Synthesis and Characterization of a Multiporous SnO2 Nanofibers-Supported Au Nanoparticles-Based Amperometric Sensor for the Nonenzymatic Detection of H2O2
Abstract
:1. Introduction
2. Methods and Materials
2.1. Materials and Reagents
2.2. Synthesis of Gold Nanoparticles (Au NPs)
2.3. Synthesis of Multiporous SnO2 Nanofiber (SnO2 NFs)
2.4. Sensing Electrode Fabrication
2.5. Preparation of Real Sample for Multiple-Step Chronoamperometry Analysis
2.6. Apparatus
3. Results and Discussion
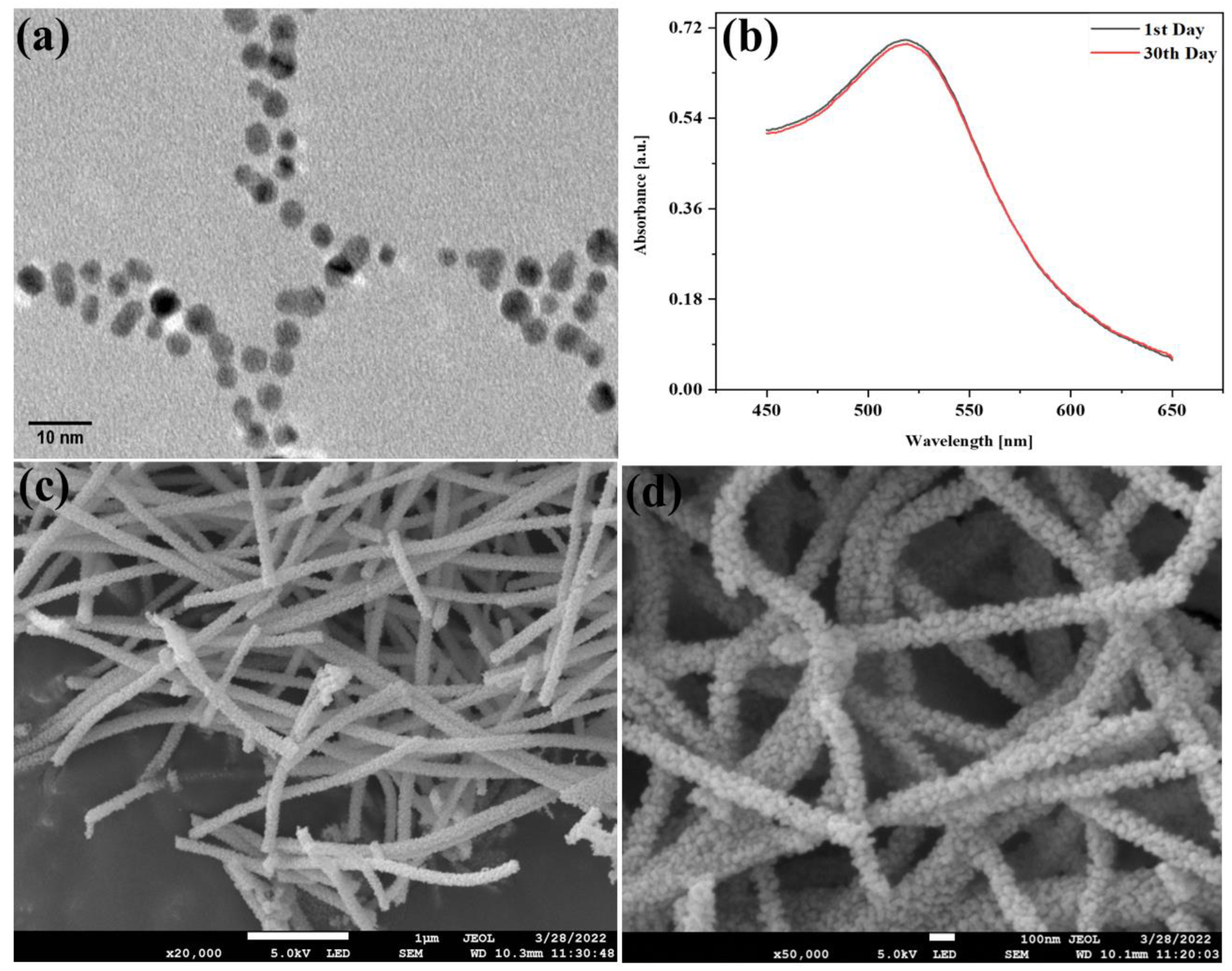
3.1. Morphological Characterization
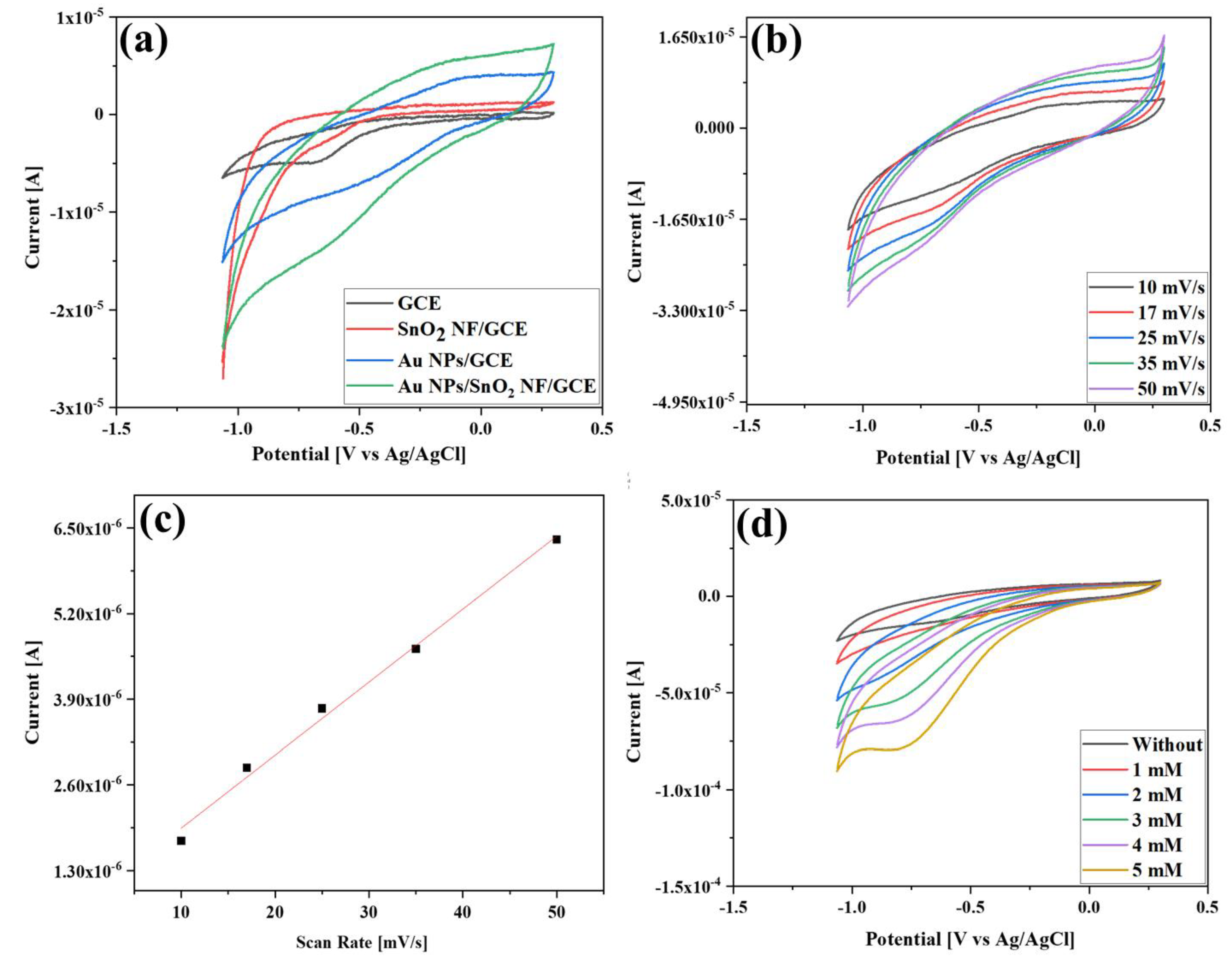
3.2. Electrochemical Properties of the Au NPs/SnO2 NFs Composite Electrode
3.3. Electrochemical Reduction of H2O2 Using the Au NPs/SnO2 NFs Composite
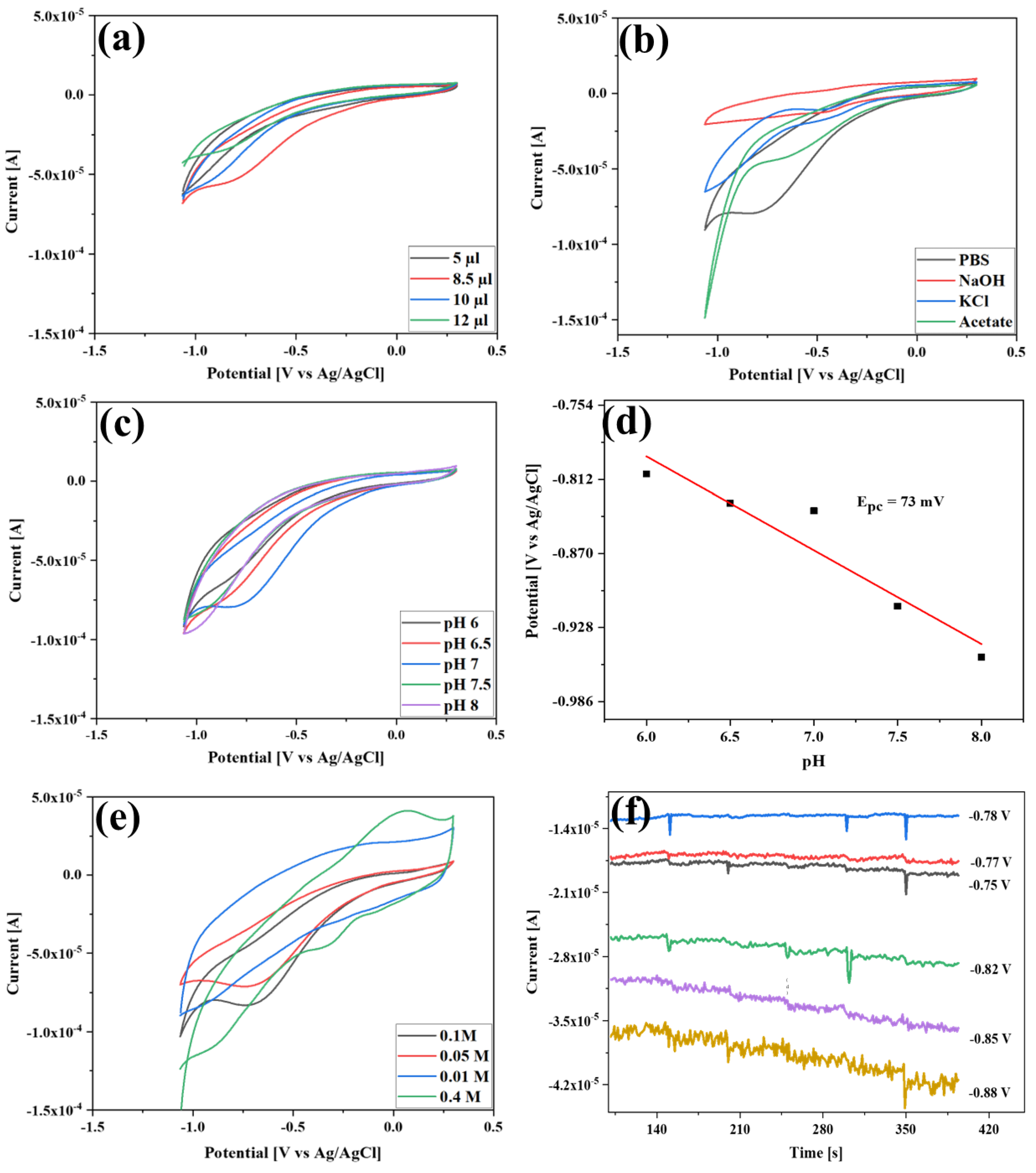
3.4. Optimization of Detection Circumstances
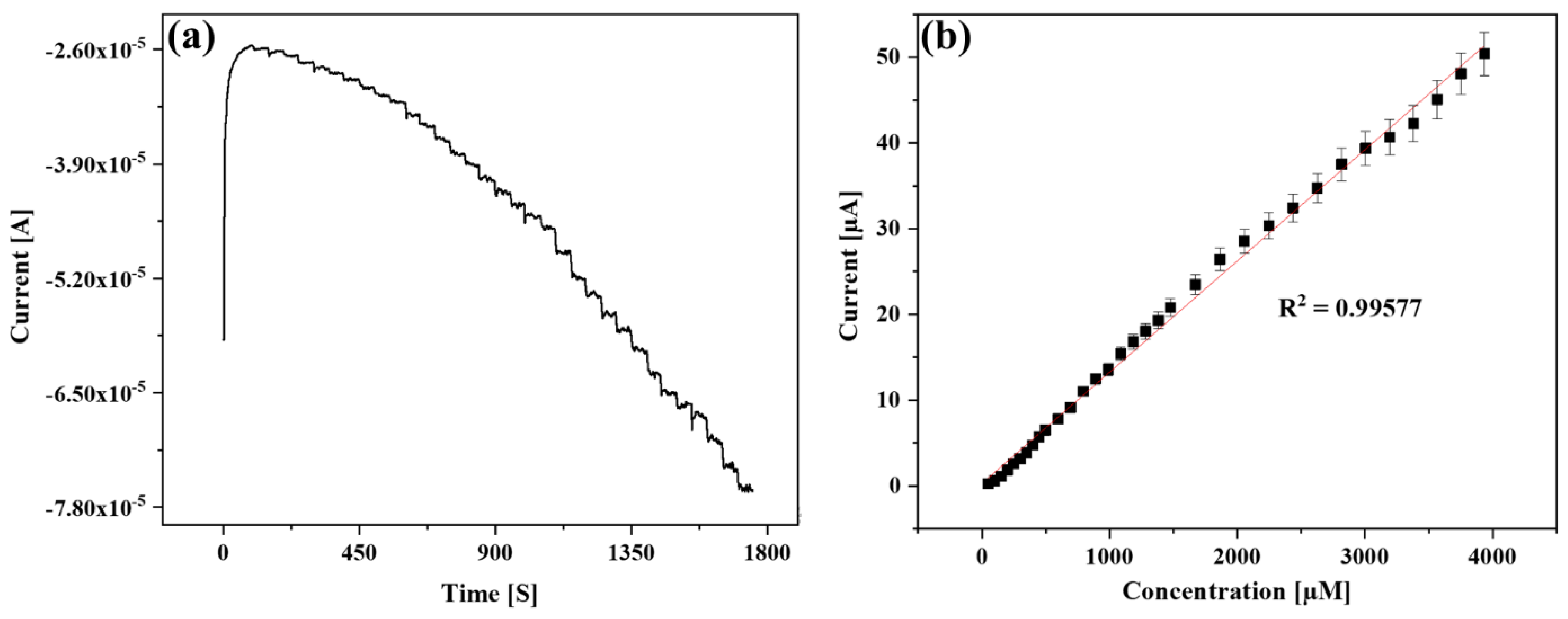
3.5. Amperometric Sensing of H2O2 Using the GCE/Au NPs/SnO2 NFs Composite
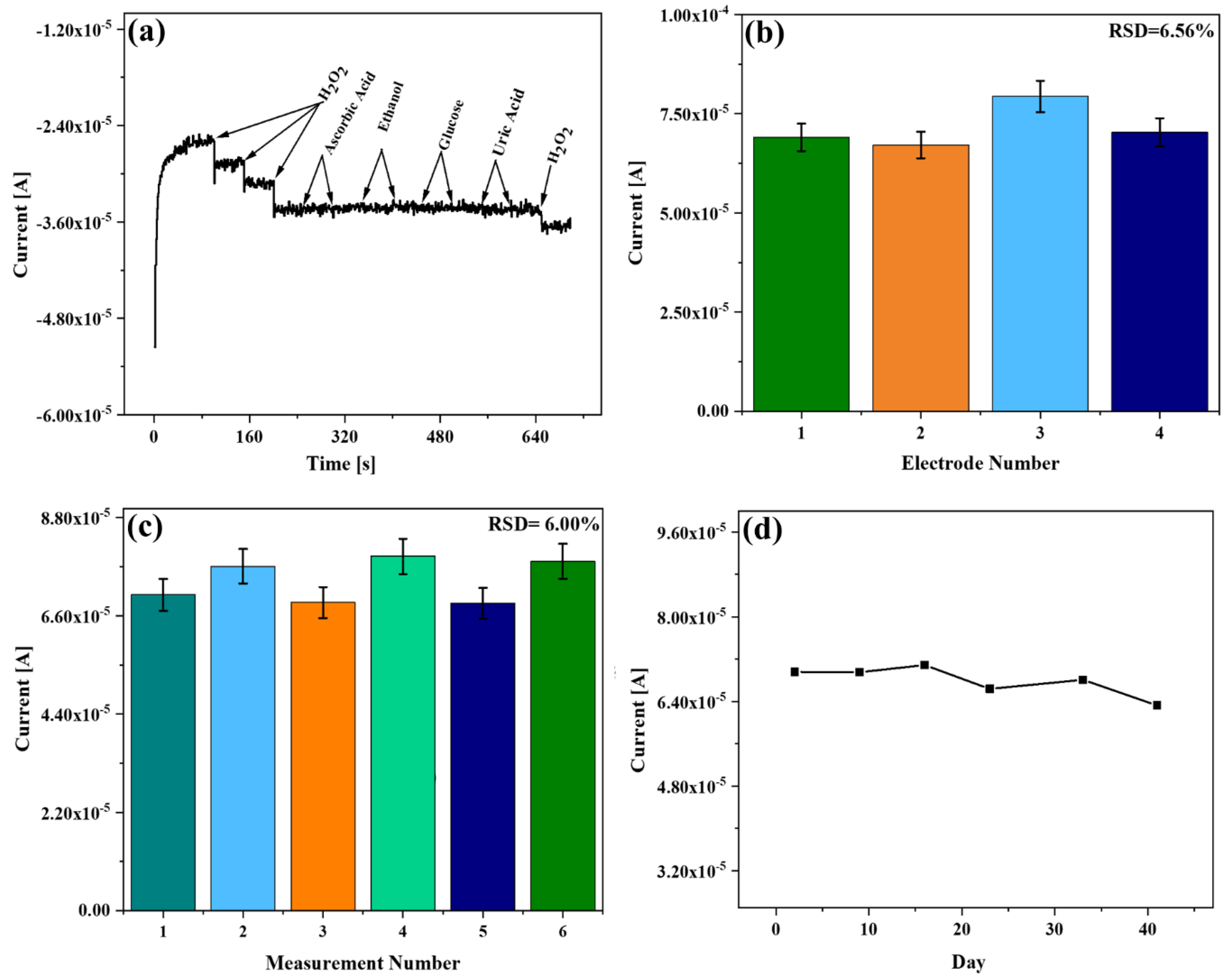
3.6. Study of Selectivity, Repeatability, Reproducibility, and Stability
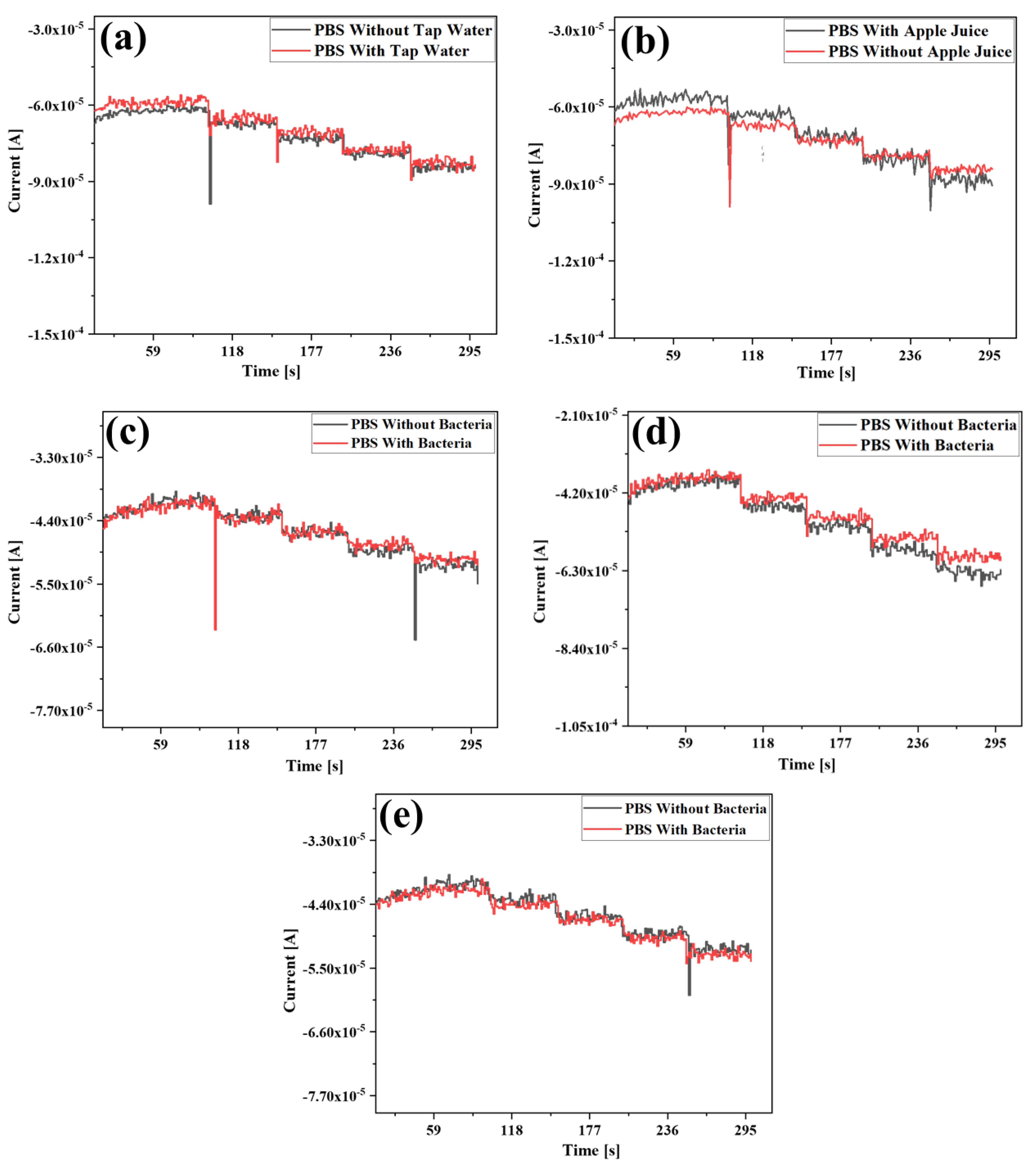
3.7. Electrochemical Detection of H2O2 in Practical Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiménez, M.J.; Lissarrague, M.S.; Bechthold, P.; González, E.A.; Jasen, P.V.; Juan, A. Ethanol adsorption on Ni doped Mo2C(001): A theoretical study. Top. Catal. 2022, 65, 839–847. [Google Scholar] [CrossRef]
- Hyslop, P.A.; Chaney, M.O. Mechanism of GAPDH Redox Signaling by H2O2 Activation of a Two-Cysteine Switch. Int. J. Mol. Sci. 2022, 23, 4604. [Google Scholar] [CrossRef] [PubMed]
- Ghanam, A.; Mohammadi, H.; Amine, A.; Haddour, N.; Buret, F. Chemical Sensors: Voltammetric and Amperometric Electrochemical Sensors. In Encyclopedia of Sensors and Biosensors; Narayan, R., Ed.; Elsevier: Oxford, UK, 2023; pp. 161–177. [Google Scholar]
- Watt, B.E.; Proudfoot, A.T.; Vale, J.A. Hydrogen Peroxide Poisoning. Toxicol. Rev. 2004, 23, 51–57. [Google Scholar] [CrossRef]
- Raffellini, S.; Schenk, M.; Guerrero, S.; Alzamora, S.M. Kinetics of Escherichia coli inactivation employing hydrogen peroxide at varying temperatures, pH and concentrations. Food Control 2011, 22, 920–932. [Google Scholar] [CrossRef]
- Swieca, M. Production of ready-to-eat lentil sprouts with improved antioxidant capacity: Optimization of elicitation conditions with hydrogen peroxide. Food Chem. 2015, 180, 219–226. [Google Scholar] [CrossRef]
- Khadhraoui, H.; Othmani, A.; Kouki, A.; Zouaoui, M. A Highly Sensitive and Selective Non-enzymatic Hydrogen Peroxide Sensor Based on Nanostructured Co3O4 Thin Films Using the Sol-gel Method. Electroanalysis 2020, 33, 152–159. [Google Scholar] [CrossRef]
- Luna-Guevara, J.J.; Arenas-Hernandez, M.M.P.; Martinez de la Pena, C.; Silva, J.L.; Luna-Guevara, M.L. The Role of Pathogenic E. coli in Fresh Vegetables: Behavior, Contamination Factors, and Preventive Measures. Int. J. Microbiol. 2019, 2019, 2894328. [Google Scholar] [CrossRef]
- Ravindra Kumar, S.; Imlay, J.A. How Escherichia coli tolerates profuse hydrogen peroxide formation by a catabolic pathway. J. Bacteriol. 2013, 195, 4569–4579. [Google Scholar] [CrossRef]
