence of gridnard compounds in electric and magnetic fields, and related electrical phenomena. J. Am. Chem. Soc. 1927, 49, 1858–1864. [Google Scholar] [CrossRef]
- Harvey, N. Luminescence during Electrolysis. J. Phys. Chem. 1929, 33, 1456. [Google Scholar] [CrossRef]
- Hercules, D.M. Chemiluminescence Resulting from Electrochemically Generated Species. Science 1964, 145, 808–809. [Google Scholar] [CrossRef] [PubMed]
- Santhanam, K.S.V.; Bard, A.J. Chemiluminescence of electrogenerated 9,10-Diphenylanthracene anion radical. J. Am. Chem. Soc. 1965, 87, 139–140. [Google Scholar] [CrossRef]
- Ma, X.; Gao, W.; Du, F.; Yuan, F.; Yu, J.; Guan, Y.; Sojic, N.; Xu, G. Rational Design of Electrochemiluminescent Devices. Acc. Chem. Res. 2021, 54, 2936–2945. [Google Scholar] [CrossRef]
- Zanut, A.; Palomba, F.; Rossi Scota, M.; Rebeccani, S.; Marcaccio, M.; Genovese, D.; Rampazzo, E.; Valenti, G.; Paolucci, F.; Prodi, L. Dye-Doped Silica Nanoparticles for Enhanced ECL-Based Immunoassay Analytical Performance. Angew. Chem. Int. Ed. 2020, 59, 21858–21863. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, S.; Tan, L.; Tan, Y.; Wang, Y.; Ye, Z.; Hou, C.; Xu, Y.; Liu, S.; Wang, G. Frontier and hot topics in electrochemiluminescence sensing technology based on CiteSpace bibliometric analysis. Biosens. Bioelectron. 2022, 201, 113932. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.M.; Xu, C.H.; Zhao, W.; Chen, H.Y.; Xu, J.J. Single Cell Imaging of Electrochemiluminescence-Driven Photodynamic Therapy. Angew. Chem. Int. Ed. 2022, 61, e202117401. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.M.; Jeon, Y.M.; Heo, S.Y. Electrochemiluminescence Systems for the Detection of Biomarkers: Strategical and Technological Advances. Biosensors 2022, 12, 738. [Google Scholar] [CrossRef] [PubMed]
- Gou, X.; Xing, Z.; Ma, C.; Zhu, J.J. A Close Look at Mechanism, Application, and Opportunities of Electrochemiluminescence Microscopy. Chem. Biomed. Imaging 2023, 1, 414–433. [Google Scholar] [CrossRef]
- Guo, M.; Du, D.; Wang, J.; Ma, Y.; Yang, D.; Haghighatbin, M.A.; Shu, J.; Nie, W.; Zhang, R.; Bian, Z.; et al. Three-Biomarker Joint Strategy for Early and Accurate Diagnosis of Acute Myocardial Infarction via a Multiplex Electrochemiluminescence Immunoarray Coupled with Robust Machine Learning. Chem. Biomed. Imaging 2023, 1, 179–185. [Google Scholar] [CrossRef]
- Trad, F.B.; Delacotte, J.; Guille-Collignon, M.; Lemaître, F.; Arbault, S.; Sojic, N.; Burlina, F.; Labbé, E.; Burie, O. Electrochemiluminescence Imaging of Liposome Permeabilization by an Antimicrobial Peptide: Melittin. Chem. Biomed. Imaging 2023, 1, 58–65. [Google Scholar] [CrossRef]
- Zhu, W.; Dong, J.; Ruan, G.; Zhou, Y.; Feng, J. Quantitative Single-Molecule Electrochemiluminescence Bioassay. Angew. Chem. Int. Ed. 2023, 62, e202214419. [Google Scholar] [CrossRef]
- Hoebe, R.A.; Van Oven, C.H.; Gadella, T.W., Jr.; Dhonukshe, P.B.; Van Noorden, C.J.; Manders, E.M. Controlled light-exposure microscopy reduces photobleaching and phototoxicity in fluorescence live-cell imaging. Nat. Biotechnol. 2007, 25, 249–253. [Google Scholar] [CrossRef]
- Liu, Z.; Qi, W.; Xu, G. Recent advances in electrochemiluminescence. Chem. Soc. Rev. 2015, 44, 3117–3142. [Google Scholar] [CrossRef]
- Forster, R.J.; Bertoncello, P.; Keyes, T.E. Electrogenerated chemiluminescence. Annu. Rev. Anal. Chem. 2009, 2, 359–385. [Google Scholar] [CrossRef]
- Richter, M.M. Electrochemiluminescence (ECL). Chem. Rev. 2004, 104, 3003–3036. [Google Scholar] [CrossRef]
