Abstract
Paper-based diagnostics offer a promising alternative to traditional diagnostic methods for point-of-care use due to their low cost, ease of use, portability, rapid results, versatility, and low environmental impact. While paper-based serology tests in the form of lateral flow assays can provide rapid test results for past pathogen exposure, they currently lack the accuracy and sensitivity offered by molecular diagnostic tests such as the polymerase chain reaction (PCR). Loop-mediated isothermal amplification (LAMP)—an isothermal nucleic acid amplification test (NAAT)—provides PCR-like performance while simultaneously reducing the instrumentation and assay complexity associated with PCR. In this review, we discuss a newly emerging class of paper-based LAMP platforms that integrates the versatility of paper microfluidics with the accuracy of NAATs. Since its first adoption in 2015, we have discussed all paper-based LAMP platforms in terms of the paper substrates, reagent incorporation techniques, paper platform design, heating hardware, detection methods, and sensitivity and specificity of paper-based LAMP assays. We conclude by identifying the current challenges and future prospects of paper-based NAATs.
1. Introduction
Early stage diagnosis is a critical component of infectious disease management systems to mitigate and arrest the propagation of pathogen outbreaks [1]. This has largely been applicable in developing countries where difficult living conditions and limited health care cause diseases to propagate more swiftly [2,3,4]. In general, the capacity to perform early detection of pathogenic infections has been primarily hindered by the difficulty in identifying and isolating infected people, owing to a lack of rapid, portable, and accurate diagnostic tests [5,6]. Serological tests play an essential role in rapidly testing infectious diseases [7]. However, designing antibodies can be challenging, leading to slower development of antigen-based assays. Furthermore, immunoassays often lack the sensitivity for direct detection of pathogens. Detection of host immune response (i.e., pathogen-specific IgG and IgM) requires seroconversion, which may lag behind the relevant window for both therapies and patient isolation by several days. Antibody tests can also lead to false positives as they may be detecting the antibodies for the pathogen that infected the body in the past [7,8]. In contrast, molecular diagnostics test such as the polymerase chain reaction (PCR) are considered the gold standard in pathogen detection as they offer high sensitivity and specificity resulting highly reliable diagnostics even in asymptomatic patients [9,10,11].
In this regard, detection methodologies based on nucleic acid amplification tests (NAATs) are expected to play a major in inhibiting the spread of viral and bacterial infections. With resource-limited settings, especially in developing countries, implementing molecular detection techniques such as NAAT at any point-of-care (POC) location is extremely challenging [12]. Conventional NAATs such as the PCR relies on thermal cycling, with a temperature regulation within a few degrees around reaction temperature (~55 °C–~95 °C), along with fluorescence measurements for assay readout [13,14]. These constraints require the use of sophisticated and bulky laboratory equipment. Furthermore, the thermal cycling requirement for PCR makes the test slow, expensive, and power intensive, making them sub-optimal for point of care (POC) diagnosis. To address these limitations, isothermal nucleic acid amplification techniques have become extremely popular recently [10,15,16]. The isothermal operation enables a greatly simplified and low-powered thermal system while simultaneously reducing the testing time. They rely on using multiple specialized primers to initiate strand displacement activity without requiring high denaturation temperatures in PCR [10]. Recently, several promising isothermal amplification strategies have been proposed which can primarily be classified based on their working temperature ranges, enzymes used, necessity for pre-heating, and the overall efficiency. Isothermal amplification techniques such as NASBA (nucleic acid sequence-based amplification) [17,18], 3SR (self-sustained sequence replication) [19,20], TMA (transcription-mediated amplification) [21], SDA (strand displacement amplification) [22,23], and SMART (a simple method for amplifying RNA targets) [24,25] requires the pre-heating procedure (65 °C to 95 °C) followed by the application of isothermal reaction temperature ranging from 37 °C to 41 °C. Although this step reduces the total number of thermal cycles compared to PCR, implementing even two temperature cycles at any POC setting is still challenging [13]. Alternatively, researchers have also developed amplification techniques that can be executed at a single temperature without any pre-heating step. These include processes such as HDA (helicase-dependent amplification) [26,27], RPA (recombinase polymerase amplification) [28,29], RCA (rolling circle amplification) [30,31], RAM (ramification amplification) [32], MDA (multiple displacement amplification) [33,34], and LAMP (loop-mediated isothermal amplification) [35,36]. While promising, isothermal processes such as NASBA, 3SR, TMA, and SDA require two to three enzymes, increasing the preservation and application cost for their use in the POC setting [37]. TMA and SDA have demonstrated an overall decrease in amplification efficiency compared to NASBA and 3SR. In addition, complications toward nuclease selection and higher inefficiency for long target sequences are the other drawbacks of the SDA process [37]. The SMART amplification process suffers from its dependence on multiple probes and enzymes for hybridization steps, thus adding to the complexity of the amplification process [38]. The HDA process utilizes expensive enzymes and thus incurs higher costs to the overall amplification process. Although RPA provides several advantages, such as simple reaction scheme, low reaction temperature, high efficiency, and high specificity, the greater length of the primers and probe limits amplification of longer templates. On the other hand, the RCA process requires circular nucleic acid templates restricting their use for particular target nucleic acid templates. RAM is an improved version of RCA, which results in an exponential amplification of target nucleic acid sequences as compared to RCA. However, RAM utilizes padlock probe whose complex secondary structure not only interferes with the assay detection but also impairs the assay detection efficiency. One of the significant drawbacks of the MDA amplification technique is the high rate of allele dropout (ADO) and extensive preferential amplification. Among all these techniques, LAMP—operating isothermally around 65 °C—has emerged as the most promising and widely used nucleic acid isothermal amplification techniques (Figure 1A,B). Although the primer design of LAMP is complex [39], the usage of 4 to 6 primers spanning 6–8 distinct sequences enhance the specificity as compared to the other amplification procedures. Moreover, the LAMP amplification process utilizes only one enzyme (BST Polymerase) unlike the other isothermal amplification processes. Finally, the amplification efficiency of LAMP is significantly higher as the entire process yields nearly 109-fold amplification in less than 30 min [35,36].