- Arasu, M.V.; Al-Dhabi, N.A.; Ilavenil, S.; Choi, K.C.; Srigopalram, S. In vitro importance of probiotic Lactobacillus plantarum related to medical field. Saudi J. Biol. Sci. 2016, 23, S6–S10. [Google Scholar] [CrossRef] [PubMed]
- Cornacchione, L.P.; Hu, L.T. Hydrogen peroxide-producing pyruvate oxidase from Lactobacillus delbrueckii is catalytically activated by phosphotidylethanolamine. BMC Microbiol. 2020, 20, 128. [Google Scholar] [CrossRef]
- Wen, F.; He, T.Y.Y.; Liu, H.C.; Chen, H.Y.; Zhang, T.; Lee, C.K. Advances in chemical sensing technology for enabling the next-generation self-sustainable integrated wearable system in the IoT era. Nano Energy 2020, 78, 105155. [Google Scholar] [CrossRef]
- Simões, F.R.; Xavier, M.G. Electrochemical Sensors. In Nanoscience and Its Applications; Da Róz, A.L., Ferreira, M., de Lima Leite, F., Oliveira, O.N., Eds.; William Andrew Publishing: Norwich, NY, USA, 2017; pp. 155–178. [Google Scholar]
- Clark, L.C., Jr.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Y.; Liu, S. Preparation of Trace Fe2P Modified N,P Co-doped Carbon Materials and their Application to Hydrogen Peroxide Detection. Electroanalysis 2020, 33, 831–837. [Google Scholar] [CrossRef]
- Cheng, D.; Li, P.P.; Zhu, X.H.; Liu, M.L.; Zhang, Y.Y.; Liu, Y. Enzyme-free Electrochemical Detection of Hydrogen Peroxide Based on the Three-Dimensional Flower-like Cu-based Metal Organic Frameworks and MXene Nanosheets. Chin. J. Chem. 2021, 39, 2181–2187. [Google Scholar] [CrossRef]
- Zuccarello, L.; Barbosa, C.; Todorovic, S.; Silveira, C.M. Electrocatalysis by Heme Enzymes-Applications in Biosensing. Catalysts 2021, 11, 218. [Google Scholar] [CrossRef]
- Ding, S.; Lyu, Z.; Fang, L.; Li, T.; Zhu, W.; Li, S.; Li, X.; Li, J.C.; Du, D.; Lin, Y. Single-Atomic Site Catalyst with Heme Enzymes-Like Active Sites for Electrochemical Sensing of Hydrogen Peroxide. Small 2021, 17, e2100664. [Google Scholar] [CrossRef]
- Hu, F.X.; Kang, Y.J.; Du, F.; Zhu, L.; Xue, Y.H.; Chen, T.; Dai, L.M.; Li, C.M. Living Cells Directly Growing on a DNA/Mn3(PO4)2-Immobilized and Vertically Aligned CNT Array as a Free-Standing Hybrid Film for Highly Sensitive In Situ Detection of Released Superoxide Anions. Adv. Funct. Mater. 2015, 25, 5924–5932. [Google Scholar] [CrossRef]
- Dhara, K.; Mahapatra, D.R. Recent advances in electrochemical nonenzymatic hydrogen peroxide sensors based on nanomaterials: A review. J. Mater. Sci. 2019, 54, 12319–12357. [Google Scholar] [CrossRef]
- Chen, W.; Cai, S.; Ren, Q.Q.; Wen, W.; Zhao, Y.D. Recent advances in electrochemical sensing for hydrogen peroxide: A review. Analyst 2012, 137, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Weng, L.; Yang, C. A review on nanomaterial-based electrochemical sensors for H2O2, H2S and NO inside cells or released by cells. Microchim. Acta 2017, 184, 1267–1283. [Google Scholar] [CrossRef]
- Wang, P.; Lin, Z.; Su, X.; Tang, Z. Application of Au based nanomaterials in analytical science. Nano Today 2017, 12, 64–97. [Google Scholar] [CrossRef]
- Xiao, T.; Huang, J.; Wang, D.; Meng, T.; Yang, X. Au and Au-Based nanomaterials: Synthesis and recent progress in electrochemical sensor applications. Talanta 2020, 206, 120210. [Google Scholar] [CrossRef] [PubMed]
