Electrochemical Sensor Based on Spent Coffee Grounds Hydrochar and Metal Nanoparticles for Simultaneous Detection of Emerging Contaminants in Natural Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Solutions and Reagents
2.3. Synthesis of the Hydrochar
2.4. Synthesis of the Hydrochar and Copper Nanoparticle Composites
2.5. Electrode Preparation
2.6. Sample Preparation and Analysis of HCS and BPA in Natural Water
3. Results
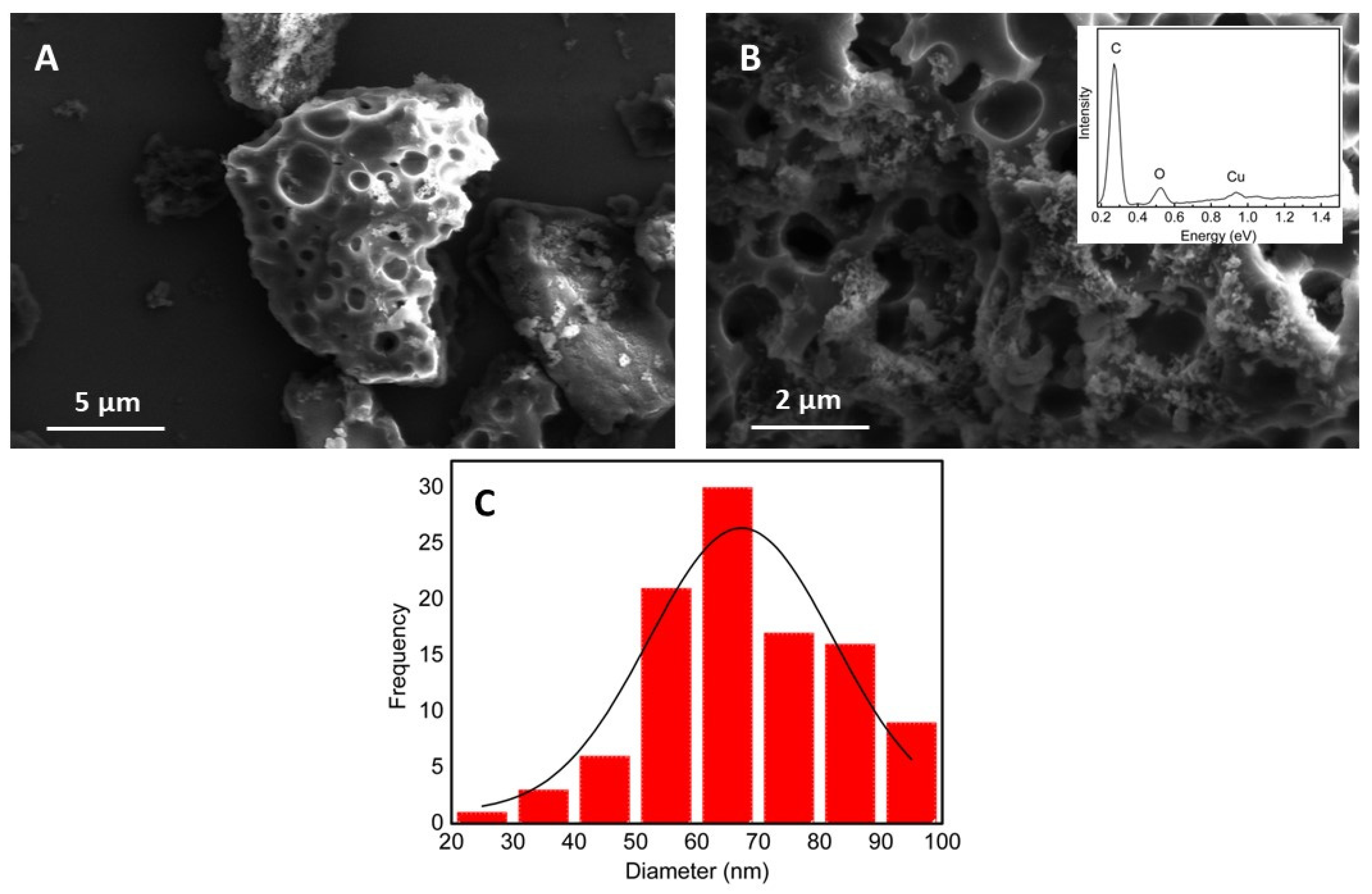
3.1. Morphological and Electrochemical Characterization of the Nanocomposites
3.2. Evaluation of Different Working Electrodes in Presence of a Redox Probe
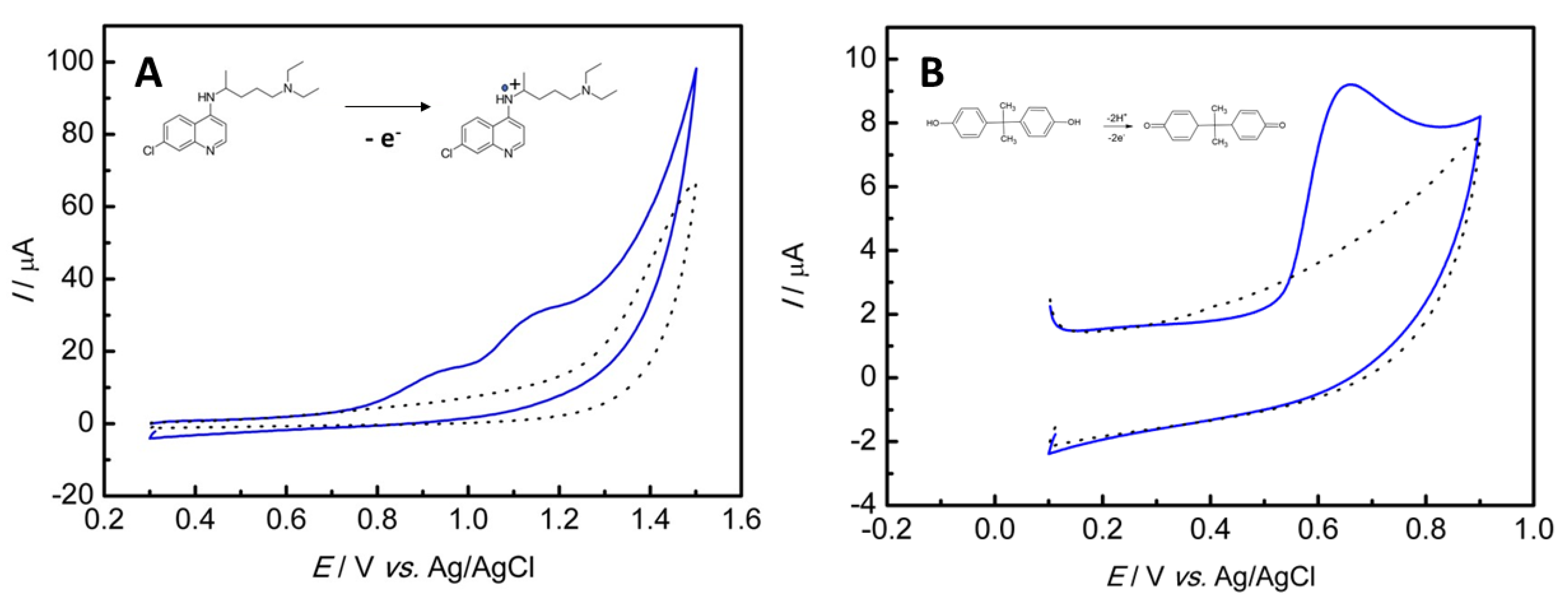
3.3. Electrochemical Oxidation of the HCS and BPA on the Nanocomposite
3.4. Optimization of Parameters
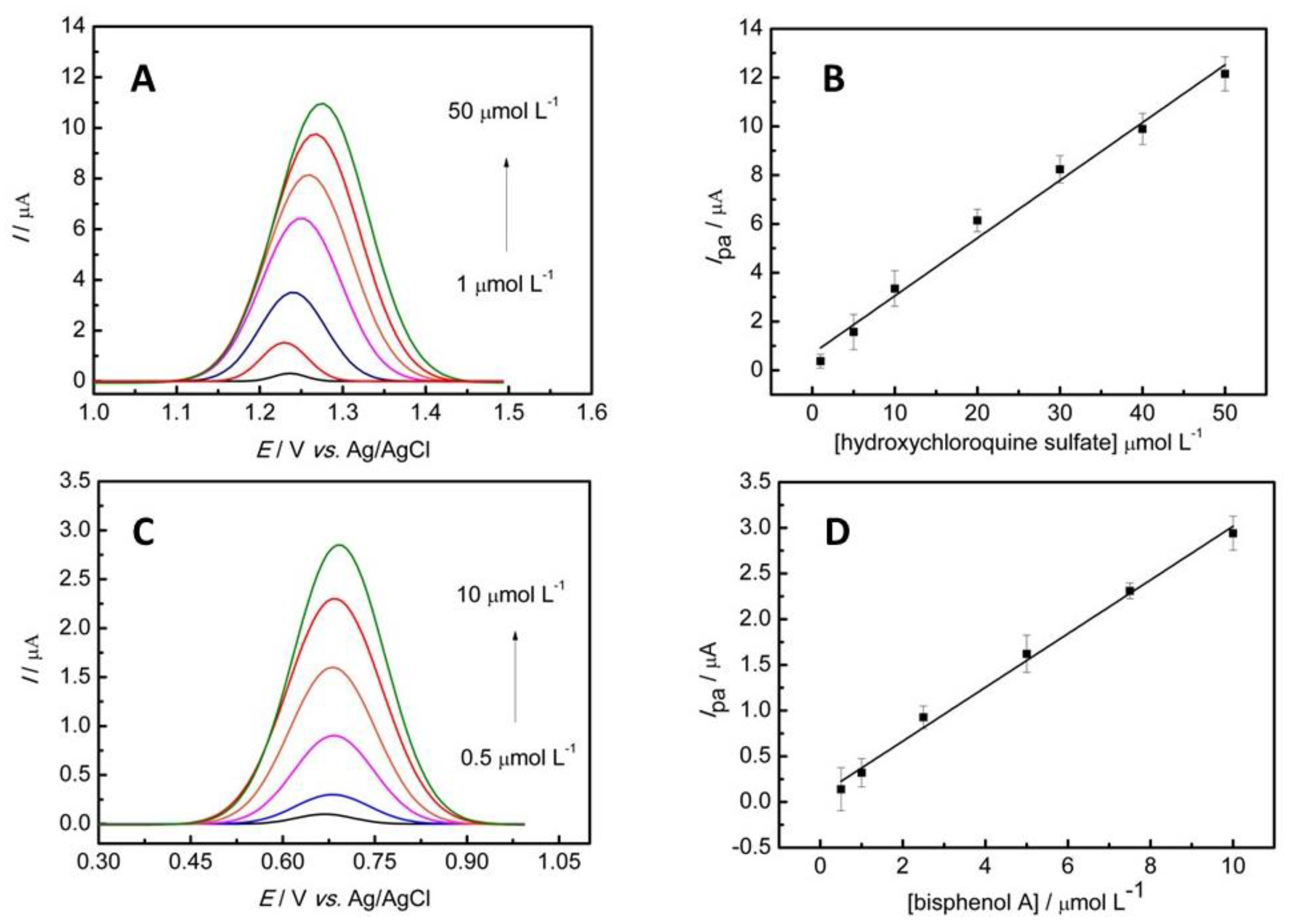
3.5. Analytical Characteristics
3.6. Simultaneous Determination of HCS and BPA in Natural Water
3.7. Simultaneous Determination of HCS and BPA in Natural Water in the Presence of Other Analytes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shahid, M.K.; Kashif, A.; Fuwad, A.; Choi, Y. Current Advances in Treatment Technologies for Removal of Emerging Contaminants from Water—A Critical Review. Coord. Chem. Rev. 2021, 442, 213993. [Google Scholar] [CrossRef]
- Montagner, C.; Sodré, F.; Acayaba, R.; Vidal, C.; Campestrini, I.; Locatelli, M.; Pescara, I.; Albuquerque, A.; Umbuzeiro, G.; Jardim, W. Ten Years-Snapshot of the Occurrence of Emerging Contaminants in Drinking, Surface and Ground Waters and Wastewaters from São Paulo State, Brazil. J. Braz. Chem. Soc. 2018, 30, 614–632. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, B.; Wu, P.; Lee, X.; Xing, Y.; Chen, M.; Gao, B. Adsorption of Emerging Contaminants from Water and Wastewater by Modified Biochar: A Review. Environ. Pollut. 2021, 273, 116448. [Google Scholar] [CrossRef] [PubMed]
- He, Q.G.; Huang, C.Y.; Chang, H.; Nie, L.B. Progress in Recycling of Plastic Packaging Wastes. Adv. Mater. Res. 2013, 660, 90–96. [Google Scholar] [CrossRef]
- Romanó de Orte, M.; Clowez, S.; Caldeira, K. Response of Bleached and Symbiotic Sea Anemones to Plastic Microfiber Exposure. Environ. Pollut. 2019, 249, 512–517. [Google Scholar] [CrossRef]