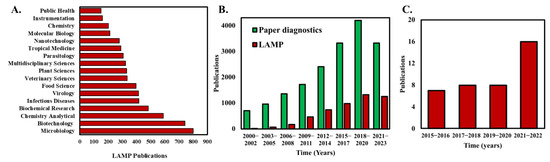
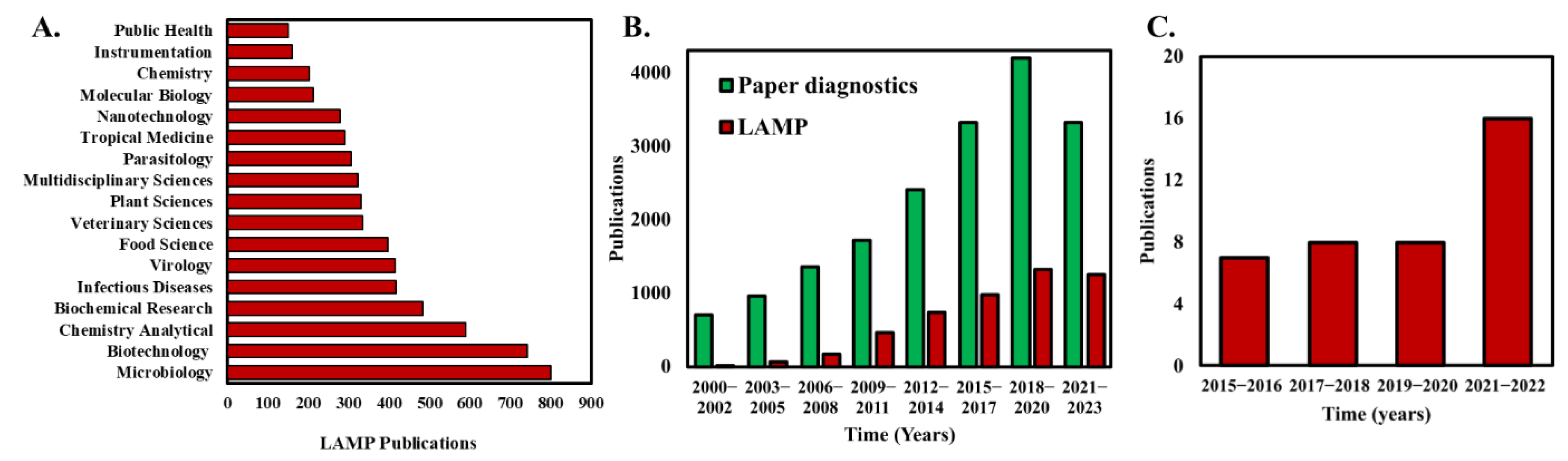
Figure 1.
(A) Analysis of the number of publications that apply LAMP categorized by the topic area. (B) Analysis of annual publications in peer reviewed journals with keywords “paper diagnostics” and “Loop-mediated isothermal amplification” in the title or abstract of the publication. (C) The number of publications that demonstrate and use paper-based LAMP assays. Publication data were compiled on 13 January 2023 from Web of Science database using the keywords “Loop-mediated isothermal amplification” in (A), “Loop-mediated isothermal amplification” and “Paper diagnostics” in (B).
Even with the advantages and simplicity of isothermal LAMP operation, the assay chip brings additional complexity to an otherwise straightforward NAAT [40]. For example, most implementation of LAMP still requires peripheral components such as fluid pumping systems and valves for fluid manipulation, increasing the cost and operational complexity of the system [41]. Alternatively, paper-microfluidics offer several advantages that traditional sample-in-tube assays do not offer such as their low cost, accessibility, biocompatibility, and portability [42]. Furthermore, the porous paper substrates enable capillary-driven passive fluid transport to reliably move liquid samples and reagents without the need for external fluid pumps [43]. Consequently, the number of publications that harness paper for diagnostics have steadily been increasing over the last decade (Figure 1B). Paper-based loop-mediated isothermal amplification (LAMP) is a method that combines the simplicity and low cost of paper-based diagnostic tests with the specificity and sensitivity of LAMP to detect nucleic acid sequences in samples. In this method, the LAMP reaction is performed on a small piece of paper, such as filter paper or chromatography paper, instead of in a test tube. The paper is then incubated at the appropriate temperature, typically ~65 °C, for the LAMP reaction to occur. Recently, researchers have explored the potential of simpler paper-based LAMP devices, well-known for their robustness, cost-effectiveness, and user-friendliness [44,45,46]. A critical aspect of paper-based LAMP detection is the paper’s material selection [40,47]. The nonspecific binding of DNA molecules to the paper’s fibers as well as paper self-fluorescence, negatively influence the noise level and thus the limit of detection (LOD). The most used paper membranes are nitrocellulose-based FTA (Flinders Technology Associates), cellulose, or glass-fiber-based [47]. Another factor in determining the efficiency of paper-based LAMP is the pre-drying of LAMP reagents with the paper matrix. Paper-based LAMP systems that utilize pre-dried reagents eliminate the need for sample preparation steps [46,48]. Dry reagents are less sensitive to storage conditions, thus preventing problems with temperature-dependent transport or storage. Taken together, paper-based LAMP devices offer significant potential to be commercially used as accurate and cost-effective NAATs, especially in resource-limited settings.
