Capacitive Humidity Sensor with a Rapid Response Time on a GO-Doped P(VDF-TrFE)/LiCl Composite for Noncontact Sensing Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the GO-Doped P(VDF-TrFE)/LiCl-Based Sensor
2.3. Humidity-Sensing Measurement
2.4. Sensor Characterization
3. Results and Discussion
3.1. Characterization of GO-Doped P(VDF-TrFE)/LiCl
3.2. Sensing Properties of the GO-Doped P(VDF-TrFE)/LiCl Composite
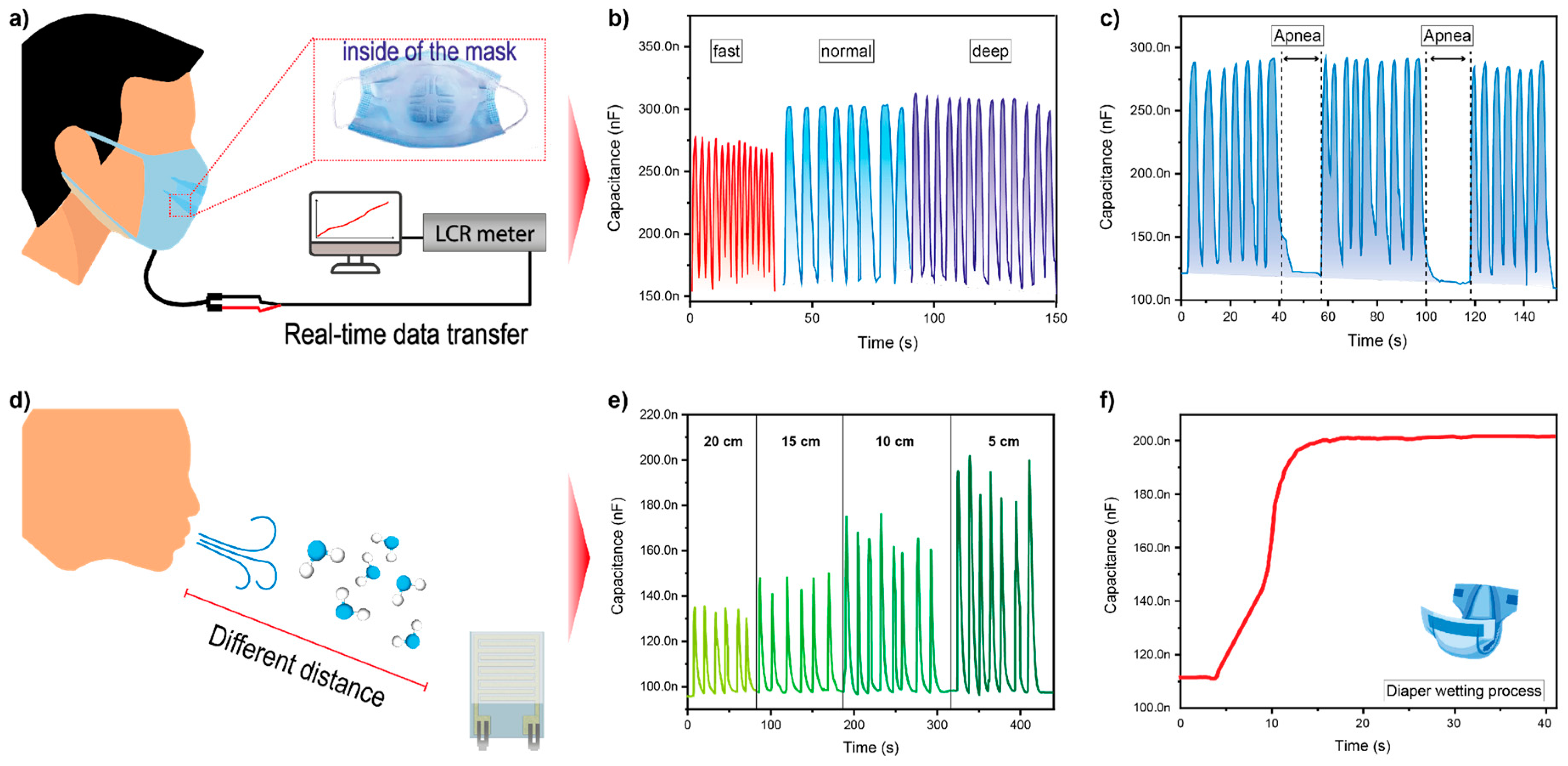
3.3. Real-Time Monitoring Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Zhuang, Z.; Qi, D.; Zhao, C. High-sensitive and fast-response humidity sensor based on polymer composite nanofibers for breath monitoring and non-contact sensing. Sens. Actuators B Chem. 2021, 330, 129239. [Google Scholar] [CrossRef]
- Pi, C.; Chen, W.; Zhou, W.; Yan, S.; Liu, Z.; Wang, C.; Guo, Q.; Qiu, J.; Yu, X.; Liu, B.; et al. Highly stable humidity sensor based on lead-free Cs3Bi2Br9 perovskite for breath monitoring. J. Mater. Chem. C 2021, 9, 11299. [Google Scholar] [CrossRef]
- Zhang, Z.-S.; Liu, J.X.; Cheng, X.F.; He, J.H.; Li, H.; Xu, Q.F.; Li, N.J.; Chen, D.Y.; Lu, J.M. Ultrasensitive humidity sensing using one-dimensional π-d conjugated coordination polymers for breath monitoring. Sens. Actuators B. Chem. 2021, 330, 129353. [Google Scholar] [CrossRef]
- Chen, G.; Guan, R.; Shi, M.; Dai, X.; Li, H.; Zhou, N.; Chen, D.; Mao, H. A nanoforest-based humidity sensor for respiration monitoring. Microsyst. Nanoeng. 2022, 8, 44. [Google Scholar] [CrossRef]
- Cho, M.Y.; Kim, I.S.; Kim, S.H.; Park, C.H.; K, N.Y.; Kim, S.W.; Kim, S.H.; Oh, J.M. Unique noncontact monitoring of human respiration and sweat evaporation using a CsPb2Br5-based sensor. ACS Appl. Mater. Interfaces 2021, 13, 5602–5613. [Google Scholar] [CrossRef]
- Sharma, P.K.; Kim, E.S.; Mishra, S.; Ganbold, E.; Seong, R.S.; Kaushik, A.K.; Kim, N.Y. Ultrasensitive and reusable graphene oxide-modified double-interdigitated capacitive (DIDC) sensing chip for detecting SARS-CoV-2. ACS Sens. 2021, 6, 3468–3476. [Google Scholar] [CrossRef]
- Hwang, C.J.; Park, N.Y.; Kim, E.S.; Kim, M.; Kim, S.D.; Park, S.J.; Kim, N.Y.; Kim, J.H. Ultra-fast and recyclable DNA biosensor for point-of-care detection of SARS-CoV-2 (COVID-19). Biosens. Bioelectron. 2021, 185, 113177. [Google Scholar] [CrossRef]
- Cho, M.Y.; Kim, I.S.; Kim, M.J.; Hyun, D.E.; Koo, S.M.; Sohn, H.; Kim, N.Y.; Kim, S.H.; Ko, S.H.; Oh, J.M. NaCl ionization- based moisture sensor prepared by aerosol deposition for monitoring respiratory patterns. Sensors 2022, 22, 5178. [Google Scholar] [CrossRef]