- Turra, A.; Manzano, A.B.; Dias, R.J.S.; Mahiques, M.M.; Barbosa, L.; Balthazar-Silva, D.; Moreira, F.T. Three-Dimensional Distribution of Plastic Pellets in Sandy Beaches: Shifting Paradigms. Sci. Rep. 2014, 4, 4435. [Google Scholar] [CrossRef]
- Kim, H.; Ji, K. Effects of Tetramethyl Bisphenol F on Thyroid and Growth Hormone-Related Endocrine Systems in Zebrafish Larvae. Ecotoxicol. Environ. Saf. 2022, 237, 113516. [Google Scholar] [CrossRef]
- Hu, S.; Fang, S.; Zhao, J.; Wang, G.; Qi, W.; Zhang, G.; Huang, C.; Qu, J.; Liu, H. Toxicity Evaluation and Effect-Based Identification of Chlorine Disinfection Products of the Anti-COVID-19 Drug Chloroquine Phosphate. Environ. Sci. Technol. 2023, 57, 7913–7923. [Google Scholar] [CrossRef]
- Barreto, F.C.; da Silva, M.K.L.; Cesarino, I. Copper Nanoparticles and Reduced Graphene Oxide as an Electrode Modifier for the Development of an Electrochemical Sensing Platform for Chloroquine Phosphate Determination. Nanomaterials 2023, 13, 1436. [Google Scholar] [CrossRef]
- Ballesteros-Gómez, A.; Rubio, S.; Pérez-Bendito, D. Analytical Methods for the Determination of Bisphenol A in Food. J. Chromatogr. A 2009, 1216, 449–469. [Google Scholar] [CrossRef]
- Ragavan, K.V.; Rastogi, N.K.; Thakur, M.S. Sensors and Biosensors for Analysis of Bisphenol-A. TrAC Trends Anal. Chem. 2013, 52, 248–260. [Google Scholar] [CrossRef]
- Galli, A.; De Souza, D.; Garbellini, G.S.; Coutinho, C.F.B.; Mazo, L.H.; Avaca, L.A.; Machado, S.A.S. Utilização de técnicas eletroanalíticas na determinação de pesticidas em alimentos. Quím. Nova 2006, 29, 105–112. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ahmed, J.; Asiri, A.M.; Alfaifi, S.Y. Ultra-Sensitive, Selective and Rapid Carcinogenic Bisphenol A Contaminant Determination Using Low-Dimensional Facile Binary Mg-SnO2 Doped Microcube by Potential Electro-Analytical Technique for the Safety of Environment. J. Ind. Eng. Chem. 2022, 109, 147–154. [Google Scholar] [CrossRef]
- Thevenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical Biosensors: Recommended Definitions and Classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhou, Y.; Ai, S.; Han, R.; Tang, T.; Zhu, L. Electrochemical Behavior of Bisphenol A at Glassy Carbon Electrode Modified with Gold Nanoparticles, Silk Fibroin, and PAMAM Dendrimers. Microchim. Acta 2010, 170, 99–105. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, Z.; Hu, Y.; Lord, A.; Cesarino, I.; Goonetilleke, A.; He, Q. Exploring the Properties and Potential Uses of Biocarbon from Spent Coffee Grounds: A Comparative Look at Dry and Wet Processing Methods. Processes 2023, 11, 2099. [Google Scholar] [CrossRef]
- Santos Santana, M.; Pereira Alves, R.; da Silva Borges, W.M.; Francisquini, E.; Guerreiro, M.C. Hydrochar Production from Defective Coffee Beans by Hydrothermal Carbonization. Bioresour. Technol. 2020, 300, 122653. [Google Scholar] [CrossRef]
- Masoumi, S.; Borugadda, V.B.; Nanda, S.; Dalai, A.K. Hydrochar: A Review on Its Production Technologies and Applications. Catalysts 2021, 11, 939. [Google Scholar] [CrossRef]
- Cao, X.; Sun, S.; Sun, R. Application of Biochar-Based Catalysts in Biomass Upgrading: A Review. RSC Adv. 2017, 7, 48793–48805. [Google Scholar] [CrossRef]
- Diaz, E.; Sanchis, I.; Coronella, C.J.; Mohedano, A.F. Activated Carbons from Hydrothermal Carbonization and Chemical Activation of Olive Stones: Application in Sulfamethoxazole Adsorption. Resources 2022, 11, 43. [Google Scholar] [CrossRef]
- Shen, R.; Lu, J.; Yao, Z.; Zhao, L.; Wu, Y. The Hydrochar Activation and Biocrude Upgrading from Hydrothermal Treatment of Lignocellulosic Biomass. Bioresour. Technol. 2021, 342, 125914. [Google Scholar] [CrossRef] [PubMed]
- Azzaz, A.A.; Khiari, B.; Jellali, S.; Ghimbeu, C.M.; Jeguirim, M. Hydrochars Production, Characterization and Application for Wastewater Treatment: A Review. Renew. Sustain. Energy Rev. 2020, 127, 109882. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, Y.; Zheng, Y.; Yang, Y.; Huang, J.; Chen, H.; Quan, G.; Gao, B. Potassium Permanganate Modification of Hydrochar Enhances Sorption of Pb(II), Cu(II), and Cd(II). Bioresour. Technol. 2023, 386, 129482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, L.; Li, A. Hydrothermal Co-Carbonization of Sewage Sludge and Pinewood Sawdust for Nutrient-Rich Hydrochar Production: Synergistic Effects and Products Characterization. J. Environ. Manag. 2017, 201, 52–62. [Google Scholar] [CrossRef]