Few review articles related to paper-based devices and nucleic acid amplification have been published in the past few years. Choi et al. [49] reviewed the advances and challenges of fully integrated paper-based point-of-care NAATs with a discussion on the implementation of NAATs on low-cost paper substrates. Tian et al. [50] illustrated the latest developments of integrated μPADs (paper-based analytical devices) and highlighted the accomplishments and challenges of each component, including sample collection/pre-treatment, signal transduction, and amplification followed by detection. This review paper provides a comprehensive yet compact discussion on the implementation of paper-based LAMP for the first time. Notably, the first work on paper-based LAMP was reported by the Whitesides group [45] in their work entitled “Paper Machines for molecular diagnostics” where they detected the malb gene in E. coli. Their work was directed toward developing an integrated paper-based LAMP device that involved the functional steps of viral nucleic acid capture, purification and isothermal amplification, and thereafter, leading to real-time fluorescence detection. Although this was the only work on paper-based LAMP reported in 2015, a steady increase in publication records can be found since then, showcasing the significance of this technology. The last couple of years, especially since the COVID-19 pandemic era, have seen a significant increase in paper-based LAMP research and publications [51,52,53,54] (Figure 1C).
First, this review focuses on the development of paper-based LAMP technology in the past decade. In the next section, details about the fabrication of the paper-based assay are discussed, including—(1) LAMP-assay preparation which reviews the different methods for preparing the Clinical/DNA samples; (2) paper membranes which illustrate the different paper membranes used for carrying out LAMP; (3) incorporation of LAMP reagents on paper matrix which discusses the imbibition techniques to introduce LAMP reagents on paper substrate; (4) design of the device which reviews the design and fabrication of paper-based LAMP platforms; (5) heating techniques which discuss the various heating instrument that have been used for paper-based LAMP assays; (6) detection techniques which discuss the various assay detection methodologies utilized for paper-based LAMP; and (7) sensitivity and specificity where the robustness and limit of detection (LOD) of paper-based LAMP assays is reviewed. Finally, the conclusive remarks, challenges, and future scope of paper-based LAMP technology is presented.
2. Fabrication and Design of Paper-Based LAMP Assays
Most NAAT systems function in conjunction with existing laboratory facilities and equipment. Conventional LAMP assays (i.e., LAMP performed in polypropylene PCR tubes) rely on the use of several manual fluid handling steps, primarily with pipettes [40]. Paper-based LAMP systems eliminate these steps resulting in a more straightforward and cost-effective NAAT assay system [9,50]. Here we discuss several aspects of paper-based LAMP assay systems.
2.1. Design of the Paper-Based LAMP Platform