- Kaushik, A.K.; Dhau, J.S.; Gohel, H.; Mishra, Y.K.; Kateb, B.; Kim, N.Y.; Goswami, D.Y. Electrochemical SARS-CoV-2 sensing at point-of-care and artificial intelligence for intelligent COVID-19 management. ACS Appl. Bio. Mater. 2020, 11, 7306–7325. [Google Scholar] [CrossRef]
- Cho, M.Y.; Kim, S.H.; Kim, I.S.; Kim, E.S.; Wang, Z.J.; K, N.Y.; Kim, S.W.; Kim, S.H.; Oh, J.M. Perovskite-induced ultrasensitive and highly stable humidity sensor systems prepared by aerosol deposition at room temperature. Adv. Func. Mater. 2019, 30, 1907449. [Google Scholar] [CrossRef]
- Yu, H.; Wang, C.; Meng, F.Y.; Liang, J.G.; Kashan, H.S.; Adhikari, K.K.; Wang, L.; Kim, E.S.; Kim, N.Y. Design and analysis of ultrafast and high- sensitivity microwave transduction humidity sensor based on belt-shaped MoO3 nanomaterial. Sens. Actuators B Chem. 2020, 304, 127138. [Google Scholar] [CrossRef]
- Qian, Z.; Li, T.; Sakthivelpathi, V.; Goodman, S.M.; Dichiara, A.B.; Mamishev, A.V.; Chung, J.H. Humidity response of a capacitive sensor based on auxeticity of carbon nanotube-paper composites. Nano Express 2020, 3, 025001. [Google Scholar] [CrossRef]
- Boudaden, J.; Steinmabl, M.; Endres, H.E.; Drost, A.; Eisele, I.; Kutter, C.; Buschbaum, P.M. Polyimide-based capacitive humidity sensor. Sensors 2018, 15, 1516. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Cho, J.H.; Lee, H.M.; Hong, S.M. Capacitive humidity sensor based on carbon black/polyimide composites. Sensors 2021, 21, 1974. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Zhang, T.; Guan, X.; Dai, J.; Liu, S.; Zhao, H.; Fei, T. Capacitive humidity sensors based on mesoporous silica and poly(3,4-ethylenedioxythiophene) composites. J. Colloid Interface Sci. 2020, 565, 592–600. [Google Scholar] [CrossRef]
- Tousi, M.M.; Zhang, Y.; Wan, S.; Yu, L.; Hou, C.; Yan, N.; Fink, Y.; Wang, A.; Jia, X. Scalable fabrication of highly flexible porous polymer-based capacitive humidity sensor using convergence fiber drawing. Polymers 2019, 11, 1985. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Q.; Xu, F.; Jiang, H.; Liu, W.; Zhang, X.; Jiang, Z.; Zhu, G. Epitaxy enhancement of piezoelectric properties in P(VDF-TrFE) copolymer films and applications in sensing and energy harvesting. Adv. Electron. Mater. 2020, 6, 2000578. [Google Scholar] [CrossRef]
- Ko, Y.J.; Jin, D.W.; Kong, D.S.; Jung, J.H. Effects of humidity on the microstructure and the ferroelectric properties of sol-gel grown P(VDF-TrFE) films. J. Korean Phys. Soc. 2020, 76, 348–351. [Google Scholar] [CrossRef]