- Ahmad, S.; Zhu, X.; Wei, X.; Zhang, S. Characterization and Potential Applications of Hydrochars Derived from P- and N-Enriched Agricultural and Antibiotic Residues via Microwave-Assisted Hydrothermal Conversion. Energy Fuels 2020, 34, 11154–11164. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Gan, X.; Zheng, L.; Peng, C.; Wang, B.; Li, C.; Zeng, G. Evaluation of the Clean Characteristics and Combustion Behavior of Hydrochar Derived from Food Waste towards Solid Biofuel Production. Bioresour. Technol. 2018, 266, 275–283. [Google Scholar] [CrossRef]
- Espro, C.; Satira, A.; Mauriello, F.; Anajafi, Z.; Moulaee, K.; Iannazzo, D.; Neri, G. Orange Peels-Derived Hydrochar for Chemical Sensing Applications. Sens. Actuators B Chem. 2021, 341, 130016. [Google Scholar] [CrossRef]
- Welch, C.M.; Compton, R.G. The Use of Nanoparticles in Electroanalysis: A Review. Anal. Bioanal. Chem. 2006, 384, 601–619. [Google Scholar] [CrossRef]
- Barreto, F.C.; Silva, M.K.L.; Cesarino, I. An Electrochemical Sensor Based on Reduced Graphene Oxide and Copper Nanoparticles for Monitoring Estriol Levels in Water Samples after Bioremediation. Chemosensors 2022, 10, 395. [Google Scholar] [CrossRef]
- Trindade, C.M.B.; Silva, M.K.L.; Cesarino, I. Copper Nanostructures Anchored on Renewable Carbon as Electrochemical Platform for the Detection of Dopamine, Fluoxetine and Escitalopram. Sens. Actuators Rep. 2022, 4, 100107. [Google Scholar] [CrossRef]
- Melaré, A.G.; Barreto, F.C.; Silva, M.K.L.; Simões, R.P.; Cesarino, I. Determination of Fluoxetine in Weight Loss Herbal Medicine Using an Electrochemical Sensor Based on RGO-CuNPs. Molecules 2023, 28, 6361. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, V.Q.; Silva, M.K.L.; Cesarino, I. Determination of Isotretinoin (13-Cis-Retinoic Acid) Using a Sensor Based on Reduced Graphene Oxide Modified with Copper Nanoparticles. J. Electroanal. Chem. 2020, 856, 113692. [Google Scholar] [CrossRef]