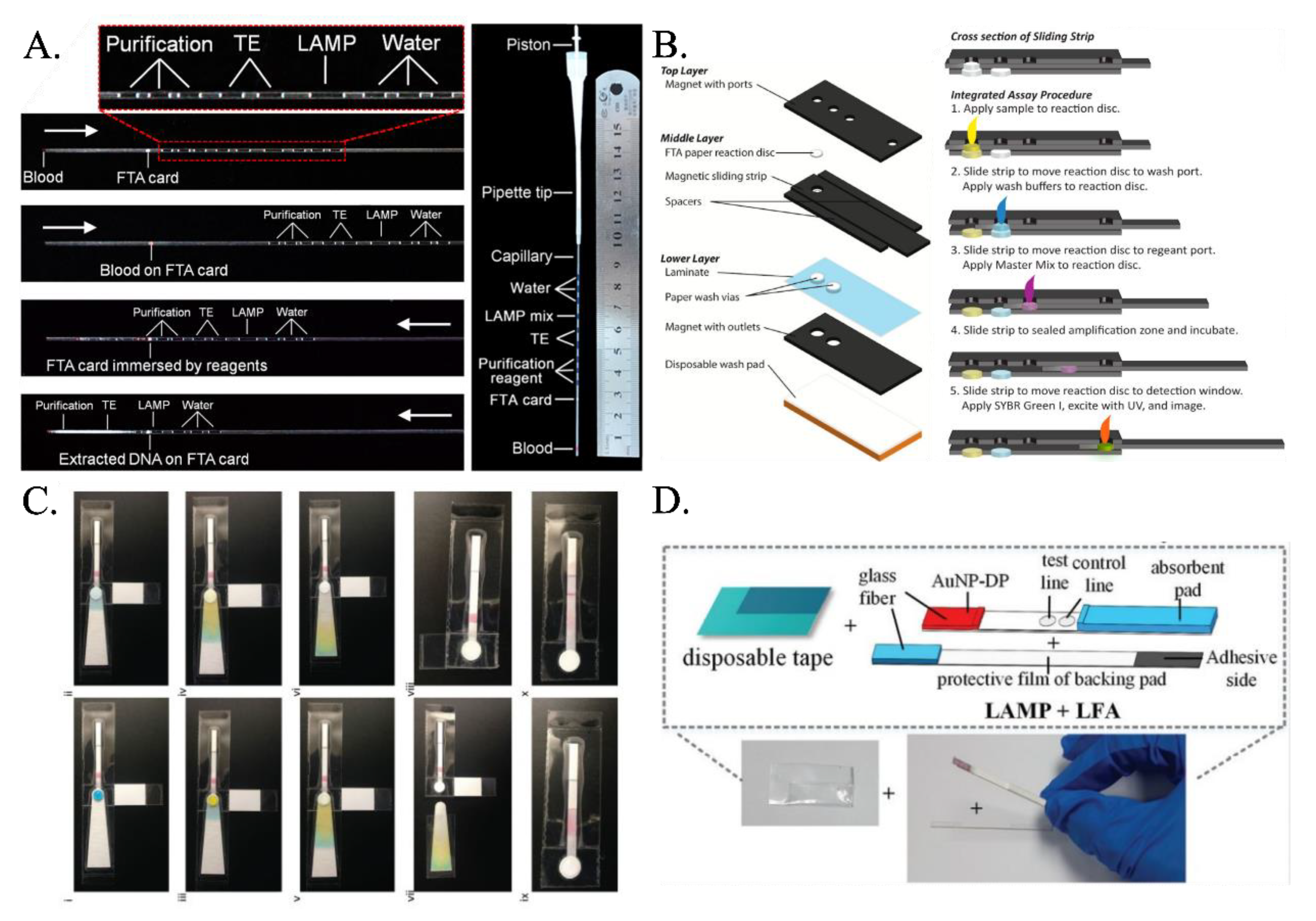
Researchers have proposed various paper-based LAMP devices that integrate the processes of extraction, purification, and amplification. Although few researchers have focused on first extracting the nucleic acid (RNA/DNA) and then amplifying it in the presence of primers and LAMP buffer mixture on paper substrates, the majority of them have used the hybrid technique of detection in lateral flow assays or dipstick after carrying out the conventional tube-based LAMP. Even though FTA card had been used extensively for nucleic acid extraction (Figure 2A) [55,56], it was the implementation by Connelly et al. [45] that first demonstrated the use of the FTA card as an effective paper-based LAMP platform. Their platform—aptly called “paper machines”—comprised of a movable reaction paper disc in a magnetic slide strip-based arrangement (Figure 2B). The reaction paper disk is first overlapped on the paper-based sample port where the clinical sample is added. The excess sample is absorbed into the paper-based sample port, and the sample imbibed reaction paper disc is next hauled to the paper-based wash port. Here, washing is carried out, and the retentate on the reaction paper disc is mainly the nucleic acid. Finally, the reaction paper disc is slid past, and the LAMP master mix is added to execute the LAMP reaction. Rodriguez et al. [57] presented a paper-based LAMP device involving amplification at the sample inlet zone followed by Lateral flow assay-based detection at the right half. The sample inlet port has an absorbent pad beneath the amplification pad which absorbs the excess liquid. It was washed with ethanol, and the wash liquid wicked through to the absorbent pad, removing impurities and leaving behind the purified precipitated DNA. The LAMP reaction mix is placed directly onto the sample port where the purified DNA remains, and the chip’s bottom tab is folded over the designated perforation to act as a cover film for the sample port and prevent evaporation during the heating step. The eluted products wick through the LFD strip toward the right (Figure 2C). Choi et al. [58] came up with an integrated device consisting of an amplification region and a lateral flow assay (LFA)-based detection part (Figure 2D). The amplification region exhibits a glass-fiber membrane protected by a PVC adhesive tape and adhesive PVC backing pad. One end of this backing pad was attached to the lateral flow assay. Later, Choi et al. [59] added the step of cell lysis and extraction of nucleic acid on the FTA paper membrane mounted on a glass-fiber membrane and multiple-layered PVC backing pad. Amplification reagents were added to the glass-fiber membrane, and this was then moved into the covered heating compartment of the handheld heating device for amplification. After amplification, this was directly pasted on the lateral flow assay sample pad, where external fluid was added for carrying over the sample toward the detection section. They also modified the LFA design, where they added PDMS drops and shunt paper layers to delay the flow and enhance the sensitivity.
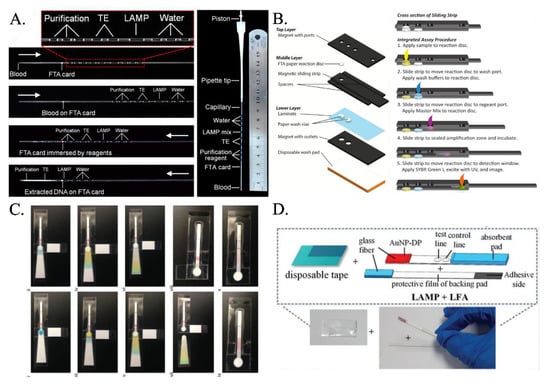
Figure 2.