- Mostafa, I.M.; Abdussalam, A.; Zholudov, Y.T.; Snizhko, D.V.; Zhang, W.; Hosseini, M.; Guan, Y.; Xu, G. Recent Applications and Future Perspectives of Chemiluminescent and Bioluminescent Imaging Technologies. Chem. Biomed. Imaging 2023, 1, 297–314. [Google Scholar] [CrossRef]
- Wang, S.; Ren, W.X.; Hou, J.T.; Won, M.; An, J.; Chen, X.; Shu, J.; Kim, J.S. Fluorescence imaging of pathophysiological microenvironments. Chem. Soc. Rev. 2021, 50, 8887–8902. [Google Scholar] [CrossRef] [PubMed]
- Elowitz, M.B.; Levine, A.J.; Siggia, E.D.; Swain, P.S. Stochastic gene expression in a single cell. Science 2002, 297, 1183–1186. [Google Scholar] [CrossRef] [PubMed]
- Keller, L.; Pantel, K. Unravelling tumour heterogeneity by single-cell profiling of circulating tumour cells. Nat. Rev. Cancer 2019, 19, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Pollen, A.A.; Nowakowski, T.J.; Shuga, J.; Wang, X.; Leyrat, A.A.; Lui, J.H.; Li, N.; Szpankowski, L.; Fowler, B.; Chen, P.; et al. Low-coverage single-cell mRNA sequencing reveals cellular heterogeneity and activated signaling pathways in developing cerebral cortex. Nat. Biotechnol. 2014, 32, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Chen, T. Probing Oxidant Effects on Superoxide Dismutase 1 Oligomeric States in Live Cells Using Single-Molecule Fluorescence Anisotropy. Chem. Biomed. Imaging 2023, 1, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Stuart, T.; Satija, R. Integrative single-cell analysis. Nat. Rev. Genet. 2019, 20, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Brandman, O.; Ferrell, J.E., Jr.; Li, R.; Meyer, T. Interlinked fast and slow positive feedback loops drive reliable cell decisions. Science 2005, 310, 496–498. [Google Scholar] [CrossRef]
- Piwecka, M.; Rajewsky, N.; Rybak-Wolf, A. Single-cell and spatial transcriptomics: Deciphering brain complexity in health and disease. Nat. Rev. Neurol. 2023, 19, 346–362. [Google Scholar] [CrossRef]
- Labib, M.; Kelley, S.O. Single-cell analysis targeting the proteome. Nat. Rev. Chem. 2020, 4, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Rush, C.; Tingey, M.; Junod, S.; Yang, W. Application of Super-resolution SPEED Microscopy in the Study of Cellular Dynamics. Chem. Biomed. Imaging 2023, 1, 356–371. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zhang, L.; Zhang, Y.; Li, Z.; Siemers, N.; Zhang, Z. Insights Gained from Single-Cell Analysis of Immune Cells in the Tumor Microenvironment. Annu. Rev. Immunol. 2021, 39, 583–609. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, S.; Bouffier, L.; Liu, B.; Jiang, D.; Sojic, N. Electrochemiluminescence microscopy: From single objects to living cells. Curr. Opin. Electrochem. 2022, 35, 101096. [Google Scholar] [CrossRef]
- Rebeccani, S.; Zanut, A.; Santo, C.I.; Valenti, G.; Paolucci, F. A Guide Inside Electrochemiluminescent Microscopy Mechanisms for Analytical Performance Improvement. Anal. Chem. 2022, 94, 336–348. [Google Scholar] [CrossRef] [PubMed]
- He, Q.-N.; Ma, Z.-Y.; Yang, Y.-X.; Xu, C.-H.; Zhao, W. Recent Advances in Electrochemiluminescence-Based Single-Cell Analysis. Chemosensors 2023, 11, 281. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y. Electrochemiluminescence Single-cell Analysis on Nanostructured Interface. Electroanalysis 2022, 34, 937. [Google Scholar]
- Ding, H.; Su, B.; Jiang, D. Recent Advances in Single Cell Analysis by Electrochemiluminescence. ChemistryOpen 2023, 12, e202200113. [Google Scholar] [CrossRef]