- Lou-Franco, J.; Das, B.; Elliott, C.; Cao, C. Gold Nanozymes: From Concept to Biomedical Applications. Nano-Micro Lett. 2020, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Dinu, L.; Pogacean, F.; Kurbanoglu, S.; Barbu-Tudoran, L.; Serban, A.; Kacso, I.; Pruneanu, S. Graphene-Gold Nanoparticles Nanozyme-Based Electrochemical Sensor with Enhanced Laccase-Like Activity for Determination of Phenolic Substrates. J. Electrochem. Soc. 2021, 168, 067523. [Google Scholar] [CrossRef]
- Berbeć, S.; Żołądek, S.; Jabłońska, A.; Pałys, B. Electrochemically reduced graphene oxide on gold nanoparticles modified with a polyoxomolybdate film. Highly sensitive non-enzymatic electrochemical detection of H2O2. Sens. Actuators B Chem. 2018, 258, 745–756. [Google Scholar] [CrossRef]
- Qi, Y.; Ma, J.; Xiu, F.-R.; Gao, X. Determination of Cr(VI) based on the peroxidase mimetic catalytic activity of citrate-capped gold nanoparticles. Microchim. Acta 2021, 188, 273. [Google Scholar] [CrossRef]
- Zhang, G. Functional gold nanoparticles for sensing applications. Nanotechnol. Rev. 2013, 2, 269–288. [Google Scholar] [CrossRef]
- He, Q.R.; Sun, H.; Shang, Y.X.; Tang, Y.N.; She, P.; Zeng, S.; Xu, K.L.; Lu, G.L.; Liang, S.; Yin, S.Y.; et al. Au@TiO2 yolk-shell nanostructures for enhanced performance in both photoelectric and photocatalytic solar conversion. Appl. Surf. Sci. 2018, 441, 458–465. [Google Scholar] [CrossRef]
- Zheng, N.; Stucky, G.D. A general synthetic strategy for oxide-supported metal nanoparticle catalysts. J. Am. Chem. Soc. 2006, 128, 14278–14280. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Chai, X.; Wang, T.; Cao, T.; Li, Y.; Zhang, L.; Fan, F.; Fu, Y.; Qi, W. An Electrochemical Sensor for H2O2 Based on Au Nanoparticles Embedded in UiO-66 Metal–Organic Framework Films. ACS Appl. Nano Mater. 2021, 4, 6103–6110. [Google Scholar] [CrossRef]
- Wang, K.; Li, J.; Li, W.; Wei, W.; Zhang, H.; Wang, L.L. Highly Active Co-Based Catalyst in Nanofiber Matrix as Advanced Sensing Layer for High Selectivity of Flexible Sensing Device. Adv. Mater. Technol.-Us 2019, 4, 1800521. [Google Scholar] [CrossRef]
- Wang, L.; Chai, R.; Lou, Z.; Shen, G. Highly sensitive hybrid nanofiber-based room-temperature CO sensors: Experiments and density functional theory simulations. Nano Res. 2018, 11, 1029–1037. [Google Scholar] [CrossRef]
- Wang, L.; Chen, S.; Li, W.; Wang, K.; Lou, Z.; Shen, G. Grain-Boundary-Induced Drastic Sensing Performance Enhancement of Polycrystalline-Microwire Printed Gas Sensors. Adv. Mater. 2019, 31, e1804583. [Google Scholar] [CrossRef]
- Horne, J.; McLoughlin, L.; Bridgers, B.; Wujcik, E.K.J.S.; Reports, A. Recent developments in nanofiber-based sensors for disease detection, immunosensing, and monitoring. Sens. Actuators Rep. 2020, 2, 100005. [Google Scholar] [CrossRef]
- Alim, S.; Kafi, A.K.M.; Rajan, J.; Yusoff, M.M. Application of polymerized multiporous nanofiber of SnO2 for designing a bienzyme glucose biosensor based on HRP/GOx. Int. J. Biol. Macromol. 2019, 123, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Di, J.; Ma, J.; Ma, Z. Highly sensitive detection of cancer cells by electrochemical impedance spectroscopy. J Electrochim. Acta 2012, 61, 179–184. [Google Scholar] [CrossRef]