(A) The microcapillary with preloaded reagents connected to a pipet tip forms all in one LAMP LFA. The white arrow indicates the direction of the fluids [from [56], copyright 2014 American Chemical Society]. (B) Schematic of sliding-strip device shows the three major layers of the sliding-strip architecture and their components [from [45], copyright 2015 American Chemical Society]. (C) Fluidic demonstration of chip operation demonstrates paper-based LAMP assay including sample lysis, capillary fluid transport, ethanol wash, buffer wash, extraction of purified DNA followed by LAMP reaction [from [57], copyright 2016 Royal Society of Chemistry]. (D) An integrated paper-based device incorporating LAMP and LFA [from [58], copyright 2016 Royal Society of Chemistry].
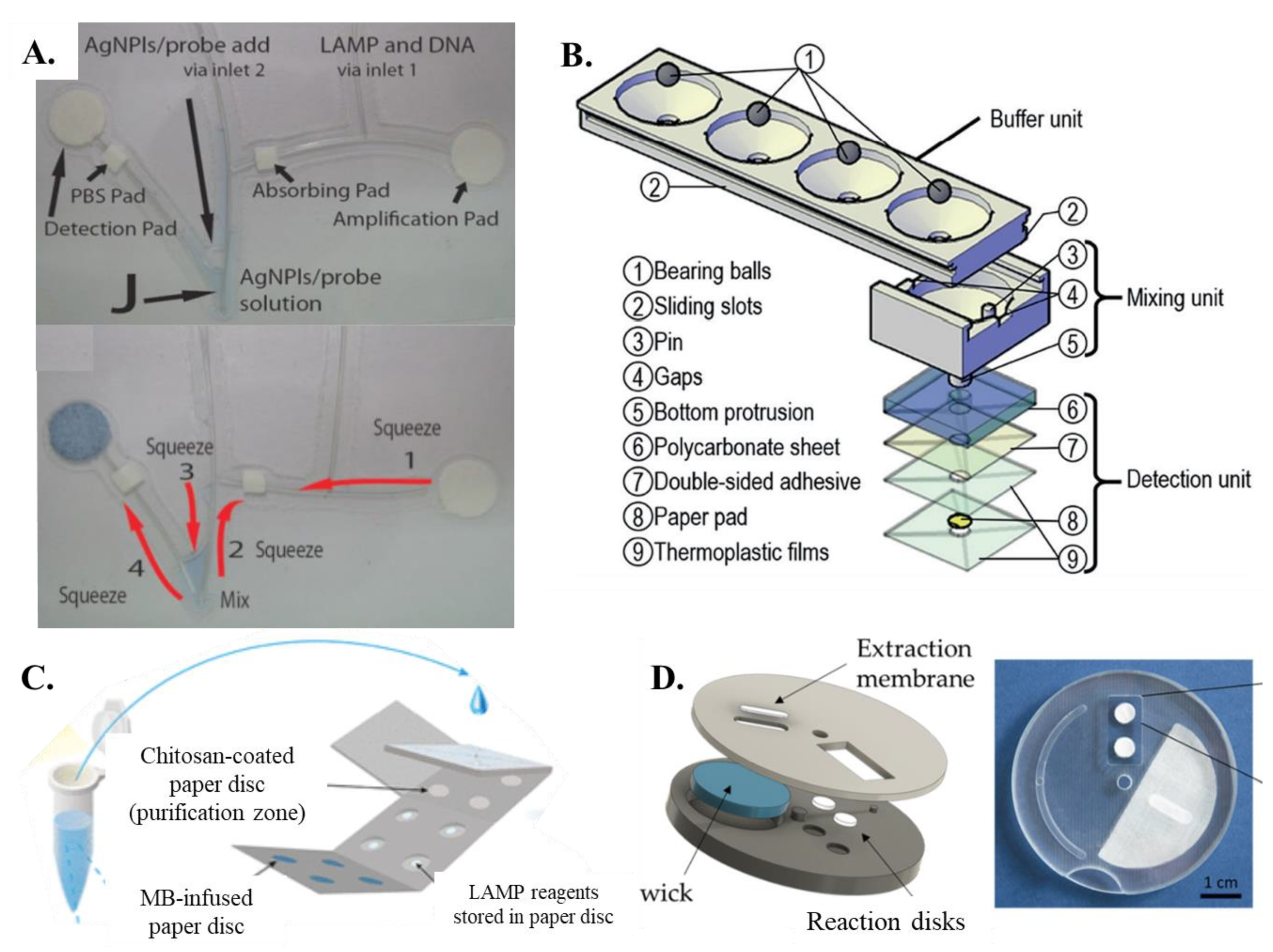
Champlauk et al. [60] established a bendable chip consisting of a paper-based reaction and detection pads connected by a fishing line. Both the amplification and detection pads are circular discs (Figure 3A). The detection part also includes a square paper pad which is coated with phosphate-buffered saline (PBS). The entire design is a fishing wire-based network coupled with paper pads for amplification and detection. Ru Choi et al. [61] proposed an integrated paper-based biosensor consisting of four layers (1) top PVC layer—lateral flow assay detection; (2) second glass fiber—for carrying out LAMP; (3) third nitrocellulose membrane layer—for sample addition and nucleic acid extraction; (4) fourth absorbent pad—for sample purification and washing. The LAMP paper device given by Seok et al. [46] and Batule et al. [48] had two layers of paper membranes sandwiched together. The bottom layer was essential in distributing the inserted sample and helping it vertically imbibe the four paper-based LAMP zones. Jiang et al. [62] developed a new concept named VLEAD which consisted of four reservoirs performing the function of lysis, wash, and purification, followed by buffering, mixing, and detection in the vertical direction in one of the reservoirs (Figure 3B). Li et al. [63] came up with a unique concept of fabricating 12 paper-based reaction pads laminated between two magnetic plates to minimize evaporation rates. An origami-paper-based DNA purification (using chitosan matrix)—LAMP amplification-detection device was fabricated by Tung Trieu and Lee [64] (Figure 3C). Wang et al. [54] proposed a new methodology in which a protruded stick can be pressed on a sponge pad-based amplification system that transfers the amplicons into the interconnected detection zones. Garneret et al. [51] proposed a movable system named COVIDISC consisting of a rectangular-shaped extraction membrane that was overlapped on two paper-reaction pads (one for test and the other for control) (Figure 3D). Recently, Rofman et al. [65] developed a design involving the sample transfer from the pumping pad to the target pad via a superhydrophobic membrane. Overall, it is seen that designs ranging from combined and lateral flow-based paper pads to disc-shaped paper arrangements have all been successful in amplifying and detecting nucleic acid targets via LAMP.
