A Non-Contact Method for Detecting and Distinguishing Chloride and Carbonate Salts Based on Dielectric Properties Using a Microstrip Patch Sensor
Abstract
1. Introduction
2. Materials and Methods
2.1. Microstrip Patch Sensor Design and Fabrication
2.2. Salt Concentration Preparation
2.3. Measurement Setup
3. Results
3.1. Reflection Coefficient of Sensor Response
3.2. The Relationship between the Concentration of Salt in Water and the Resulting Values of S11 and Fr
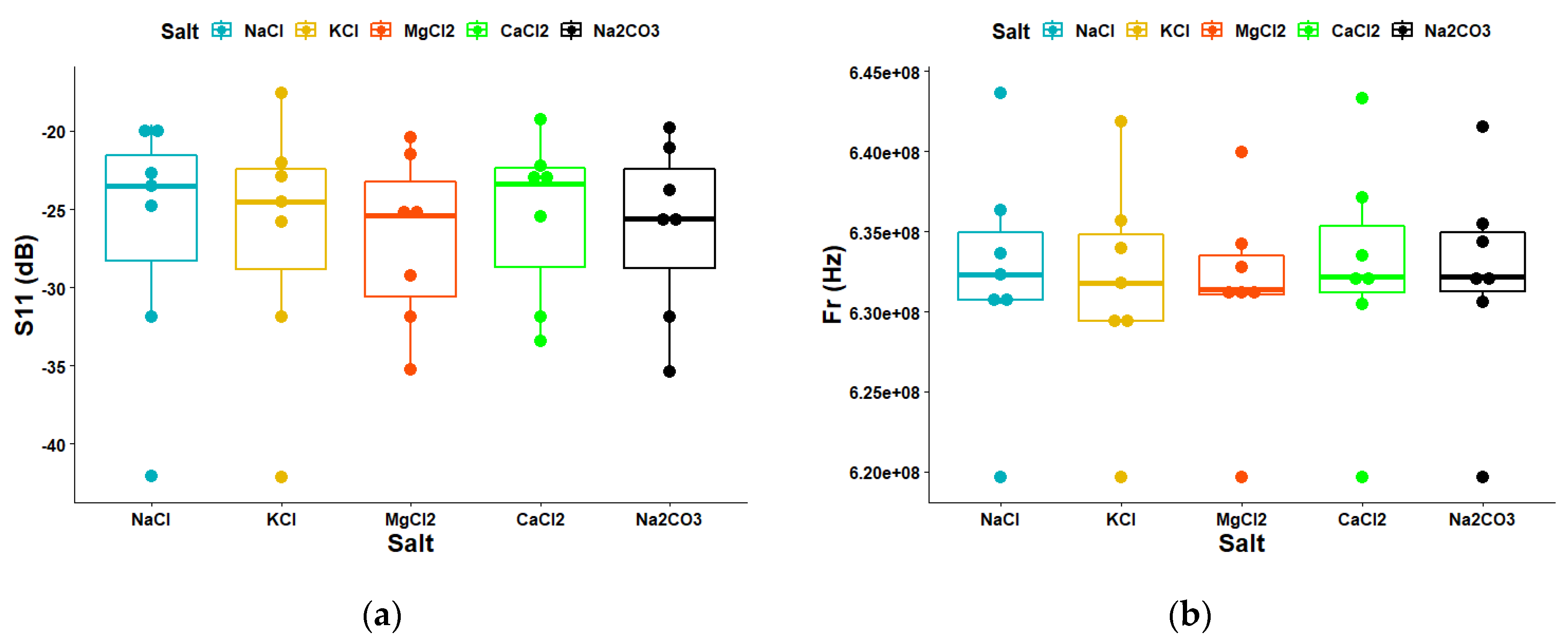
3.3. Statistical Analysis
4. Limitations of the Work
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mata-Contreras, J.; Herrojo, C.; Martín, F. Detecting the rotation direction in contactless angular velocity sensors implemented with rotors loaded with multiple chains of resonators. IEEE Sens. J. 2018, 18, 7055–7065. [Google Scholar] [CrossRef]
- Naqui, J.; Martín, F. Angular displacement and velocity sensors based on electric-LC (ELC) loaded microstrip lines. IEEE Sens. J. 2014, 14, 939–940. [Google Scholar] [CrossRef]
- Horestani, A.; Fumeaux, C.; Al-Sarawi, S.; Abbott, D. Displacement sensor based on diamond-shaped tapered split ring resonator. IEEE Sens. J. 2013, 13, 1153–1160. [Google Scholar] [CrossRef]
- Horestani, A.; Fumeaux, C.; Al-Sarawi, S.; Abbott, D. Rotation sensor based on horn-shaped split ring resonator. IEEE Sens. J. 2013, 13, 3014–3015. [Google Scholar] [CrossRef]
- Wang, L.; Chung, K.L.; Zong, W.; Feng, B. A highly sensitive microwave patch sensor for multidirectional strain sensing based on near orthogonal modes. IEEE Access 2021, 9, 24669–24681. [Google Scholar] [CrossRef]
- Daliri, A.; Galehdar, A.; John, S.; Wang, C.H.; Rowe, W.S.T.; Ghorbani, K. Wireless strain measurement using circular microstrip patch antennas. Sens. Actuators A Phys. 2012, 184, 86–92. [Google Scholar] [CrossRef]
- Cho, C.; Yi, X.; Li, D.; Wang, Y.; Tentzeris, M.M. Passive wireless frequency doubling antenna sensor for strain and crack sensing. IEEE Sens. J. 2016, 16, 5725–5733. [Google Scholar] [CrossRef]
- Ibrahim, A.; Cumming, D.R.S. Passive single chip wireless microwave pressure sensor. Sens. Actuators A Phys. 2011, 165, 200–206. [Google Scholar] [CrossRef]
- Zarifi, M.H.; Wiltshire, B.; Mahdi, N.; Kar, P.; Shankar, K.; Daneshmand, M. Ultraviolet sensing using a TiO2 nanotube integrated high resolution planar microwave resonator device. Nanoscale 2018, 10, 4882–4889. [Google Scholar] [CrossRef]
- Kang, T.G.; Park, J.K.; Yun, G.H.; Choi, H.H.; Lee, H.J.; Yook, J.G. A real-time humidity sensor based on a microwave oscillator with conducting polymer PEDOT: PSS film. Sens. Actuators B Chem. 2019, 282, 145–151. [Google Scholar] [CrossRef]