- Dong, J.; Feng, J. Electrochemiluminescence from Single Molecule to Imaging. Anal. Chem. 2023, 95, 374–387. [Google Scholar] [CrossRef]
- Yang, Q.; Huang, X.; Gao, B.; Gao, L.; Yu, F.; Wang, F. Advances in electrochemiluminescence for single-cell analysis. Analyst 2022, 148, 9–25. [Google Scholar] [CrossRef]
- Meng, C.; Knežević, S.; Du, F.X.; Guan, Y.R.; Kanoufi, F.; Sojic, N.; Xu, G.B. Recent advances in electrochemiluminescence imaging analysis. eScience 2022, 2, 591–605. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, C.; Xu, Q.; Zhu, J.J. Recent progress in electrochemiluminescence microscopy analysis of single cells. Analyst 2022, 147, 2884–2894. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Zhou, J.; Tian, C.; Jiang, D.; Fang, D.; Chen, H. Luminol electrochemiluminescence for the analysis of active cholesterol at the plasma membrane in single mammalian cells. Anal. Chem. 2013, 85, 3912–3917. [Google Scholar] [CrossRef]
- Huang, S.; Liu, K.; Jiang, D.; Fang, D. Codetermination of Sphingomyelin and Cholesterol in Cellular Plasma Membrane in Sphingomyelin-Depletion-Induced Cholesterol Efflux. Anal. Chem. 2019, 91, 1501–1506. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Zhou, B.; Yang, X.; Long, D.; Hao, Y.; Yang, P. Novel Single-Cell Analysis Platform Based on a Solid-State Zinc-Coadsorbed Carbon Quantum Dots Electrochemiluminescence Probe for the Evaluation of CD44 Expression on Breast Cancer Cells. ACS Appl. Mater. Interfaces 2017, 9, 16848–16856. [Google Scholar] [CrossRef] [PubMed]
- Long, D.; Chen, C.; Cui, C.; Yao, Z.; Yang, P. A high precision MUA-spaced single-cell sensor for cellular receptor assay based on bifunctional Au@Cu-PbCQD nanoprobes. Nanoscale 2018, 10, 18597–18605. [Google Scholar] [CrossRef]
- Dong, S.; Gao, X.; Fu, L.; Jia, J.; Zou, G. Low-Triggering-Potential Electrochemiluminescence from Surface-Confined CuInS2@ZnS Nanocrystals and their Biosensing Applications. Anal. Chem. 2021, 93, 12250–12256. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhao, L.; Zhang, N.; Liu, L.; Ren, X.; Ma, H.; Kuang, X.; Li, Y.; Luo, C.; Wei, Q. Ultrasensitive Electrochemiluminescence Biosensor with Silver Nanoclusters as a Novel Signal Probe and α-Fe2O3-Pt as an Efficient Co-reaction Accelerator for Procalcitonin Immunoassay. Anal. Chem. 2023, 95, 1582–1588. [Google Scholar] [CrossRef]
- Liu, L.; Yang, A.; Luo, W.; Liu, H.; Liu, X.; Zhao, W. Ultrasensitive detection of cyclin D1 by a self-enhanced ECL immunosensor based on Bi2S3 quantum dots. Analyst 2021, 146, 2057–2064. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, G.; Gao, C.; Prudnikau, A.; Hübner, R.; Zhan, J.; Zou, G.; Eychmüller, A.; Cai, B. Interparticle Charge-Transport-Enhanced Electrochemiluminescence of Quantum-Dot Aerogels. Angew. Chem. Int. Ed. 2023, 62, e202214487. [Google Scholar] [CrossRef]
- Yang, Y.T.; Liu, J.L.; Sun, M.F.; Yuan, R.; Chai, Y.Q. Highly Efficient Electrochemiluminescence of MnS:CdS@ZnS Core-Shell Quantum Dots for Ultrasensitive Detection of MicroRNA. Anal. Chem. 2022, 94, 6874–6881. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, Y.L.; Lin, X.; Ma, S.H.; Cao, J.T.; Liu, Y.M. Cu-MOFs/GOx Bifunctional Probe-Based Synergistic Signal Amplification Strategy: Toward Highly Sensitive Closed Bipolar Electrochemiluminescence Immunoassay. ACS Appl. Mater. Interfaces 2023, 15, 22959–22966. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Huang, Z.; Sheng, Y.; Zhang, X.; Deng, H.; Chen, W.; Liu, J. Pre-oxidation of Gold Nanoclusters Results in a 66% Anodic Electrochemiluminescence Yield and Drives Mechanistic Insights. Angew. Chem. Int. Ed. 2019, 58, 11691–11694. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Huang, Z.; Deng, H.; Wu, W.; Huang, K.; Li, Z.; Chen, W.; Liu, J. Dual Enhancement of Gold Nanocluster Electrochemiluminescence: Electrocatalytic Excitation and Aggregation-Induced Emission. Angew. Chem. Int. Ed. 2020, 59, 9982–9985. [Google Scholar] [CrossRef] [PubMed]