- Venkatesan, S.; Baloch, H.A.; Jamro, I.A.; Rafique, N. Evaluation of the Production of Hydrochar from Spent Coffee Grounds under Different Operating Conditions. J. Water Process Eng. 2022, 49, 103037. [Google Scholar] [CrossRef]
- Afolabi, O.O.D.; Sohail, M.; Cheng, Y.-L. Optimisation and Characterisation of Hydrochar Production from Spent Coffee Grounds by Hydrothermal Carbonisation. Renew. Energy 2020, 147, 1380–1391. [Google Scholar] [CrossRef]
- Çalışkan, M.; Akay, S.; Kayan, B.; Baran, T.; Kalderis, D. Preparation and Application of a Hydrochar-Based Palladium Nanocatalyst for the Reduction of Nitroarenes. Molecules 2021, 26, 6859. [Google Scholar] [CrossRef]
- Ianesko, F.; Alves de Lima, C.; Antoniazzi, C.; Santana, E.R.; Piovesan, J.V.; Spinelli, A.; Galli, A.; Guimarães de Castro, E. Simultaneous Electrochemical Determination of Hydroquinone and Bisphenol A Using a Carbon Paste Electrode Modified with Silver Nanoparticles. Electroanalysis 2018, 30, 1946–1955. [Google Scholar] [CrossRef]
- Silva, M.K.L.; Sousa, G.S.; Simoes, R.P.; Cesarino, I. Fabrication of Paper-Based Analytical Devices Using a PLA 3D-Printed Stencil for Electrochemical Determination of Chloroquine and Escitalopram. J. Solid. State Electrochem. 2022, 26, 581–586. [Google Scholar] [CrossRef]
- Pushpanjali, P.A.; Manjunatha, J.G.; Hareesha, N.; Girish, T.; Al-Kahtani, A.A.; Tighezza, A.M.; Ataollahi, N. Electrocatalytic Determination of Hydroxychloroquine Using Sodium Dodecyl Sulphate Modified Carbon Nanotube Paste Electrode. Top. Catal. 2022. [Google Scholar] [CrossRef]
- George, J.M.; Mathew, B. Cyclodextrin-Mediated Gold Nanoparticles as Multisensing Probe for the Selective Detection of Hydroxychloroquine Drug. Korean J. Chem. Eng. 2021, 38, 624–634. [Google Scholar] [CrossRef]
- Deroco, P.B.; Vicentini, F.C.; Oliveira, G.G.; Rocha-Filho, R.C.; Fatibello-Filho, O. Square-Wave Voltammetric Determination of Hydroxychloroquine in Pharmaceutical and Synthetic Urine Samples Using a Cathodically Pretreated Boron-Doped Diamond Electrode. J. Electroanal. Chem. 2014, 719, 19–23. [Google Scholar] [CrossRef]