- Kim, S.R.; Dong, Y.C.; Hossain, M.M.; Gorman, S.; Towfeeq, I.; Gajula, D.; Childress, A.; Rao, A.M.; Koley, G. Piezoresistive graphene/P(VDF-TrFE) heterostructure based highly sensitive and flexible pressure sensor. ACS Appl. Mater. Interfaces 2019, 11, 16006–16017. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Zhang, T.; Qi, R.; Zhao, H.; Fei, T.; Lu, G. LiCl loaded cross-linked polymer composites by click reaction for humidity sensing. Sens. Actuators B Chem. 2017, 253, 361–367. [Google Scholar] [CrossRef]
- Fei, T.; Jiang, K.; Liu, S.; Zhang, T. Humidity sensors based on Li-loaded nanoporous polymers. Sens. Actuators B Chem. 2014, 190, 523–528. [Google Scholar] [CrossRef]
- Feng, M.H.; Wang, W.C.; Li, X.J. LiCl-enhanced capacitive humidity-sensing properties of cadmium sulfide grown on silicon nanoporous pillar array. J. Mater. Sci. 2016, 52, 3841–3848. [Google Scholar] [CrossRef]
- Zhuang, Z.; Li, Y.; Li, X.; Zhao, C. A novel polymer salt complex based on LiCl doped SPEEK & poly ether ketone co polyethylene glycol for humidity sensors. IEEE Sens. J. 2021, 21, 8886–8895. [Google Scholar]
- Khan, S.A.; Saqib, M.; Rahman, M.M.; Mutee Ur Rehman, H.M.; Rahman, S.A.; Yang, Y.; Kim, S.W. A full-range flexible and printed humidity sensor based on a solution-processed P(VDF-TrFE)/graphene-flower composite. Nanomaterials 2021, 11, 1915. [Google Scholar] [CrossRef]
- Gomez, E.S.; Martinez, R.M.; Roldan, G.R.; Rivera, D.H. A capacitive humidity sensor based on an electrospun PVDF/graphene membrane. Sensors 2017, 17, 1009. [Google Scholar]
- Shahzad, A.; Chen, Z.; Haidry, A.A.; Yang, L.; Mehmood, A.; Khan, Z.M. GO-modified P(VDF-TrFE) fibrous membrane for humidity sensing applications in vacuum insulation panels. Mater. Lett. 2022, 313, 131773. [Google Scholar] [CrossRef]
- Zhang, D.; Chang, H.; Li, P.; Liu, R.; Xue, Q. Fabrication and characterization of an ultrasensitive humidity sensor based on metal oxide/graphene hybrid nanocomposite. Sens. Actuators B Chem. 2016, 225, 233–240. [Google Scholar] [CrossRef]
- Priyadharshini, B.; Valsalal, P. An improved humidity sensor with GO-Mn-doped ZnO nanocomposite and dimensional orchestration of comb electrode for effective bulk manufacturing. Nanomaterials 2022, 12, 1659. [Google Scholar] [CrossRef]
- Liu, M.Q.; Wang, C.; Kim, N.Y. High-sensitivity and low-hysteresis porous MIM-type capacitive humidity sensor using functional polymer mixed with TiO2 microparticles. Sensors 2017, 17, 284. [Google Scholar] [CrossRef]
- Liang, J.G.; Kim, E.S.; Wang, C.; Cho, M.Y.; Oh, J.M.; Kim, N.Y. Thickness effects of aerosol deposited hygroscopic films on ultra-sensitive humidity sensors. Sens. Actuat. B Chem. 2018, 265, 632–643. [Google Scholar] [CrossRef]