- Hesari, M.; Ding, Z. Identifying Highly Photoelectrochemical Active Sites of Two Au21 Nanocluster Isomers toward Bright Near-Infrared Electrochemiluminescence. J. Am. Chem. Soc. 2021, 143, 19474–19485. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ding, J.; Zhou, P.; Liu, J.; Qiao, Z.; Yu, K.; Jiang, J.; Su, B. Electrochemiluminescence Distance and Reactivity of Coreactants Determine the Sensitivity of Bead-Based Immunoassays. Angew. Chem. Int. Ed. 2023, 62, e202216525. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Wu, Y.; Guo, L.; Li, L.; Zhou, M.; Li, X.; Liu, T.; Ding, Y.; Bu, H.; Xie, G.; et al. Self-assembly Induced Enhanced Electrochemiluminescence of Copper Nanoclusters Using DNA Nanoribbon Templates. Angew. Chem. Int. Ed. 2023, 62, e202300893. [Google Scholar] [CrossRef]
- Wu, Z.; Wen, J.; Qin, Y.; Ling, L.; Jiao, L.; Zhang, R.; Luo, Z.; Xi, M.; Hu, L.; Gu, W.; et al. Dual-Site Activation Coupling with a Schottky Junction Boosts the Electrochemiluminescence of Carbon Nitride. Angew. Chem. Int. Ed. 2023, 62, e202308257. [Google Scholar] [CrossRef]
- Han, D.; Goudeau, B.; Jiang, D.; Fang, D.; Sojic, N. Electrochemiluminescence Microscopy of Cells: Essential Role of Surface Regeneration. Anal. Chem. 2021, 93, 1652–1657. [Google Scholar] [CrossRef]
- Chong, Y.T.; Koh, J.L.; Friesen, H.; Duffy, S.K.; Cox, M.J.; Moses, A.; Moffat, J.; Boone, C.; Andrews, B.J. Yeast Proteome Dynamics from Single Cell Imaging and Automated Analysis. Cell 2015, 161, 1413–1424. [Google Scholar] [CrossRef]
- Valm, A.M.; Cohen, S.; Legant, W.R.; Melunis, J.; Hershberg, U.; Wait, E.; Cohen, A.R.; Davidson, M.W.; Betzig, E.; Lippincott-Schwartz, J. Applying systems-level spectral imaging and analysis to reveal the organelle interactome. Nature 2017, 546, 162–167. [Google Scholar] [CrossRef]
- Royce, C.; Kirk, W.; Scott, D. Characterization of electrode heterogeneity with electrogenerated chemiluminescence. Anal. Chem. 1987, 59, 670–673. [Google Scholar]
- Robert, J.; Richard, L.; Christine, M.; Royce, C. Observation of kinetic heterogeneity on highly ordered pyrolytic graphite using electrogenerated chemiluminescence. Anal. Chem. 1989, 61, 2763–2766. [Google Scholar]
- Shultz, L.L.; Stoyanoff, J.S.; Nieman, T.A. Temporal and Spatial Analysis of Electrogenerated Ru(bpy)33+ Chemiluminescent Reactions in Flowing Streams. Anal. Chem. 1996, 68, 349–354. [Google Scholar] [CrossRef]
- Zu, Y.; Ding, Z.; Zhou, J.; Lee, Y.; Bard, A.J. Scanning optical microscopy with an electrogenerated chemiluminescent light source at a nanometer tip. Anal. Chem. 2001, 73, 2153–2156. [Google Scholar] [CrossRef] [PubMed]
- Szunerits, S.; Tam, J.M.; Thouin, L.; Amatore, C.; Walt, D.R. Spatially resolved electrochemiluminescence on an array of electrode tips. Anal. Chem. 2003, 75, 4382–4388. [Google Scholar] [CrossRef] [PubMed]
- Amatore, C.; Pebay, C.; Servant, L.; Sojic, N.; Szunerits, S.; Thouin, L. Mapping electrochemiluminescence as generated at double-band microelectrodes by confocal microscopy under steady state. ChemPhysChem 2006, 7, 1322–1327. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, Y.; Wu, S.; Liu, X.; Su, B. Imaging latent fingerprints by electrochemiluminescence. Angew. Chem. Int. Ed. 2012, 51, 8068–8072. [Google Scholar] [CrossRef]