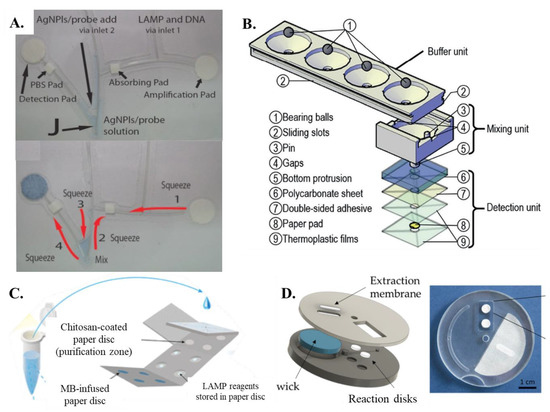
Figure 3.
(A) The bendable chip has two parts, one for DNA amplification and one for detection that are connected with channels made of fishing line [from [60], copyright 2016 Wiley-VCH GmbH]. (B) Exploded view of VLEAD consisting of three components, buffer unit at the top, mixing unit in the middle, and detection unit at the bottom [from [62], copyright 2018 Wiley-VCH GmbH]. (C) Schematic representation of the concept of sample preparation for the performance of the PMA–LAMP assay on the origami paper microdevice [from [64], copyright 2018 American Chemistry Society]. (D) A–COVIDISC workflow decomposed in three steps: (1)—injection, washing (fluids flow through the capture membrane and get absorbed by capillarity in the absorbent wick (in blue)), drying. (2)—Disk rotation and elution. (3)—Disk counter-rotation, coverage of the reaction zone by a PCR sealing film, heating, amplification, and readout [from [51], CC BY License].
2.2. Paper-Based LAMP Assay Preparation
The sample preparation step involves processing LAMP reaction either from extracted nucleic acid or directly from crude clinical samples [35,36]. Optimal paper-based LAMP should (i) enable integrated sample preparation steps such as extracting DNA/RNA from crude samples, (ii) enable on-paper reagent storage, and (iii) enable automated fluid transport and assay readout. In general, LAMP reaction requires four to six target-specific primers. In addition, for the execution of LAMP, a master reaction consisting of tris-hydrochloric acid (pH 8.8), potassium chloride, ammonium sulfate, magnesium sulphate, Tween 20, betaine, Bst DNA polymerase, and reverse transcriptase (only for the detection of viral pathogens), and dNTPs is required [36]. In addition to this, detection reagents such as intercalating dyes or fluorescent probes are also added.
Efficient extraction and purification of nucleic acids from complex biological samples are challenging in paper-based NAATs. First, paper-based methods often require smaller sample volumes, potentially limiting the amount of genetic material that can be extracted and processed. The porous structure of the paper can lead to incomplete recovery of genetic material and contamination from impurities. Additionally, achieving consistent and reproducible nucleic acid extractions can be difficult due to variations in the physical and chemical properties of the paper substrate, which can impact the efficiency of sample preparation steps. Incomplete sample recovery may reduce the sensitivity of the assay, and the quality of the genetic material extracted may be more variable compared to the traditional methods, affecting the accuracy and reproducibility of the assay. Most proof-of-concept work uses nucleic acid extraction kits or laboratory-based sample processing to isolate nucleic acids from crude biological samples before performing paper-based LAMP. Alternatively, researchers have also opted for spiking DNA templates in clinical samples before performing paper-based LAMP reactions. On the other hand, a few researchers used paper membranes to demonstrate a sample-to-answer test that performs nucleic acid extraction followed by LAMP reaction and detection on paper. Connelly et al. [45] introduced the sliding strip-based paper-LAMP assay, which can perform all the steps of sample preparation involving E. coli cell lysis, DNA isolation, and purification, as well as LAMP amplification and detection. Rodriguez et al. [57] developed single-step chaotropic cell lysis and DNA extraction methodology based on the alcohol precipitation method for extracting DNA from the clinical cervical sample. The entire procedure, along with ethanol washing, was executed on the paper membrane, which left DNA precipitate on the surface of the paper substrate. LAMP mixture was added next to initiate the reaction, followed by the detection of the target amplicons. Kaarj et al. [44] demonstrated ZIKA virus lysis and subsequent filtration followed by detection on a LAMP-paper assay. They demonstrated that the input sample could even be human urine, human blood plasma, or solution spiked with the ZIKA virus. Jiang et al. [62] introduced a paper-based unit for sample lysis, RNA enrichment, and purification, followed by RT-LAMP. Overall, it is found that these multi-functional single-step processes are relatively cost-effective and can be easily operated. Even though such sample-in answer-out type paper-based LAMP has been demonstrated, systems that can seamlessly integrate single-step extraction, purification, and detection process on paper are rare and considerable future scope exists toward engineering these systems.