- Liang, J.G.; Wang, C.; Yao, Z.; Liu, M.Q.; Kim, H.K.; Oh, J.M.; Kim, N.Y. Preparation of ultrasensitive humidity-sensing films by aerosol deposition. ACS Appl. Mater. Interfaces 2018, 10, 851–863. [Google Scholar] [CrossRef]
- Kumar, A.; Wang, C.; Meng, F.Y.; Liang, J.G.; Xie, B.F.; Zhou, Z.L.; Zhao, M.; Kim, N.Y. Aerosol deposited BaTiO3 film based interdigital capacitor and squared spiral capacitor for humidity sensing application. Ceram. Int. 2021, 47, 510–520. [Google Scholar] [CrossRef]
- Cunha, B.B.; Greenshields, M.W.C.C.; Mamo, M.G.; Coville, N.J.; Hummelgen, I.A. A surfactant dispersed N-doped carbon sphere-poly (vinyl alcohol) composite as relative humidity sensor. J. Mater. Sci. Mater. Electron. 2015, 26, 4198–4201. [Google Scholar] [CrossRef]
- Kim, E.S.; Liang, J.G.; Wang, C.; Cho, M.C.; Oh, J.M.; Kim, N.Y. Inter-digital capacitors with aerosol-deposited high-K dielectric layer for highest capacitance value in capacitive super-sensing applications. Sci. Rep. 2019, 9, 9680. [Google Scholar] [CrossRef]
- Mishra, S.; Kim, E.S.; Sharma, P.K.; Wang, Z.J.; Yang, S.H.; Kaushik, A.K.; Wang, C.; Li, Y.; Kim, N.Y. Tailored biofunctionalized biosensor for the label-free sensing of prostate-specific antigen. ACS Appl. Bio. Mater. 2020, 3, 7821–7830. [Google Scholar] [CrossRef]
- Babu Reddy, L.P.; Rajprakash, H.G.; Ravikiran, Y.T. Synthesis of α-MoO3 nanorods by sol gel synthesis and to investigate its room temperature humidity sensing properties. AIP Conf. Proc. 2019, 2142, 070022. [Google Scholar]
- Kim, K.S.; Lee, S.; Kim, Y.I. Solvent-controlled crystalline beta-phase formation in electrospun P(VDF-TrFE) fibers for enhanced piezoelectric energy harvesting. APL Mater. 2020, 8, 071109. [Google Scholar] [CrossRef]
- Meng, N.; Ren, X.; Santagiuliana, G.; Ventura, L.; Zhang, H.; Wu, J.; Yan, H.; Reece, M.J. Ultrahigh β-phase content poly(vinylidene fluoride) with relaxor-like ferroelectricity for high energy density capacitors. Nat. Commun. 2019, 10, 4535. [Google Scholar] [CrossRef]
- Hu, X.; You, M.; Yi, N.; Zhang, X.; Xiang, Y. Enhanced piezoelectric coefficient of PVDF-TrFE films via in situ polarization. Front. Energy Res. 2021, 9, 321540. [Google Scholar] [CrossRef]
- King, A.A.; Davies, B.R.; Noorbehest, N.; Newman, P.; Church, T.L.; Harris, A.T.; Razal, J.M.; Minett, A.I. A new Raman metric for the characterisation of graphene oxide and its derivatives. Sci. Rep. 2016, 6, 19491. [Google Scholar] [CrossRef]