- Mohd Bahar, A.A.; Zakaria, Z.; Isa AA, M.; Dasril, Y.; Alahnomi, R.A. Real time microwave biochemical sensor based on circular SIW approach for aqueous dielectric detection. Sci. Rep. 2019, 9, 5467. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.Y.; Yesiloz, G.; Boybay, M.S.; Ren, C.L. Microwave temperature measurement in microfluidic devices. Lab Chip 2016, 16, 2192–2197. [Google Scholar] [CrossRef]
- Rydosz, A.; Maciak, E.; Wincza, K.; Gruszczynski, S. Microwave-based sensors with phthalocyanine films for acetone, ethanol and methanol detection. Sens. Actuators B Chem. 2016, 237, 876–886. [Google Scholar] [CrossRef]
- Bailly, G.; Harrabi, A.; Rossignol, J.; Stuerga, D.; Pribetich, P. Microwave gas sensing with a microstrip interdigital capacitor: Detection of NH3 with TiO2 nanoparticles. Sens. Actuators B Chem. 2016, 236, 554–564. [Google Scholar] [CrossRef]
- Zarifi, M.H.; Shariaty, P.; Hashisho, Z.; Daneshmand, M. A non-contact microwave sensor for monitoring the interaction of zeolite 13X with CO2 and CH4 in gaseous streams. Sens. Actuators B Chem. 2017, 238, 1240–1247. [Google Scholar] [CrossRef]
- Withayachumnankul, W.; Jaruwongrungsee, K.; Tuantranont, A.; Fumeaux, C.; Abbott, D. Metamaterial-based microfluidic sensor for dielectric characterization. Sens. Actuators A Phys. 2013, 189, 233–237. [Google Scholar] [CrossRef]
- Withayachumnankul, W.; Jaruwongrungsee, K.; Fumeaux, C.; Abbott, D. Metamaterial-inspired multichannel thin-film sensor. IEEE Sens. J. 2012, 12, 1455–1458. [Google Scholar] [CrossRef]
- Melikyan, H.; Danielyan, E.; Kim, S.; Kim, J.; Babajanyan, A.; Lee, J.; Friedman, B.; Lee, K. Non-invasive in vitro sensing of d-glucose in pig blood. Med. Eng. Phys. 2012, 34, 299–304. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Withayachumnankul, W.; Al-Sarawi, S.; Abbott, D. High-sensitivity metamaterial-inspired sensor for microfluidic dielectric characterization. IEEE Sens. J. 2014, 14, 1345–1351. [Google Scholar] [CrossRef]
- Kim, N.Y.; Dhakal, R.; Adhikari, K.K.; Kim, E.S.; Wang, C. A reusable robust radio frequency biosensor using microwave resonator by integrated passive device technology for quantitative detection of glucose level. Biosens. Bioelectron. 2015, 67, 687–693. [Google Scholar] [CrossRef]
- Zarifi, M.H.; Farsinezhad, S.; Shankar, K.; Daneshmand, M. Liquid sensing using active feedback assisted planar microwave resonator. IEEE Microw. Wirel. Compon. Lett. 2015, 25, 621–623. [Google Scholar] [CrossRef]
- Zarifi, M.H.; Sadabadi, H.; Hejazi, S.H.; Daneshmand, M.; Sanati-Nezhad, M. Noncontact and nonintrusive microwave-microfuidic flow sensor for energy and biomedical engineering. Sci. Rep. 2018, 8, 139. [Google Scholar] [CrossRef]