- Rajendran, J.; Kannan, T.S.; Dhanasekaran, L.S.; Murugan, P.; Atchudan, R.; ALOthman, Z.A.; Ouladsmane, M.; Sundramoorthy, A.K. Preparation of 2D Graphene/MXene Nanocomposite for the Electrochemical Determination of Hazardous Bisphenol A in Plastic Products. Chemosphere 2022, 287, 132106. [Google Scholar] [CrossRef]
- Wang, X.; Li, M.; Wu, M.; Shi, Y.; Yang, J.; Shan, J.; Liu, L. Simultaneous Determination of Bisphenol A and Bisphenol S Using Multi-Walled Carbon Nanotubes Modified Electrode. Int. J. Electrochem. Sci. 2018, 13, 11906–11922. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Yan, B.; Zhang, H. An Electrochemical Sensor for the Determination of Bisphenol A Using Glassy Carbon Electrode Modified with Reduced Graphene Oxide-Silver/Poly-l-Lysine Nanocomposites. J. Electroanal. Chem. 2017, 805, 39–46. [Google Scholar] [CrossRef]
- Özcan, L.; Altuntaş, M.; Büyüksağiş, A.; Türk, H.; Yurdakal, S. Electrochemical Determination of Bisphenol A with Pencil Graphite Electrodes Modified with Co(II), Ni(II), Cu(II) and Fe(II) Phthalocyaninetetrasulfonates. Anal. Sci. 2016, 32, 881–886. [Google Scholar] [CrossRef]
- Mirzaei Karazan, Z.; Roushani, M. Electrochemical Determination of Mercury Ions in Different Food Samples Using Glassy Carbon Electrode Modified with Poly (Crocin). Microchem. J. 2023, 195, 109402. [Google Scholar] [CrossRef]
- Song, X.; Zhong, H.; Li, X.; Yu, R.; Chen, M.; Liu, J.; Cheng, Z.; Qian, H. Noncovalent Assembly of Non-Metallic Porphyrin on Graphene Oxide Nanosheets and Its Application for Simultaneous Determination of Cu2+ and Pb2+ in Real Water. J. Electroanal. Chem. 2023, 946, 117748. [Google Scholar] [CrossRef]
- Ostroushko, A.A.; Russkikh, O.V. Oxide material synthesis by combustion of organic-inorganic compositions. Nanosyst. Phys. Chem. Math. 2017, 8, 476–502. [Google Scholar] [CrossRef]
- Fleischmann, M.; Pletcher, D.; Race, G.M. The Electrochemical Oxidation of The Mercury(II)-Propene Complex. Electroanal. Chem. Interfacial Electrochem. 1969, 23, 369–374. [Google Scholar] [CrossRef]