- Kafi, A.K.M.; Wali, Q.; Jose, R.; Biswas, T.; Yusoff, M. A glassy carbon electrode modified with SnO2 nanofibers, polyaniline and hemoglobin for improved amperometric sensing of hydrogen peroxide. Microchim. Acta 2017, 184, 4443–4450. [Google Scholar] [CrossRef]
- Sophia, J.; Muralidharan, G. Gold nanoparticles for sensitive detection of hydrogen peroxide: A simple non-enzymatic approach. J. Appl. Electrochem. 2015, 45, 963–971. [Google Scholar] [CrossRef]
- Hu, M.; Chen, J.; Li, Z.-Y.; Au, L.; Hartland, G.V.; Li, X.; Marquez, M.; Xia, Y. Gold nanostructures: Engineering their plasmonic properties for biomedical applications. Chem. Soc. Rev. 2006, 35, 1084–1094. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M. Size Evaluation of Gold Nanoparticles by UV−vis Spectroscopy. J. Phys. Chem. C 2009, 113, 4277–4285. [Google Scholar] [CrossRef]
- Suchomel, P.; Kvitek, L.; Prucek, R.; Panacek, A.; Halder, A.; Vajda, S.; Zboril, R. Simple size-controlled synthesis of Au nanoparticles and their size-dependent catalytic activity. Sci. Rep. 2018, 8, 4589. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, W.; Liu, G.; Panda, D.; Chen, P. Size-Dependent Catalytic Activity and Dynamics of Gold Nanoparticles at the Single-Molecule Level. J. Am. Chem. Soc. 2010, 132, 138–146. [Google Scholar] [CrossRef]
- Wali, Q.; Fakharuddin, A.; Ahmed, I.; Ab Rahim, M.H.; Ismail, J.; Jose, R. Multiporous nanofibers of SnO2 by electrospinning for high efficiency dye-sensitized solar cells. J. Mater. Chem. A 2014, 2, 17427–17434. [Google Scholar] [CrossRef]
- Babu, B.; Koutavarapu, R.; Harish, V.V.N.; Shim, J.; Yoo, K. Novel in-situ synthesis of Au/SnO2 quantum dots for enhanced visible-light-driven photocatalytic applications. Ceram. Int. 2019, 45, 5743–5750. [Google Scholar] [CrossRef]
- Wang, L.; Dou, H.; Lou, Z.; Zhang, T. Encapsuled nanoreactors (Au@SnO2): A new sensing material for chemical sensors. Nanoscale 2013, 5, 2686–2691. [Google Scholar] [CrossRef]
- Duan, C.Q.; Bai, W.S.; Zheng, J.B. Non-enzymatic sensors based on a glassy carbon electrode modified with Au nanoparticles/polyaniline/SnO2 fibrous nanocomposites for nitrite sensing. New J. Chem. 2018, 42, 11516–11524. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, J.; Gong, H.; Ju, D.; Cao, B. Au nanoparticle-functionalized 3D SnO2 microstructures for high performance gas sensor. Sens. Actuators B Chem. 2016, 226, 266–272. [Google Scholar] [CrossRef]
- Tripathy, S.K.; Mishra, A.; Jha, S.K.; Wahab, R.; Al-Khedhairy, A.A. Synthesis of thermally stable monodispersed Au@SnO2 core–shell structure nanoparticles by a sonochemical technique for detection and degradation of acetaldehyde. Anal. Methods 2013, 5, 1456–1462. [Google Scholar] [CrossRef]
- Zhu, Y.; Lu, S.; Gowri Manohari, A.; Dong, X.; Chen, F.; Xu, W.; Shi, Z.; Xu, C. Polydopamine interconnected graphene quantum dots and gold nanoparticles for enzymeless H2O2 detection. J. Electroanal. Chem. 2017, 796, 75–81. [Google Scholar] [CrossRef]