- Zarifi, M.H.; Rahimi, M.; Daneshmand, M.; Thundat, T. Microwave ring resonator-based non-contact interface sensor for oil sands applications. Sens. Actuators B Chem. 2016, 224, 632–639. [Google Scholar] [CrossRef]
- Zarifi, M.H.; Daneshmand, M. Liquid sensing in aquatic environment using high quality planar microwave resonator. Sens. Actuators B Chem. 2016, 225, 517–521. [Google Scholar] [CrossRef]
- Harnsoongnoen, S.; Wanthong, A. Coplanar waveguides loaded with a split ring resonator-based microwave sensor for aqueous sucrose solutions. Meas. Sci. Technol. 2016, 27, 015103. [Google Scholar] [CrossRef]
- Harnsoongnoen, S.; Wanthong, A. Real-time monitoring of sucrose, sorbitol, D-glucose and D-fructose concentration by electromagnetic sensing. Food Chem. 2017, 232, 566–570. [Google Scholar] [CrossRef]
- Harnsoongnoen, S.; Wanthong, A.; Charoen-In, U.; Siritaratiwat, A. Planar microwave sensor for detection and discrimination of aqueous organic and inorganic solutions. Sens. Actuators B Chem. 2018, 271, 300–305. [Google Scholar] [CrossRef]
- Harnsoongnoen, S.; Wanthong, A.; Charoen-In, U.; Siritaratiwat, A. Microwave sensor for nitrate and phosphate concentration sensing. IEEE Sens. J. 2019, 19, 2950–2955. [Google Scholar] [CrossRef]
- Harnsoongnoen, S.; Wanthong, A. A non-contact planar microwave sensor for detection of high-salinity water containing NaCl, KCl, CaCl2, MgCl2 and Na2CO3. Sens. Actuators B Chem. 2021, 331, 129355. [Google Scholar] [CrossRef]
- Srisai, S.; Harnsoongnoen, S. Noncontact planar microwave sensor for liquid interface detection by a pixelated CSRR-loaded microstrip line. J. RF Microw. Comput.-Aided Eng. 2021, 31, e22557. [Google Scholar] [CrossRef]
- Harnsoongnoen, S. Metamaterial-inspired microwave sensor for detecting the concentration of mixed phosphate and nitrate in water. IEEE Trans. Instrum. Meas. 2021, 70, 9509906. [Google Scholar] [CrossRef]
- Harnsoongnoen, S. Microwave Sensors Based on Coplanar Waveguide Loaded with Split Ring Resonators: A Review. Appl. Sci. Eng. Prog. 2019, 12, 224–234. [Google Scholar] [CrossRef]
- Vélez, P.; Muñoz-Enano, J.; Grenier, K.; Mata-Contreras, J.; Dubuc, D.; Martín, F. Split ring resonator-based microwave fluidic sensors for electrolyte concentration measurements. IEEE Sens. J. 2019, 19, 2562–2569. [Google Scholar] [CrossRef]
- Vélez, P.; Grenier, K.; Mata-contreras, J.; Dubuc, D.; Martin, F. Highly-sensitive microwave sensors based on open complementary split ring resonators (OCSRRs) for dielectriccharacterization and solute concentration measurement in liquids. IEEE Access 2018, 6, 48324–48338. [Google Scholar] [CrossRef]