2.3. Paper Membranes
Since LAMP reaction and detection, along with nucleic acid extraction/purification, is being carried out on paper membranes, the composition of the paper matrix plays a very important role in determining the efficiency of the amplification reaction. Researchers have used wide variety of paper pads ranging from polyethersulfone membrane (PES) and FTA to Whatman filter papers and chromatography papers [66]. However, the broad range of classification involves paper membranes composed of cellulose and nitrocellulose [47]. Cellulose-based paper matrices are primarily composed of hydroxyl groups and a relatively lesser amount of carboxyl group, which renders them negatively charged. These are essentially hydrophilic in nature, so the retention time of fluids on these paper membranes is comparatively less. The primarily used cellulose paper membranes are chromatography and filter papers. The other widely used cellulose-based paper membrane is FTA (fast technology analysis) cards [9,45]. FTA cards are good retainers of dried lysis agents and stabilizers, which are desirable for a single-step nucleic acid extraction prior to performing the LAMP reaction [45].
When the nitrate groups replace the hydroxy group in the cellulose, nitrocellulose is created, which is usually cast into membrane sheets after dissolving into solvents. The porosity of the paper membranes can be controlled by changing the rate of evaporation of the added solvent. In addition, the permeability of the nitrocellulose membranes can be adjusted by varying the concentration ratio of nitrocellulose and the organic solvent. Nitrocellulose membranes are primarily hydrophobic in nature, and the extent of binding of proteins on these membranes is dependent on the mechanism of electrostatic or hydrophobic interaction. It has been observed that dsDNA generally does not bind, whereas denatured single stranded DNA easily binds on the nitrocellulose membranes. Recently, there has been significant development in enhancing the hydrophilicity of the nitrocellulose membrane by adding several surfactants at different compositions [67]. Other than cellulose and nitrocellulose membranes, PES (polyether sulphone), PC (polycarbonate), and glass fiber membranes have been used for executing LAMP [66]. Linnes et al. [66] compared the extent of LAMP amplification on cellulose chromatography (CHR), PES, NC, PC, and unbound glass fiber paper membranes. Among all the membranes, PES demonstrated positive LAMP amplification every time, while PC, on rare occasions, exhibited false negative LAMP amplification. Cellulose sometimes demonstrated successful, positive LAMP amplification, whereas NC and unbound glass fiber membranes failed to show any positive amplification. Seok et al. [46] used PES as transfer pads while glass fiber paper membranes were implemented for the detection of multiple pathogens using the LAMP. Glass fibers have larger pores and are biocompatible, which makes them highly effective. Moreover, LAMP reagents are provided with sufficient reaction space on the glass fibers and these paper substances are devoid of any water-soluble material which may hamper LAMP. It was inferred that the addition of PVA (polyvinyl alcohol) on glass fiber membranes was deemed to be more effective in amplification and visual detection. They also mentioned that other membranes that contain hydrophilic fibers, such as polyether sulfone and cellulose acetate fibers, inhibited the LAMP reaction.
Several paper-based LAMP devices consist of a combination of different types of paper membranes. These are more common for devices involving lateral flow assay-based detection just after the LAMP reaction. Choi et al. [58] developed the integrated lateral flow assay for DNA amplification to detection. The developed device consisted of a glass fiber pad for LAMP, a glass fiber pad for LFA, a nitrocellulose membrane, an absorbent pad, and two PVC backing pads. Rodriguez et al. [57] combined PES and cellulose blotting paper as the sample pad for isolating and purifying the DNA from the sample. After initiating the LAMP amplification process in the combined membrane setup, the amplified products are swept away into the commercially brought lateral flow assay strip consisting of glass fiber-based conjugate pad followed by the nitrocellulose-based detection pad and, finally, the absorbent pad. Choi et al. [59] developed an integrated paper-based biosensor consisting of four layers. The top PVC layer is the lateral flow layer supported by a PVC backing pad, which consists of a glass fiber, a nitrocellulose membrane, and an absorbent pad. The second layer is composed of glass fiber for a highly specific and sensitive nucleic acid amplification technique (i.e., LAMP). The third layer consists of a piece of FTA card with a diameter of 0.25 cm for sample addition and nucleic acid extraction. The bottom layer is composed of an absorbent pad for sample purification and washing. Batule et al. [48] developed a handheld paper device by integrating multiple pads for loading, transferring, and binding the sample. Specifically, an asymmetric polyethersulfone membrane was used as the transfer pad, and a GF/C grade glass pad, glass fiber, SS DNA-modified glass fiber, and an absorbent pad were used to fabricate loading, sample, binding pads, and the absorbent pad, respectively. The paper-strip-based extracted viral RNAs were directly added to the RT-LAMP reaction buffer, and then viral RNA containing RT-LAMP buffer was pipetted into the sample hole of the ready-to-use paper chip, consisting of a PES transfer pad and glass fiber LAMP reaction pad. Other than the lateral flow assay, Champlauk et al. [60] used a bendable plastic laminated chip consisting of separate paper-based LAMP amplification and detection zones connected by a fishing line. The amplification zone was made up of amplification and absorption pads, while the detection zone comprised a PBS (phosphate buffered saline) pad and detection pad. All the paper pads were made up of Whatman filter paper no. 1 grade membrane except the PBS pad, which was prepared from a square polystyrene pad. Thus, it is well understood that combining paper pads for performing separate functions in a paper-based LAMP amplification device renders them more effective.