- Naseri, A.; Hormozi-Nezhad, M.R.; Shahrokhian, S.; Asadian, E. Silver nanowires immobilized on gold-modified glassy carbon electrode for electrochemical quantification of atorvastatin. J. Electroanal. Chem. 2020, 876, 114540. [Google Scholar] [CrossRef]
- Honeychurch, K. Trace Voltammetric Determination of Lead at a Recycled Battery Carbon Rod Electrode. Sensors 2019, 19, 770. [Google Scholar] [CrossRef]
- Ni, X.; Tian, M.; Sun, J.; Chen, X. A Novel Nonenzymatic Hydrogen Peroxide Sensor Based on Magnetic Core-Shell Fe3O4@C/Au Nanoparticle Nanocomposite. Int. J. Anal. Chem. 2021, 2021, 8839895. [Google Scholar] [CrossRef] [PubMed]
- Simioni, N.B.; Silva, T.A.; Oliveira, G.G.; Fatibello-Filho, O. A nanodiamond-based electrochemical sensor for the determination of pyrazinamide antibiotic. Sens. Actuators B Chem. 2017, 250, 315–323. [Google Scholar] [CrossRef]
- Karikalan, N.; Karthik, R.; Chen, S.-M.; Velmurugan, M.; Karuppiah, C. Electrochemical properties of the acetaminophen on the screen printed carbon electrode towards the high performance practical sensor applications. J. Colloid Interface Sci. 2016, 483, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.Y.; Yang, C.K.; Zhang, M.R. A Novel Electrochemical Hydrogen Peroxide Sensor Based on AuNPs/n-Type GaN Electrode. Chem. Lett. 2020, 49, 656–658. [Google Scholar] [CrossRef]
- Patella, B.; Buscetta, M.; Di Vincenzo, S.; Ferraro, M.; Aiello, G.; Sunseri, C.; Pace, E.; Inguanta, R.; Cipollina, C. Electrochemical sensor based on rGO/Au nanoparticles for monitoring H2O2 released by human macrophages. Sens. Actuat. B-Chem. 2021, 327, 128901. [Google Scholar] [CrossRef]
- Teker, M.S.; Karaca, E.; Pekmez, N.O.; Tamer, U.; Pekmez, K. An Enzyme-free H2O2 Sensor Based on Poly(2-Aminophenylbenzimidazole)/Gold Nanoparticles Coated Pencil Graphite Electrode. Electroanalysis 2019, 31, 75–82. [Google Scholar] [CrossRef]
- Yang, Z.; Li, J.; Liu, P.; Zhang, A.; Wang, J.; Huang, Y.; Wang, J.; Wang, Z. Highly sensitive non-enzymatic hydrogen peroxide monitoring platform based on nanoporous gold via a modified solid-phase reaction method. RSC Adv. 2021, 11, 36753–36759. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Chen, Y.; Dong, W.; Han, B.; Liu, M.; Chen, Q.; Zhou, J. A nanocomposite-based electrochemical sensor for non-enzymatic detection of hydrogen peroxide. Oncotarget 2017, 8, 13039–13047. [Google Scholar] [CrossRef]
- Bharathi, S.; Nogami, M.; Ikeda, S. Novel Electrochemical Interfaces with a Tunable Kinetic Barrier by Self-Assembling Organically Modified Silica Gel and Gold Nanoparticles. Langmuir 2001, 17, 1–4. [Google Scholar] [CrossRef]
- Elewi, A.S.; Al-Shammaree, S.A.W.; Al Sammarraie, A.K.M.A. Hydrogen peroxide biosensor based on hemoglobin-modified gold nanoparticles–screen printed carbon electrode. Sens. Bio-Sens. Res. 2020, 28, 100340. [Google Scholar] [CrossRef]
- Rao Vusa, C.S.; Manju, V.; Berchmans, S.; Arumugam, P. Electrochemical amination of graphene using nanosized PAMAM dendrimers for sensing applications. RSC Adv. 2016, 6, 33409–33418. [Google Scholar] [CrossRef]
- Xie, H.; Luo, G.; Niu, Y.; Weng, W.; Zhao, Y.; Ling, Z.; Ruan, C.; Li, G.; Sun, W. Synthesis and utilization of Co3O4 doped carbon nanofiber for fabrication of hemoglobin-based electrochemical sensor. Mater. Sci. Eng. C 2020, 107, 110209. [Google Scholar] [CrossRef]