- Vélez, P.; Su, L.; Grenier, K.; Mata-Contreras, J.; Dubuc, D.; Martín, F. Microwave microfluidic sensor based on a microstrip splitter/combiner configuration and split ring resonators (SRRs) for dielectric characterization of liquids. IEEE Sens. J. 2017, 17, 6589–6598. [Google Scholar] [CrossRef]
- Zarifi, M.H.; Shariaty, P.; Abdolrazzaghi, M.; Hashisho, Z.; Daneshmand, M. Particle size characterization using a high resolution planar resonator sensor in a lossy medium. Sens. Actuators B Chem. 2016, 234, 332–337. [Google Scholar] [CrossRef]
- Zarifi, M.H.; Thundat, T.; Daneshmand, M. High resolution microwave microstrip resonator for sensing applications. Sens. Actuators A Phys. 2015, 233, 224–230. [Google Scholar] [CrossRef]
- Trabelsi, S.; Nelson, S.O. Microwave sensing of quality attributes of agricultural and food products. IEEE Instrum. Meas. Mag. 2016, 19, 36–41. [Google Scholar] [CrossRef]
- Li, F.; Zheng, Y.; Hua, C.; Jian, J. Gas sensing by microwave transduction: Review of progress and challenges. Front. Mater. 2019, 6, 101. [Google Scholar] [CrossRef]
- Martín, F.; Vélez, P.; Gil, M. Microwave sensors based on resonant elements. Sensors 2020, 20, 3375. [Google Scholar] [CrossRef] [PubMed]
- Haq, T.; Ruan, C.; Zhang, X.; Ullah, S.; Fahad, A.K.; He, W. Extremely sensitive microwave sensor for evaluation of dielectric characteristics of low-permittivity materials. Sensors 2020, 20, 1916. [Google Scholar] [CrossRef]
- Fayaz, M.; Zarifi, M.H.; Abdolrazzaghi, M.; Shariaty, P.; Hashisho, Z.; Daneshmand, M. A novel technique for determining the adsorption capacity and breakthrough time of adsorbents using a noncontact high-resolution microwave resonator sensor. Environ. Sci. Technol. 2017, 51, 427–435. [Google Scholar] [CrossRef]
- Kozak, R.; Wiltshire, B.D.; Khandoker, M.A.R.; Golovin, K.; Zarifi, M.H. Modified microwave sensor with a patterned ground heater for detection and prevention of ice accumulation. ACS Appl. Mater. Interfaces 2020, 12, 55483–55492. [Google Scholar] [CrossRef] [PubMed]
- Elias, M.; Laranjo, M.; Agulheiro-Santos, A.C.; Potes, M.E. The role of salt on food and human health. In Salt in the Earth; Cinku, M., Karabulut, S., Eds.; IntechOpen: London, UK. [CrossRef]
- Durack, E.; Alonso-Gomez, M.; Wilkinson, M.G. Salt: A review of its role in food science and public health. Curr. Nutr. Food Sci. 2008, 4, 290–297. [Google Scholar] [CrossRef]
- Islam, M.T.; Rahaman, M.N.; Singh, M.S.J.; Samsuzzaman, M. Detection of salt and sugar contents in water on the basis of dielectric properties. IEEE Access. 2018, 6, 4118–4126. [Google Scholar] [CrossRef]