- Rigolot, V.; Biot, C.; Lion, C. To View Your Biomolecule, Click inside the Cell. Angew. Chem. Int. Ed. 2021, 60, 23084–23105. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, G.; Chen, Y.; Fang, D.; Jiang, D.; Chen, H.Y. Electrochemiluminescence imaging for parallel single-cell analysis of active membrane cholesterol. Anal. Chem. 2015, 87, 8138–8143. [Google Scholar] [CrossRef]
- Ding, H.; Guo, W.; Zhou, P.; Su, B. Nanocage-confined electrochemiluminescence for the detection of dopamine released from living cells. Chem. Commun. 2020, 56, 8249–8252. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Guo, W.; Ding, L.; Su, B. Confined Electrochemiluminescence at Microtube Electrode Ensembles for Local Sensing of Single Cells. Chin. J. Chem. 2021, 39, 2911–2916. [Google Scholar] [CrossRef]
- Liu, G.; Ma, C.; Jin, B.K.; Chen, Z.; Zhu, J.J. Direct Electrochemiluminescence Imaging of a Single Cell on a Chitosan Film Modified Electrode. Anal. Chem. 2018, 90, 4801–4806. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jin, R.; Chen, Y.; Fang, D.; Jiang, D. Enhanced electrochemiluminescence at single lithium iron phosphate nanoparticles for the local sensing of hydrogen peroxide efflux from single living cell under a low voltage. Sens. Actuators B 2021, 329, 129208. [Google Scholar] [CrossRef]
- Cui, C.; Chen, Y.; Jiang, D.; Chen, H.Y.; Zhang, J.; Zhu, J.J. Steady-State Electrochemiluminescence at Single Semiconductive Titanium Dioxide Nanoparticles for Local Sensing of Single Cells. Anal. Chem. 2019, 91, 1121–1125. [Google Scholar] [CrossRef] [PubMed]
- Hiramoto, K.; Ino, K.; Komatsu, K.; Nashimoto, Y.; Shiku, H. Electrochemiluminescence imaging of respiratory activity of cellular spheroids using sequential potential steps. Biosens. Bioelectron. 2021, 181, 113123. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Qin, Y.; Xia, J.; Jiang, D.; Chen, H.Y. C3N4 Nanosheet Modified Microwell Array with Enhanced Electrochemiluminescence for Total Analysis of Cholesterol at Single Cells. Anal. Chem. 2017, 89, 2216–2220. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ao, H.; Xiao, W.; Chen, W.; Li, G.; Wu, J.; Ju, H. Confined electrochemiluminescence imaging microarray for high-throughput biosensing of single cell-released dopamine. Biosens. Bioelectron. 2022, 201, 113959. [Google Scholar] [CrossRef]
- Poudineh, M.; Sargent, E.H.; Pantel, K.; Kelley, S.O. Profiling circulating tumour cells and other biomarkers of invasive cancers. Nat. Biomed. Eng. 2018, 2, 72–84. [Google Scholar] [CrossRef]
- Swiecicki, J.M.; Santana, J.T.; Imperiali, B. A Strategic Approach for Fluorescence Imaging of Membrane Proteins in a Native-like Environment. Cell Chem. Biol. 2020, 27, 245–251. [Google Scholar] [CrossRef]
- Xu, H.; Cai, M.; Gao, J.; Shi, Y.; Chen, J.; Wu, Q.; Zhang, J.; Jiang, J.; Wang, H. Membrane protein density determining membrane fusion revealed by dynamic fluorescence imaging. Talanta 2021, 226, 122091. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Rakhimbekova, A.; Duan, X.; Yin, Q.; Foss, C.A.; Fan, Y.; Xu, Y.; Li, X.; Cai, X.; Kutil, Z.; et al. A prostate-specific membrane antigen activated molecular rotor for real-time fluorescence imaging. Nat. Commun. 2021, 12, 5460. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhu, D.; Liao, Y.; Liu, W.; Liu, H.; Ma, Z.; Xing, D. Synthesis, labeling and bioanalytical applications of a tris(2,2’-bipyridyl)ruthenium(II)-based electrochemiluminescence probe. Nat. Protoc. 2014, 9, 1146–1159. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Choi, J.P.; Bard, A.J. Electrogenerated chemiluminescence 69: The tris(2,2’-bipyridine)ruthenium(II), (Ru(bpy)32+)/tri-n-propylamine (TPrA) system revisited-a new route involving TPrA*+ cation radicals. J. Am. Chem. Soc. 2002, 124, 14478–14485. [Google Scholar] [CrossRef] [PubMed]