- Asif, S.A.B.; Khan, S.B.; Asiri, A.M. Electrochemical sensor for H2O2 using a glassy carbon electrode modified with a nanocomposite consisting of graphene oxide, cobalt(III) oxide, horseradish peroxidase and nafion. Microchim. Acta 2016, 183, 3043–3052. [Google Scholar] [CrossRef]
- Lee, Y.J.; Park, J.Y.; Kim, Y.; Ko, J.W. Amperometric sensing of hydrogen peroxide via highly roughened macroporous Gold-/Platinum nanoparticles electrode. Curr. Appl. Phys. 2011, 11, 211–216. [Google Scholar] [CrossRef]
- Tomassetti, M.; Leonardi, C.; Pezzilli, R.; Prestopino, G.; Di Natale, C.; Medaglia, P.G. New Voltammetric Sensor Based on LDH and H2O2 for L-Proline Determination in Red and White Wines. Crystals 2022, 12, 1474. [Google Scholar] [CrossRef]
- Mazzotta, E.; Di Giulio, T.; Mastronardi, V.; Pompa, P.P.; Moglianetti, M.; Malitesta, C. Bare Platinum Nanoparticles Deposited on Glassy Carbon Electrodes for Electrocatalytic Detection of Hydrogen Peroxide. ACS Appl. Nano Mater. 2021, 4, 7650–7662. [Google Scholar] [CrossRef]
- Rashed, M.A.; Ahmed, J.; Faisal, M.; Alsareii, S.A.; Jalalah, M.; Tirth, V.; Harraz, F.A. Surface modification of CuO nanoparticles with conducting polythiophene as a non-enzymatic amperometric sensor for sensitive and selective determination of hydrogen peroxide. Surf. Interfaces 2022, 31, 101998. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, C.; Li, X.; Du, X. An Electrochemical Sensor Based on Chalcogenide Molybdenum Disulfide-Gold-Silver Nanocomposite for Detection of Hydrogen Peroxide Released by Cancer Cells. Sensors 2020, 20, 6817. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.Y.; Wu, Q.; Wei, W.; Liu, X.Y.; Li, X.J. Preparation and electrochemical application of an AgNW/graphene/SU-8 composite conductive photoresist. J. Appl. Polym. Sci. 2021, 138, 51205. [Google Scholar] [CrossRef]
- Lete, C.; Spinciu, A.M.; Alexandru, M.G.; Moreno, J.C.; Leau, S.A.; Marin, M.; Visinescu, D. Copper(II) Oxide Nanoparticles Embedded within a PEDOT Matrix for Hydrogen Peroxide Electrochemical Sensing. Sensors 2022, 22, 8252. [Google Scholar] [CrossRef] [PubMed]
- Rashed, M.A.; Harraz, F.A.; Faisal, M.; El-Toni, A.M.; Alsaiari, M.; Al-Assiri, M.S. Gold nanoparticles plated porous silicon nanopowder for nonenzymatic voltammetric detection of hydrogen peroxide. Anal. Biochem. 2021, 615, 114065. [Google Scholar] [CrossRef] [PubMed]
- Elancheziyan, M.; Theyagarajan, K.; Saravanakumar, D.; Thenmozhi, K.; Senthilkumar, S. Viologen-terminated polyamidoamine (PAMAM) dendrimer encapsulated with gold nanoparticles for nonenzymatic determination of hydrogen peroxide. Mater. Today Chem. 2020, 16, 100274. [Google Scholar] [CrossRef]
- Divya, P.M.; Roopa, B.S.; Manusha, C.; Balannara, P. A concise review on oil extraction methods, nutritional and therapeutic role of coconut products. J. Food Sci. Tech. Mys. 2022, 33, 8576. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, Y.; Zeng, M.; Zhao, Y.; Zuo, X.; Meng, F.; Lv, F.; Lu, Y. Zr(IV)-based metal-organic framework nanocomposites with enhanced peroxidase-like activity as a colorimetric sensing platform for sensitive detection of hydrogen peroxide and phenol. Environ. Res. 2022, 203, 111818. [Google Scholar] [CrossRef]