- Naqui, J.; Martín, F. Transmission lines loaded with bisymmetric resonators and their application to angular displacement and velocity sensors. IEEE Trans. Microw. Theory Tech. 2013, 61, 4700–4712. [Google Scholar] [CrossRef]
- Lugli, S.M.; Lutz, W.K. Stimulation of cell division in the rat by NaCl, KCl, MgCl2, and CaCl2, and inhibition of the sodium chloride effect on the glandular stomach by ascorbic acid and beta-carotene. J. Cancer Res. Clin. Oncol. 1999, 125, 209–213. [Google Scholar] [CrossRef]
- Zhao, Y.; Liao, Y.; Zhang, B.; Lai, S. Monitoring technology of salinity in water with optical fiber sensor. J. Light. Technol. 2003, 21, 1334–1338. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Wang, X.; Li, P.; Hao, Q.; Xiao, B. Survey and evaluation of equations for thermophysical properties of binary/ternary eutectic salts from NaCl, KCl, MgCl2, CaCl2, ZnCl2 for heat transfer and thermal storage fluids in CSP. Sol. Energy 2017, 152, 57–79. [Google Scholar] [CrossRef]
- Kilpijärvi, J.; Halonen, N.; Juuti, J.; Hannu, J. Microfluidic microwave sensor for detecting saline in biological range. Sensors 2019, 19, 819. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Hassan, A.; Lee, C.H.; Bae, J. Microstrip patch sensor for salinity determination. Sensors 2017, 17, 2941. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.N.; Islam, M.T.; Samsuzzaman, M. Detection of different concentrated salt and sugar solution based on dielectric properties using microstrip technology. Microw. Opt. Technol. Lett. 2018, 60, 1573–1577. [Google Scholar] [CrossRef]
- Rahman, M.N.; Hassan, S.A.; Samsuzzaman, M.; Singh, M.S.J.; Islam, M.T. Determination of salinity and sugar concentration using microwave sensor. Microw. Opt. Technol. Lett. 2018, 61, 361–364. [Google Scholar] [CrossRef]
- Rahman, M.N.; Islam, M.T.; Samsuzzaman, M. Development of a microstrip based sensor aimed at salinity and sugar detection in water considering dielectric properties. Microw. Opt. Technol. Lett. 2018, 60, 667–672. [Google Scholar] [CrossRef]
- Rahman, M.N.; Islam, M.T.; Sobuz, M.S. Microwave measurement system to detect salt and sugar concentration. Microw. Opt. Technol. Lett. 2018, 60, 1772–1774. [Google Scholar] [CrossRef]
- Rahman, M.N.; Islam, M.T.; Samsuzzaman, M. Salinity and sugar detection system using microstrip patch antenna. Microw. Opt. Technol. Lett. 2018, 60, 1092–1096. [Google Scholar] [CrossRef]
- Buchner, R. Dielectric Spectroscopy of Solutions, In Novel Approaches to the Structure and Dynamics of Liquids: Experimets, Theories and Simulations; NATO Science Series (Series II: Mathematics, Physics and Chemistiry); Samios, J., Durov, V., Eds.; Springer: Dordrecht, The Netherlands, 2004; Volume 133, pp. 265–288. [Google Scholar]