- Sornambigai, M.; Bouffier, L.; Sojic, N.; Kumar, S.S. Tris(2,2’-bipyridyl)ruthenium (II) complex as a universal reagent for the fabrication of heterogeneous electrochemiluminescence platforms and its recent analytical applications. Anal. Bioanal. Chem. 2023, 415, 5875–5898. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Han, S.; Hu, L.; Parveen, S.; Xu, G. Coreactants of tris(2,2′-bipyridyl)ruthenium(II) Electrogenerated Chemiluminescence. Electrochim. Acta 2012, 82, 484–492. [Google Scholar] [CrossRef]
- Valenti, G.; Scarabino, S.; Goudeau, B.; Lesch, A.; Jović, M.; Villani, E.; Sentic, M.; Rapino, S.; Arbault, S.; Paolucci, F.; et al. Single Cell Electrochemiluminescence Imaging: From the Proof-of-Concept to Disposable Device-Based Analysis. J. Am. Chem. Soc. 2017, 139, 16830–16837. [Google Scholar] [CrossRef] [PubMed]
- Voci, S.; Goudeau, B.; Valenti, G.; Lesch, A.; Jović, M.; Rapino, S.; Paolucci, F.; Arbault, S.; Sojic, N. Surface-Confined Electrochemiluminescence Microscopy of Cell Membranes. J. Am. Chem. Soc. 2018, 140, 14753–14760. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Li, B.; Liu, J.; Jiang, D.; Liu, B.; Sojic, N. Single Biomolecule Imaging by Electrochemiluminescence. J. Am. Chem. Soc. 2021, 143, 17910–17914. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, X.; Wang, S.; Li, B.; Liu, B. Nanoconfinement-Enhanced Electrochemiluminescence for in Situ Imaging of Single Biomolecules. ACS Nano 2023, 17, 3809–3817. [Google Scholar] [CrossRef]
- Li, B.; Huang, X.; Lu, Y.; Fan, Z.; Li, B.; Jiang, D.; Sojic, N. High Electrochemiluminescence from Ru(bpy)32+ Embedded Metal-Organic Frameworks to Visualize Single Molecule Movement at the Cellular Membrane. Adv. Sci. 2022, 9, 2204715. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.T.; Wang, Y.L.; Zhang, J.J.; Dong, Y.X.; Liu, F.R.; Ren, S.W.; Liu, Y.M. Immuno-Electrochemiluminescent Imaging of a Single Cell Based on Functional Nanoprobes of Heterogeneous Ru(bpy)32+@SiO2/Au Nanoparticles. Anal. Chem. 2018, 90, 10334–10339. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gou, X.; Ma, C.; Jiang, D.; Zhu, J.J. A Synergistic Coreactant for Single-Cell Electrochemiluminescence Imaging: Guanine-Rich ssDNA-Loaded High-Index Faceted Gold Nanoflowers. Anal. Chem. 2021, 93, 7682–7689. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Gao, H.; Li, Y.; Li, G.; Chen, W.; Jin, Z.; Lei, J.; Wei, Q.; Ju, H. Dual Intramolecular Electron Transfer for In Situ Coreactant-Embedded Electrochemiluminescence Microimaging of Membrane Protein. Angew. Chem. Int. Ed. 2021, 60, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jin, R.; Jiang, D.; Chen, H.Y. Electrochemiluminescence-Based Capacitance Microscopy for Label-Free Imaging of Antigens on the Cellular Plasma Membrane. J. Am. Chem. Soc. 2019, 141, 10294–10299. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Colin, C.; Descamps, J.; Arbault, S.; Sojic, N. Shadow Electrochemiluminescence Microscopy of Single Mitochondria. Angew. Chem. Int. Ed. 2021, 60, 18742–18749. [Google Scholar] [CrossRef] [PubMed]