- Ullah, R.; Rasheed, M.A.; Abbas, S.; Rehman, K.U.; Shah, A.; Ullah, K.; Khan, Y.; Bibi, M.; Ahmad, M.; Ali, G. Electrochemical sensing of H2O2 using cobalt oxide modified TiO2 nanotubes. Curr. Appl. Phys. 2022, 38, 40–48. [Google Scholar] [CrossRef]
- Mohammad, A.; Zamzami, M.A. Construction of carbon cloth modified-Al2O3-g-C3N4 sensor for non-enzymatic electrochemical detection of hydrogen peroxide. Diam. Relat. Mater. 2023, 132, 109600. [Google Scholar] [CrossRef]
- Liu, Y.H.; Cui, L.P.; Yu, K.; Wang, M.L.; Ma, Y.J.; Lv, J.H.; Liu, X.Z.; Zhou, B.B. The reduced phosphomolybdate as dual-functional electrocatalyst and electrochemical sensor for detecting hydrogen peroxide and dopamine. J. Solid State Chem. 2022, 312, 123209. [Google Scholar] [CrossRef]
- Hooch Antink, W.; Choi, Y.; Seong, K.-d.; Piao, Y. Simple synthesis of CuO/Ag nanocomposite electrode using precursor ink for non-enzymatic electrochemical hydrogen peroxide sensing. Sens. Actuators B Chem. 2018, 255, 1995–2001. [Google Scholar] [CrossRef]
- Mathew, M.; Sandhyarani, N. A novel electrochemical sensor surface for the detection of hydrogen peroxide using cyclic bisureas/gold nanoparticle composite. Biosens. Bioelectron. 2011, 28, 210–215. [Google Scholar] [CrossRef]
- Ma, J.; Zheng, J. Voltammetric determination of hydrogen peroxide using AuCu nanoparticles attached on polypyrrole-modified 2D metal-organic framework nanosheets. Microchim. Acta 2020, 187, 389. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Tang, K.-l.; Lu, K.; Zhang, C.; Shi, W.; Zhao, W. Structural Engineering of Hollow Microflower-like CuS@C Hybrids as Versatile Electrochemical Sensing Platform for Highly Sensitive Hydrogen Peroxide and Hydrazine Detection. ACS Appl. Mater. Interfaces 2021, 13, 40942–40952. [Google Scholar] [CrossRef] [PubMed]
- Płócienniczak, P.; Rębiś, T.; Nowicki, M.; Milczarek, G. A green approach for hybrid material preparation based on carbon nanotubes/lignosulfonate decorated with silver nanostructures for electrocatalytic sensing of H2O2. J. Electroanal. Chem. 2021, 880, 114896. [Google Scholar] [CrossRef]
- Kader, M.A.; Azmi, N.S.; Kafi, A.K.M.; Hossain, M.S.; Jose, R.; Goh, K.W. Ultrasensitive Nonenzymatic Real-Time Hydrogen Peroxide Monitoring Using Gold Nanoparticles Decorated Titanium Dioxide Nanotube Electrodes. Biosensors 2023, 2071464. [Google Scholar]


| Electrode Materials | Linear Range | Detection Limit (µM) | Stability (Days) | Ref. |
|---|---|---|---|---|
| GCE/Au NPs–SnO2 NFs composites | 49.98–3937.21 µM | 6.67 µM | 41 | Current study |
| GCE/CtRGO/PAMAM/GA/HRP | 50–800 μM | 29.86 μM | 33 | [64] |
| Nafion/Hb/Co3O4–CNF/CILE | 1–12 mM | 330 μM | 15 | [65] |
| HRP/GO-Co3O4-Nafion/GCE | 1–30 mM | 2 mM | 30 | [66] |
| Fe2P/NP C/GCE | 0.1–1 mM | 60 μM | 7 | [15] |
| Porous Au-PtNP | 0.3–10 mM | 50 μM | [67] | |
| P2AB/AuNPs/PGE | 0.06–100 μM | 36.7 μM | 3 | [59] |
| GC-Ag(paste)-LDH | 125–3200 μM | 85 µM | 5 | [68] |
| 4 nm PtNPs/GCE | 0.025–0.75 mM | 10 µM | 10 | [69] |