- Capewell, S.G.; Buchner, R.; Hefter, G.; May, P.M. Dielectric relaxation of aqueous Na2CO3 solutions. Phys. Chem. Chem. Phys. 1999, 1, 1933–1937. [Google Scholar] [CrossRef]
- Buchner, R.; Hefter, G.T.; May, P.M. Dielectric relaxation of aqueous NaCl solutions. J. Phys. Chem. A. 1999, 103, 1–9. [Google Scholar] [CrossRef]
- Chen, T.; Hefter, G.; Buchner, R. Dielectric spectroscopy of aqueous solutions of KCl and CsCl. J. Phys. Chem. A 2003, 107, 4025–4031. [Google Scholar] [CrossRef]
- Friesen, S.; Hefter, G.; Buchner, R. Cation hydration and ion pairing in aqueous solutions of MgCl2 and CaCl2. J. Phys. Chem. A 2019, 123, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Sihvola, A. Mixing rules with complex dielectric coefficients. Subsurf. Sens. Technol. Appl. 2000, 1, 393–415. [Google Scholar] [CrossRef]
- Nortemann, K.; Hilland, J.; Kaatze, U. Dielectric properties of aqueous NaCl solutions at microwave frequencies. J. Phys. Chem. A 1997, 101, 6864–6869. [Google Scholar] [CrossRef]
- Gavish, N.; Promislow, K. Dependence of the dielectric constant of electrolyte solutions on ionic concentration: A microfield approach. Phys. Rev. E 2016, 94, 012611. [Google Scholar] [CrossRef] [PubMed]
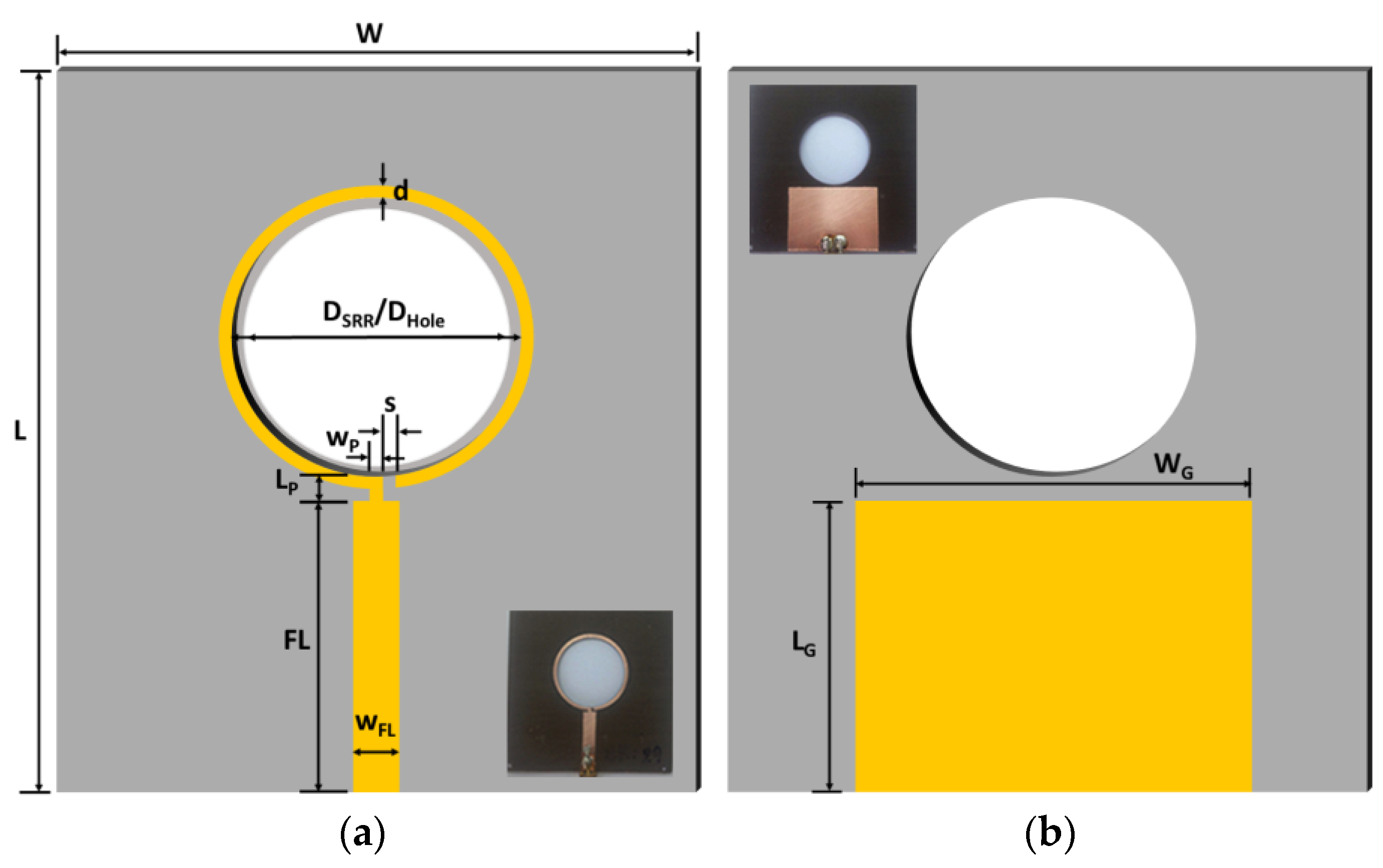
















| Parameter | W | L | WG | LG | WFL | FL | LP | WP | DSRR | DHole | d | s |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Value (mm) | 70 | 75 | 35 | 25 | 5 | 25 | 2 | 1 | 29 | 28.20 | 1 | 1 |
| Sample | Variable | Equation | Parameters | R2 |
|---|---|---|---|---|
| Sodium chloride | S11 | 0.9777 | ||
| Potassium chloride | 0.9418 | |||
| Magnesium chloride | 0.8393 | |||
| Calcium chloride | 0.8387 | |||
| Sodium carbonate | 0.9652 | |||
| Sodium chloride | Fr | 0.9256 | ||
| Potassium chloride | 0.9531 | |||
| Magnesium chloride | 0.9880 | |||
| Calcium chloride | 0.9101 | |||
| Sodium carbonate | 0.9654 |
| Sample | Variable | Equation | Parameters | R2 |
|---|---|---|---|---|
| Sodium chloride | S11 | 0.9747 | ||
| Potassium chloride | 0.9941 | |||
| Magnesium chloride | 0.9954 | |||
| Calcium chloride | 0.9743 | |||
| Sodium carbonate | 0.8600 | |||
| Sodium chloride | Fr | 0.9955 | ||
| Potassium chloride | 0.9702 | |||
| Magnesium chloride | 0.9768 | |||
| Calcium chloride | 0.9578 | |||
| Sodium carbonate | 0.9691 |
| Sample | Variable | Equation | Parameters | R2 |
|---|---|---|---|---|
| Sodium chloride | S11 | 0.9956 | ||
| Potassium chloride | 0.9721 | |||
| Magnesium chloride | 0.9759 | |||
| Calcium chloride | 0.9837 | |||
| Sodium carbonate | 0.9880 | |||
| Sodium chloride | Fr | 0.9770 | ||