- Nestke, S.; Ronge, E.; Siewert, I. Electrochemical water oxidation using a copper complex. Dalton Trans. 2018, 47, 10737. [Google Scholar] [CrossRef]
- Bond, A.M.; Colton, R.; Hollenkamp, A.F.; Hoskins, B.F.; McGregor, K. Voltammetric, coulometric, mercury-199 NMR, and other studies characterizing new and unusual mercury complexes produced by electrochemical oxidation of mercury(II) diethyldithiocarbamate. Crystal and molecular structure of octakis(N,N-diethyldithiocarbamato)pentamercury(II) perchlorate. J. Am. Chem. Soc. 1987, 109, 1969–1980. [Google Scholar] [CrossRef]
- Costa, D.J.E.; Santos, J.C.S.; Sanches-Brandão, F.A.C.; Ribeiro, W.F.; Salazar-Banda, G.R.; Araujo, M.C.U. Boron-Doped Diamond Electrode Acting as a Voltammetric Sensor for the Detection of Methomyl Pesticide. J. Electroanal. Chem. 2017, 789, 100–107. [Google Scholar] [CrossRef]
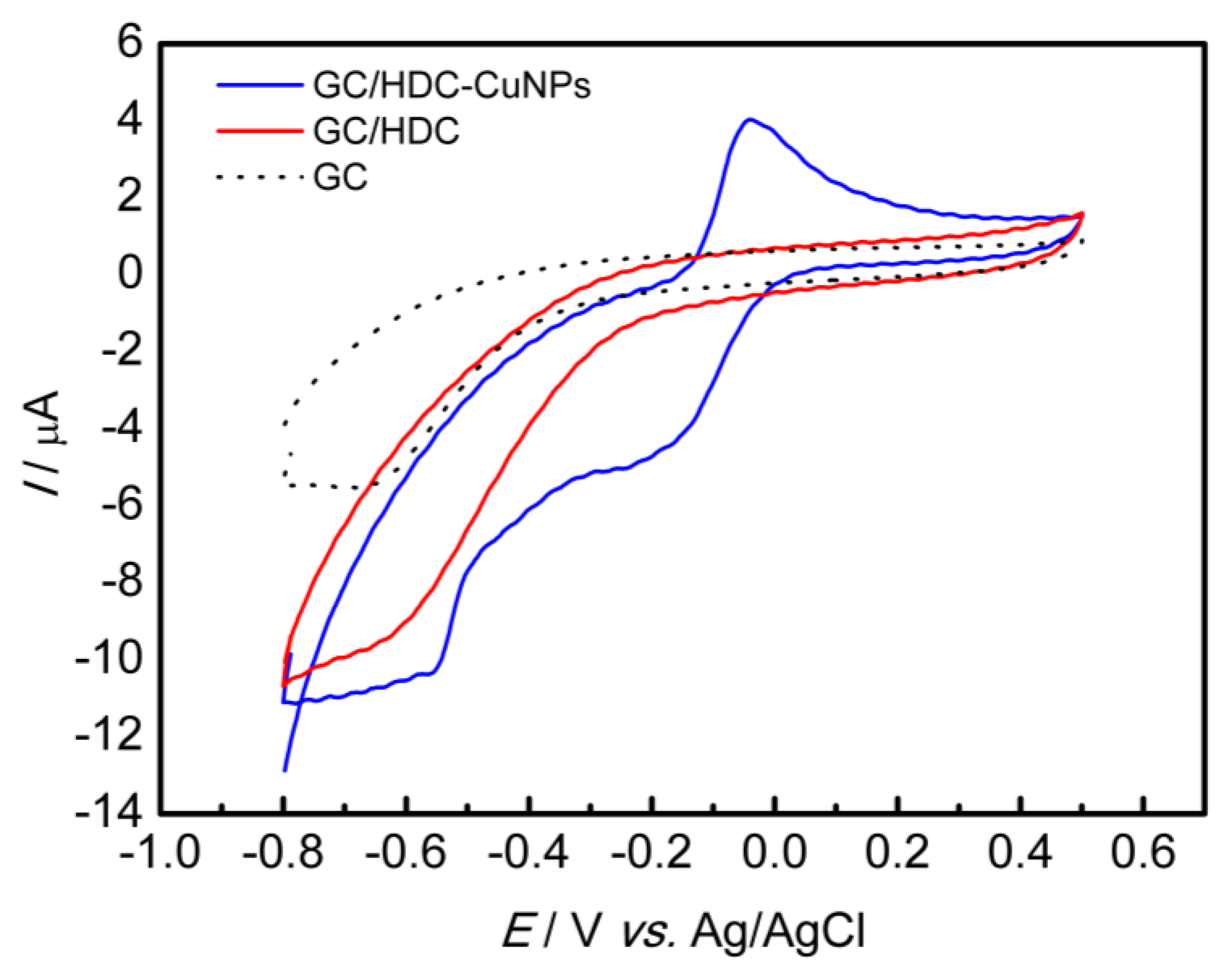


| Modified Electrode | Epa (mV) | Epc (mV) | ∆Ep (mV) | Ipa (µA) | Ipc (µA) | Ipa/Ipc |
|---|---|---|---|---|---|---|
| GC | 500 | 7 | 493 | 69.24 | −65.96 | 1.05 |
| GC/HDC | 396 | 79 | 317 | 86.93 | −72.26 | 1.20 |
| GC/HDC-CuNPs | 318 | 189 | 129 | 99.57 | −98.29 | 1.01 |
| Parameters | Optimization Range | HCS—Optimized Values | BPA—Optimized Values |
|---|---|---|---|
| HDC-CuNPs concentration (mg/mL) | 0.02–1.00 | 1.00 | 1.00 |
| Cu/HDC proportion in the synthesis (%) | 20–40 | 40 | 40 |
| Frequency (Hz) | 20–45 | 40 | 40 |
| Modulation Amplitude (V) | 0.01–0.05 | 0.05 | 0.05 |
| Step Potential (V) | 0.001–0.007 | 0.007 | 0.007 |
| pH | 5–9 | 5 | 6 |
| Electrode | Method | Linear Range (μmol L−1) | LOD (μmol L−1) | Ref |
|---|---|---|---|---|
| ePADs | DPV | 5–75 | 4.0 | [37] |
| SDSMCNTPE | CV | 10–40 | 0.85 | [38] |
| β-CD-AuNP | DPV | 0.01–0.05 | 0.00085 | [39] |
| BDD | SWV | 0.1–1.9 | 0.06 | [40] |
| HDC-CuNPs | SWV | 1–50 | 0.46 | This work |
| Electrode | Method | Linear Range (μmol L−1) | LOD (μmol L−1) | Ref |
|---|---|---|---|---|
| Gr/MXene/GCE | DPV | 1–10 | 0.35 | [41] |
| MWCNT/GCE | DPV | 2–30 | 0.51 | [42] |
| RGO-Ag/PLL/GCE | DPV | 1–80 | 0.54 | [43] |