- Ganbold, E.; Kim, E.S.; Li, Y.; Yin, F.F.; Sharma, P.K.; Jeon, J.B.; Oh, J.M.; Lee, D.N.; Kim, N.Y. Highly Sensitive Interdigitated Capacitive Humidity Sensors Based on Sponge-Like Nanoporous PVDF/LiCl Composite for Real-Time Monitoring. ACS App. Mater. Interfaces 2023, 15, 4559–4568. [Google Scholar] [CrossRef]
- Niu, H.; Gao, S.; Yue, W.; Li, Y.; Zhou, W.; Liu, H. Highly Morphology-Controllable and Highly Sensitive Capacitive Tactile Sensor Based on Epidermis-Dermis-Inspired Interlocked Asymmetric-Nanocone Arrays for Detection of Tiny Pressure. Small 2020, 16, 1904774. [Google Scholar] [CrossRef]




| Sensors | Conductivity (nS) |
|---|---|
| P(VDF-TrFE) | 27.2 |
| P(VDF-TrFE)/LiCl | 86.4 |
| GO-doped P(VDF-TrFE)/LiCl | 121.3 |
| Sensing Material | Preparation Method | Measuring Range | Sensitivity | Response and Recovery Time | Ref. #/Publishing Year |
|---|---|---|---|---|---|
| SnO2/rGO | Hydrothermal synthesis | 11–97% RH | 1604.89 pF/%RH | 120 s | 27/2016 |
| PVDF/Graphene | Electrospinning | 35–85% RH | 0.0372 pF/%RH | 19.8 s | 25/2017 |
| P(VDF-TrFE)/Graphene flower | Spin coating | 8–98% RH | 0.0558 pF/RH% | 0.8 s/2.5 s | 24/2021 |
| GO-modified P(VDF-TrFE) | Electrospinning | 9–90% RH | N/A | 100 s | 26/2022 |
| GO-Zn1−xMnxO | Drop casting | 10–90% RH | 2901 pF/%RH | 4.5 s and 21 s | 28/2022 |
| GO-doped P(VDF-TrFE)/LiCl | Drop casting | 25–95% RH | 1708.8 pF/%RH | 7.8 s and 4.5 s | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganbold, E.; Sharma, P.K.; Kim, E.-S.; Lee, D.-N.; Kim, N.-Y. Capacitive Humidity Sensor with a Rapid Response Time on a GO-Doped P(VDF-TrFE)/LiCl Composite for Noncontact Sensing Applications. Chemosensors 2023, 11, 122. https://doi.org/10.3390/chemosensors11020122
Ganbold E, Sharma PK, Kim E-S, Lee D-N, Kim N-Y. Capacitive Humidity Sensor with a Rapid Response Time on a GO-Doped P(VDF-TrFE)/LiCl Composite for Noncontact Sensing Applications. Chemosensors. 2023; 11(2):122. https://doi.org/10.3390/chemosensors11020122
Chicago/Turabian StyleGanbold, Enkhzaya, Parshant Kumar Sharma, Eun-Seong Kim, Do-Nam Lee, and Nam-Young Kim. 2023. "Capacitive Humidity Sensor with a Rapid Response Time on a GO-Doped P(VDF-TrFE)/LiCl Composite for Noncontact Sensing Applications" Chemosensors 11, no. 2: 122. https://doi.org/10.3390/chemosensors11020122
APA StyleGanbold, E., Sharma, P. K., Kim, E.-S., Lee, D.-N., & Kim, N.-Y. (2023). Capacitive Humidity Sensor with a Rapid Response Time on a GO-Doped P(VDF-TrFE)/LiCl Composite for Noncontact Sensing Applications. Chemosensors, 11(2), 122. https://doi.org/10.3390/chemosensors11020122