| Potassium chloride | 0.9327 | |||
| Magnesium chloride | 0.8477 | |||
| Calcium chloride | 0.9410 | |||
| Sodium carbonate | 0.9032 |
| Structures | Method | Frequency | Materials | Concentrations | Measurement | References |
|---|---|---|---|---|---|---|
| CPW loaded with a circular SRR | Contact | 2.3 GHz–2.6 GHz | Sucrose, sorbitol, glucose, fructose, CaCl2, NaCl, KCl, MgCl2, Na2CO3, and citric acid | 4–20% (w/v) | S21 | [27] |
| Microstrip and SRR with hole in middle | Non-contact | 0.5 GHz–2.2 GHz | CaCl2, NaCl, KCl, MgCl2, and Na2CO3 | 40–200% (w/v) | S11 | [29] |
| Microstrip antenna consists of a crescent-shaped patch and slotted partial ground | Contact | 2.50 GHz–18 GHz | NaCl and sucrose | 20–80% (w/v) | S11 | [46] |
| Interdigitated electrode(IDE) | Contact | 150 kHz–6 GHz | Isopropanol and NaCl | 125 mmol–155 mmol | S11 | [51] |
| Microstrip patch antenna | Contact | 2.5 GHz–3.2 GHz | NaCl | 20 ppt–30 ppt | S11 | [52] |
| Microstrip patch antenna | Contact | 7 GHz–12 GHz | NaCl and sucrose | 20–80% (w/v) | S11 | [53] |
| Microstrip patch antenna | Contact | 3.5 GHz–5.5 GHz | NaCl and sucrose | 20–80% (w/v) | S11 | [54] |
| Microstrip patch antenna | Contact | 2.50 GHz–18 GHz | NaCl and sucrose | 20–80% (w/v) | S11 | [55] |
| Microstrip patch antenna | Contact | 4 GHz–8 GHz | NaCl and sucrose | 20–80% (w/v) | S11 | [56] |
| Microstrip patch antenna | Contact | 14 GHz–18 GHz | NaCl and sucrose | 20–80% (w/v) | S11 | [57] |
| Microstrip and SRR with hole in middle | Non-contact | 0.6 GHz–0.8 GHz | CaCl2, NaCl, KCl, MgCl2, and Na2CO3 | 0–20% (w/v) | S11 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harnsoongnoen, S. A Non-Contact Method for Detecting and Distinguishing Chloride and Carbonate Salts Based on Dielectric Properties Using a Microstrip Patch Sensor. Chemosensors 2023, 11, 158. https://doi.org/10.3390/chemosensors11030158
Harnsoongnoen S. A Non-Contact Method for Detecting and Distinguishing Chloride and Carbonate Salts Based on Dielectric Properties Using a Microstrip Patch Sensor. Chemosensors. 2023; 11(3):158. https://doi.org/10.3390/chemosensors11030158
Chicago/Turabian StyleHarnsoongnoen, Supakorn. 2023. "A Non-Contact Method for Detecting and Distinguishing Chloride and Carbonate Salts Based on Dielectric Properties Using a Microstrip Patch Sensor" Chemosensors 11, no. 3: 158. https://doi.org/10.3390/chemosensors11030158
APA StyleHarnsoongnoen, S. (2023). A Non-Contact Method for Detecting and Distinguishing Chloride and Carbonate Salts Based on Dielectric Properties Using a Microstrip Patch Sensor. Chemosensors, 11(3), 158. https://doi.org/10.3390/chemosensors11030158