| CoPCTS | DPV | 0.5–10 | 0.43 | [44] |
| HDC-CuNPs | SWV | 0.5–10 | 0.31 | This work |
| Repetition | HCS (μmol L−1) | BPA (μmol L−1) | HCS—Relative Errors (%) | BPA—Relative Errors (%) |
|---|---|---|---|---|
| 1 | 2.37 | 2.49 | −5.2 | −0.4 |
| 2 | 2.54 | 2.57 | 1.6 | 2.8 |
| 3 | 2.67 | 2.63 | 6.8 | 5.2 |
| Mean ± SD | 2.53 ± 0.12 | 2.56 ± 0.06 | - | - |
| Interferent | Concentration (µmol L−1) | % HCS Signal | % BPA Signal |
|---|---|---|---|
| Cu(II) | 1 | 104.0 | 96.2 |
| 2 | 106.9 | 88.1 | |
| 4 | 109.3 | 84.4 | |
| Hg(II) | 1 | 102.2 | 97.2 |
| 2 | 104.1 | 94.2 | |
| 4 | 105.3 | 84.5 | |
| Methomyl | 1 | 101.0 | 90.6 |
| 2 | 102.2 | 84.7 | |
| 4 | 103.6 | 82.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreto, F.C.; Ito, E.Y.; Mounienguet, N.K.; Dal’ Evedove Soares, L.; Yang, J.; He, Q.; Cesarino, I. Electrochemical Sensor Based on Spent Coffee Grounds Hydrochar and Metal Nanoparticles for Simultaneous Detection of Emerging Contaminants in Natural Water. Chemosensors 2023, 11, 562. https://doi.org/10.3390/chemosensors11110562
Barreto FC, Ito EY, Mounienguet NK, Dal’ Evedove Soares L, Yang J, He Q, Cesarino I. Electrochemical Sensor Based on Spent Coffee Grounds Hydrochar and Metal Nanoparticles for Simultaneous Detection of Emerging Contaminants in Natural Water. Chemosensors. 2023; 11(11):562. https://doi.org/10.3390/chemosensors11110562
Chicago/Turabian StyleBarreto, Francisco Contini, Erika Yukie Ito, Naelle Kita Mounienguet, Letícia Dal’ Evedove Soares, Jie Yang, Quan (Sophia) He, and Ivana Cesarino. 2023. "Electrochemical Sensor Based on Spent Coffee Grounds Hydrochar and Metal Nanoparticles for Simultaneous Detection of Emerging Contaminants in Natural Water" Chemosensors 11, no. 11: 562. https://doi.org/10.3390/chemosensors11110562
APA StyleBarreto, F. C., Ito, E. Y., Mounienguet, N. K., Dal’ Evedove Soares, L., Yang, J., He, Q., & Cesarino, I. (2023). Electrochemical Sensor Based on Spent Coffee Grounds Hydrochar and Metal Nanoparticles for Simultaneous Detection of Emerging Contaminants in Natural Water. Chemosensors, 11(11), 562. https://doi.org/10.3390/chemosensors11110562