| Pth-CuO/GCE | 0–3300 μM | 3.86 μM | 15 | [70] |
| MoS2-Au-Ag | 0.05–20 mM | 7.19 μM | [71] | |
| CuNPs/AgNW/GR/SU-8/ITO | 1–25 mM | 9 μM | 15 | [72] |
| PEDOT-CuO | 0.04–10 mM | 8.5 μM | 90 | [73] |
| AuNPs-PSi | 2.0–13.81 mM (LSV) 0.5–6.91 mM (SWV) | 14.84 μM (LSV) 15.16 μM (SWV) | 12 | [74] |
| G3.0 Vio-PAMAM-AuNPs/GCE | 0.1 mM–6.2 mM | 27 μM | 30 | [75] |
| ITO-rGO-AuNPs | 25 μM–3 mM | 6.55 μM | [58] | |
| AQ-PF6-IL/SPE | 10–1228 μM | 2.87 µM | 30 | [76] |
| Zr-MOF-PVP | 10–800 μM | 2.76 μM | [77] | |
| Co3O4/ATNTs | 1.27–26.80 mM | 6.71 μM | 35 | [78] |
| Al2O3/CC | 0.002–0.035 mM | 110 μM | [79] | |
| 2-AB-GCE | 21 μM–34.648 mM | 7 μM | 31 | [80] |
| Addition No. | H2O2 Added (μM) | H2O2 Found (μM) | Recovery (%) |
|---|---|---|---|
| 1 | 100.05 | 108.71 | 108.66 |
| 2 | 199.90 | 184.22 | 92.16 |
| 3 | 299.55 | 289.25 | 96.56 |
| 4 | 399.00 | 422.92 | 105.99 |
| Addition No. | H2O2 Added (μM) | H2O2 Found (μM) | Recovery (%) |
|---|---|---|---|
| 1 | 100.05 | 110.14 | 110.08 |
| 2 | 199.90 | 198.93 | 99.51 |
| 3 | 299.55 | 318.42 | 106.30 |
| 4 | 399.00 | 460.59 | 115.43 |
| Addition No. | H2O2 Added (μM) | H2O2 Found (μM) | Recovery (%) |
|---|---|---|---|
| 1 | 200.29 | 202.01 | 100.86 |
| 2 | 400.19 | 426.18 | 106.50 |
| 3 | 599.69 | 576.49 | 96.13 |
| 4 | 798.79 | 772.80 | 96.75 |
| Addition No. | H2O2 Added (μM) | H2O2 Found (μM) | Recovery (%) |
|---|---|---|---|
| 1 | 199.70 | 196.97 | 98.64 |
| 2 | 398.61 | 396.91 | 99.57 |
| 3 | 596.73 | 578.07 | 96.87 |
| 4 | 794.07 | 767.58 | 96.66 |
| Addition No. | H2O2 Added (μM) | H2O2 Found (μM) | Recovery (%) |
|---|---|---|---|
| 1 | 200.29 | 195.74 | 97.73 |
| 2 | 400.19 | 406.29 | 101.525 |
| 3 | 599.69 | 611.11 | 101.90 |
| 4 | 798.79 | 839.78 | 105.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kader, M.A.; Azmi, N.S.; Kafi, A.K.M.; Hossain, M.S.; Masri, M.F.B.; Ramli, A.N.M.; Tan, C.S. Synthesis and Characterization of a Multiporous SnO2 Nanofibers-Supported Au Nanoparticles-Based Amperometric Sensor for the Nonenzymatic Detection of H2O2. Chemosensors 2023, 11, 130. https://doi.org/10.3390/chemosensors11020130
Kader MA, Azmi NS, Kafi AKM, Hossain MS, Masri MFB, Ramli ANM, Tan CS. Synthesis and Characterization of a Multiporous SnO2 Nanofibers-Supported Au Nanoparticles-Based Amperometric Sensor for the Nonenzymatic Detection of H2O2. Chemosensors. 2023; 11(2):130. https://doi.org/10.3390/chemosensors11020130
Chicago/Turabian StyleKader, Md. Ashraful, Nina Suhaity Azmi, A. K. M. Kafi, Md. Sanower Hossain, Mohd Faizulnazrie Bin Masri, Aizi Nor Mazila Ramli, and Ching Siang Tan. 2023. "Synthesis and Characterization of a Multiporous SnO2 Nanofibers-Supported Au Nanoparticles-Based Amperometric Sensor for the Nonenzymatic Detection of H2O2" Chemosensors 11, no. 2: 130. https://doi.org/10.3390/chemosensors11020130
APA StyleKader, M. A., Azmi, N. S., Kafi, A. K. M., Hossain, M. S., Masri, M. F. B., Ramli, A. N. M., & Tan, C. S. (2023). Synthesis and Characterization of a Multiporous SnO2 Nanofibers-Supported Au Nanoparticles-Based Amperometric Sensor for the Nonenzymatic Detection of H2O2. Chemosensors, 11(2), 130. https://doi.org/10.3390/chemosensors11020130




.png)