- Knežević, S.; Kerr, E.; Goudeau, B.; Valenti, G.; Paolucci, F.; Francis, P.S.; Kanoufi, F.; Sojic, N. Bimodal Electrochemiluminescence Microscopy of Single Cells. Anal. Chem. 2023, 95, 7372–7378. [Google Scholar] [CrossRef]
- Ding, H.; Guo, W.; Su, B. Imaging Cell-Matrix Adhesions and Collective Migration of Living Cells by Electrochemiluminescence Microscopy. Angew. Chem. Int. Ed. 2020, 59, 449–456. [Google Scholar] [CrossRef]
- Ding, H.; Zhou, P.; Fu, W.; Ding, L.; Guo, W.; Su, B. Spatially Selective Imaging of Cell-Matrix and Cell-Cell Junctions by Electrochemiluminescence. Angew. Chem. Int. Ed. 2021, 60, 11769–11773. [Google Scholar] [CrossRef]
- Qin, X.; Jin, H.J.; Li, X.; Li, J.; Pan, J.B.; Wang, K.; Liu, S.; Xu, J.J.; Xia, X.H. Label-Free Electrochemiluminescence Imaging of Single-Cell Adhesions by Using Bipolar Nanoelectrode Array. Chemistry 2022, 28, e202103964. [Google Scholar] [CrossRef]
- Liu, G.; Jin, B.K.; Ma, C.; Chen, Z.; Zhu, J.J. Potential-Resolved Electrochemiluminescence Nanoprobes for Visual Apoptosis Evaluation at Single-Cell Level. Anal. Chem. 2019, 91, 6363–6370. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wang, M.X.; Wei, H.F.; Wu, S.; Zhang, J.R.; Zhu, J.J.; Chen, Z. Catalytic route electrochemiluminescence microscopy of cell membranes with nitrogen-doped carbon dots as nano-coreactants. Chem. Commun. 2021, 57, 2168–2171. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Goudeau, B.; Manojlovic, D.; Jiang, D.; Fang, D.; Sojic, N. Electrochemiluminescence Loss in Photobleaching. Angew. Chem. Int. Ed. 2021, 60, 7686–7690. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Tang, H.; Jiang, D.; Chen, H.Y. Electrochemical Visualization of Intracellular Hydrogen Peroxide at Single Cells. Anal. Chem. 2016, 88, 2006–2009. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, R.; Sojic, N.; Jiang, D.; Chen, H.Y. Intracellular Wireless Analysis of Single Cells by Bipolar Electrochemiluminescence Confined in a Nanopipette. Angew. Chem. Int. Ed. 2020, 59, 10416–10420. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, W.; Liu, Y.; Sun, Y.; Jiang, Y.; Zhang, S. Electrochemiluminescence-Microscopy for microRNA Imaging in Single Cancer Cell Combined with Chemotherapy-Photothermal Therapy. Anal. Chem. 2019, 91, 12581–12586. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wu, S.; Zhou, Y.; Wei, H.F.; Zhang, J.; Chen, Z.; Zhu, J.J.; Lin, Y.; Zhu, W. Bio-Coreactant-Enhanced Electrochemiluminescence Microscopy of Intracellular Structure and Transport. Angew. Chem. Int. Ed. 2021, 60, 4907–4914. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, D.; Chen, H.Y. Wireless Electrochemical Visualization of Intracellular Antigens in Single Cells. CCS Chem. 2022, 4, 2221–2227. [Google Scholar] [CrossRef]
- Xu, J.; Huang, P.; Qin, Y.; Jiang, D.; Chen, H.Y. Analysis of Intracellular Glucose at Single Cells Using Electrochemiluminescence Imaging. Anal. Chem. 2016, 88, 4609–4612. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Zhu, J.J. Recent Advances in Electrochemiluminescence Analysis. Anal. Chem. 2017, 89, 358–371. [Google Scholar] [CrossRef]
- Bard, A.J. A life in electrochemistry. Annu. Rev. Anal. Chem. 2014, 7, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Y.; Yao, M.; Han, W.; Zhang, S. Cathodic Electrochemiluminesence Microscopy for Imaging of Single Carbon Nanotube and Nucleolin at Single Tumor Cell. Anal. Chem. 2023, 95, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Valenti, G.; Fiorani, A.; Li, H.; Sojic, N.; Paolucci, F. Essential Role of Electrode Materials in Electrochemiluminescence Applications. ChemElectroChem 2016, 3, 1990–1997. [Google Scholar] [CrossRef]
- Gao, W.; Liu, Y.; Zhang, H.; Wang, Z. Electrochemiluminescence Biosensor for Nucleolin Imaging in a Single Tumor Cell Combined with Synergetic Therapy of Tumor. ACS Sens. 2020, 5, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Ma, Q. Recent developments in electrochemiluminescence nanosensors for cancer diagnosis applications. Nanoscale 2020, 12, 13879–13898. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Zheng, Y.; Chen, R.; Zhou, Z.; Liu, S.; Shen, Y.; Zhang, Y. Advances in electrochemiluminescence luminophores based on small organic molecules for biosensing. Biosens. Bioelectron. 2023, 223, 115031. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.M.; Xu, C.H.; Zhao, W.; Chen, H.Y.; Xu, J.J. Super-Resolution Electrogenerated Chemiluminescence Microscopy for Single-Nanocatalyst Imaging. J. Am. Chem. Soc. 2021, 143, 18511–18518. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Lu, Y.; Xu, Y.; Chen, F.; Yang, J.; Chen, Y.; Feng, J. Direct imaging of single-molecule electrochemical reactions in solution. Nature 2021, 596, 244–249. [Google Scholar] [CrossRef]
- Zhao, T.; Zhou, Q.; Lv, Y.; Han, D.; Wu, K.; Zhao, L.; Shen, Y.; Liu, S.; Zhang, Y.J. Ultrafast Condensation of Carbon Nitride on Electrodes with Exceptional Boosted Photocurrent and Electrochemiluminescence. Angew. Chem. Int. Ed. 2020, 59, 1139–1143. [Google Scholar] [CrossRef]
- Gu, W.; Wang, H.; Jiao, L.; Wu, Y.; Chen, Y.; Hu, L.; Gong, J.; Du, D.; Zhu, C. Single-Atom Iron Boosts Electrochemiluminescence. Angew. Chem. Int. Ed. 2020, 59, 3534–3538. [Google Scholar] [CrossRef]
- Chen, M.M.; Zhao, W.; Zhu, M.J.; Li, X.L.; Xu, C.H.; Chen, H.Y.; Xu, J.J. Spatiotemporal imaging of electrocatalytic activity on single 2D gold nanoplates via electrogenerated chemiluminescence microscopy. Chem. Sci. 2019, 10, 4141–4147. [Google Scholar] [CrossRef]
- Guo, W.; Ding, H.; Zhou, P.; Wang, Y.; Su, B. Electrochemiluminescence Waveguide in Single Crystalline Molecular Wires. Angew. Chem. Int. Ed. 2020, 59, 6745–6749. [Google Scholar] [CrossRef]
- Gao, N.; Zeng, H.; Wang, X.; Zhang, Y.; Zhang, S.; Cui, R.; Zhang, M.; Mao, L. Graphdiyne: A New Carbon Allotrope for Electrochemiluminescence. Angew. Chem. Int. Ed. 2022, 61, e202204485. [Google Scholar] [CrossRef]
- Chinnadayyala, S.R.; Park, J.; Le, H.T.N.; Santhosh, M.; Kadam, A.N.; Cho, S. Recent advances in microfluidic paper-based electrochemiluminescence analytical devices for point-of-care testing applications. Biosens. Bioelectron. 2019, 126, 68–81. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Deng, Y.; Jiang, D.; Fang, D. Electrochemiluminescence Detection and Imaging of Biomolecules at the Single-Cell Level. Chemosensors 2023, 11, 538. https://doi.org/10.3390/chemosensors11100538
He X, Deng Y, Jiang D, Fang D. Electrochemiluminescence Detection and Imaging of Biomolecules at the Single-Cell Level. Chemosensors. 2023; 11(10):538. https://doi.org/10.3390/chemosensors11100538
Chicago/Turabian StyleHe, Xiaofan, Yufei Deng, Dechen Jiang, and Danjun Fang. 2023. "Electrochemiluminescence Detection and Imaging of Biomolecules at the Single-Cell Level" Chemosensors 11, no. 10: 538. https://doi.org/10.3390/chemosensors11100538
APA StyleHe, X., Deng, Y., Jiang, D., & Fang, D. (2023). Electrochemiluminescence Detection and Imaging of Biomolecules at the Single-Cell Level. Chemosensors, 11(10), 538. https://doi.org/10.3390/chemosensors11100538