2.4. Incorporation of LAMP Reagents on Paper Matrix
There are several ways through which LAMP reagents can be integrated and stored onto paper matrices. Connelly et al. [45] applied purification buffer and nuclease-free water on the FTA-based LAMP detection pad. The disc was then dried completely by being placed in an oven at 65 °C for 5 min and 10 μL of LAMP Master Mix was applied. Champlauk et al. [60] fed the Whatman filter paper-based LAMP amplification pad with the LAMP reagents and DNA sample through one of the inlet tubes. In both of their works on paper-based LAMP assay, Choi et al. [58,59] pipetted out the mixture of the samples and LAMP reagents onto the glass fiber pad for LAMP protected by a disposable tape. Linnes et al. [66] first added the nucleic acid sample and then followed it up with the LAMP reagents onto the circular discs made from different paper membranes. Zhang et al. [56] inserted FTA card in the icLAMP microcapillary system followed by three segments of purification reagent, two segments of TE buffer, one segment of LAMP reaction mix, and three segments of water droplets. Hence, no reagent is directly added to the FTA card. Rodriguez et al. [57] added the liquid LAMP reaction mix directly onto the sample port of the PES membrane (LAMP amplification pad). Seok et al. [46] and Batule et al. [48] used dried LAMP reagent pre-imbibed on the glass pad for LAMP amplification. The solution-treated glass pad was dried for 20 min at 37 °C in a drying oven. Then, the reaction buffer without primer, polymerase, and HNB was pipetted onto a glass pad and heated for 60 min at 63 °C. Jiang et al. [62] used a fluid-control ball valve to trigger the release of reagent from the buffer to the mixing unit in the coffee mug-paper-based LAMP amplification unit. In order to detect ZIKA virus on paper assay, Kaarj et al. [44] inserted 15 μL of RT-LAMP reaction mixture directly on the excised paper and covered it with glass slide to sandwich the paper, followed by sealing with Parafilm M to prevent evaporation. Li et al. [63] performed a multiplex LAMP assay in the paper-based chip where isothermal amplification buffer and the bacteria genome specimen were added, and then 5 μL of mineral oil was applied to each hole to prevent liquid evaporation during the amplification. Naik et al. [68] added LAMP reaction mixture containing the bacterial culture to a 5 mm diameter paper disc, heat-sealed into a plastic pouch, and incubated it at 60 °C for varying durations. Trieu and Lee [64] developed an origami all-in-one paper structure which consisted of a chitosan-based DNA purification pad and a separate LAMP reaction pad. Due to the foldable structure, the purification pad was overlapped on the reaction pad thereby initiating the LAMP reaction. Wang et al. [54] developed an on-chip RT-LAMP assay where amplification mixtures were loaded onto the sponge-like PVA pad. Then the sample-loaded RT-LAMP chip was sealed using transparent pressure-sensitive adhesive tape and placed on the thermal plate at 50 °C for 35 min and then at 65 °C for 40 min. A slightly different process was adopted by Suea-Ngam et al. [69] for their developed paper-based LAMP assay where LAMP primer solution was first added to the paper disc without the forward inner primer. Thereafter, the polymerase, target DNA and LAMP buffer was added to the paper substrate. Choopara et al. [70] inserted LAMP mixture onto the reaction pad, dried in a sterile air flow and stored at low temperatures. To use the paper-based LAMP device, the reaction pad was placed on the reaction layer of the sandwich-like bottom base, 1 μL of DNA sample and 14 μL of sterile water were pipetted onto the reaction pad, covered by the clear top seal to prevent evaporation during the LAMP incubation. Trinh et al. [71] mixed agarose gel and LAMP reagents for an effective storage at low gelling temperature. When this mixture was deposited on the surface, it solidified at room temperature. Thereafter, the DNA solution was added. Paper-based LAMP reactions can also operate effectively at different ambient humidity conditions [72]. The various methods used to incorporate LAMP reagents on paper have been summarized in Table 1. Overall, it is found that adding the LAMP reagents directly onto the paper substrate followed by drying is an effective procedure for achieving LAMP-based detection.

Table 1.
Methods to integrate LAMP reagents on paper matrices.
3. Heating Technique
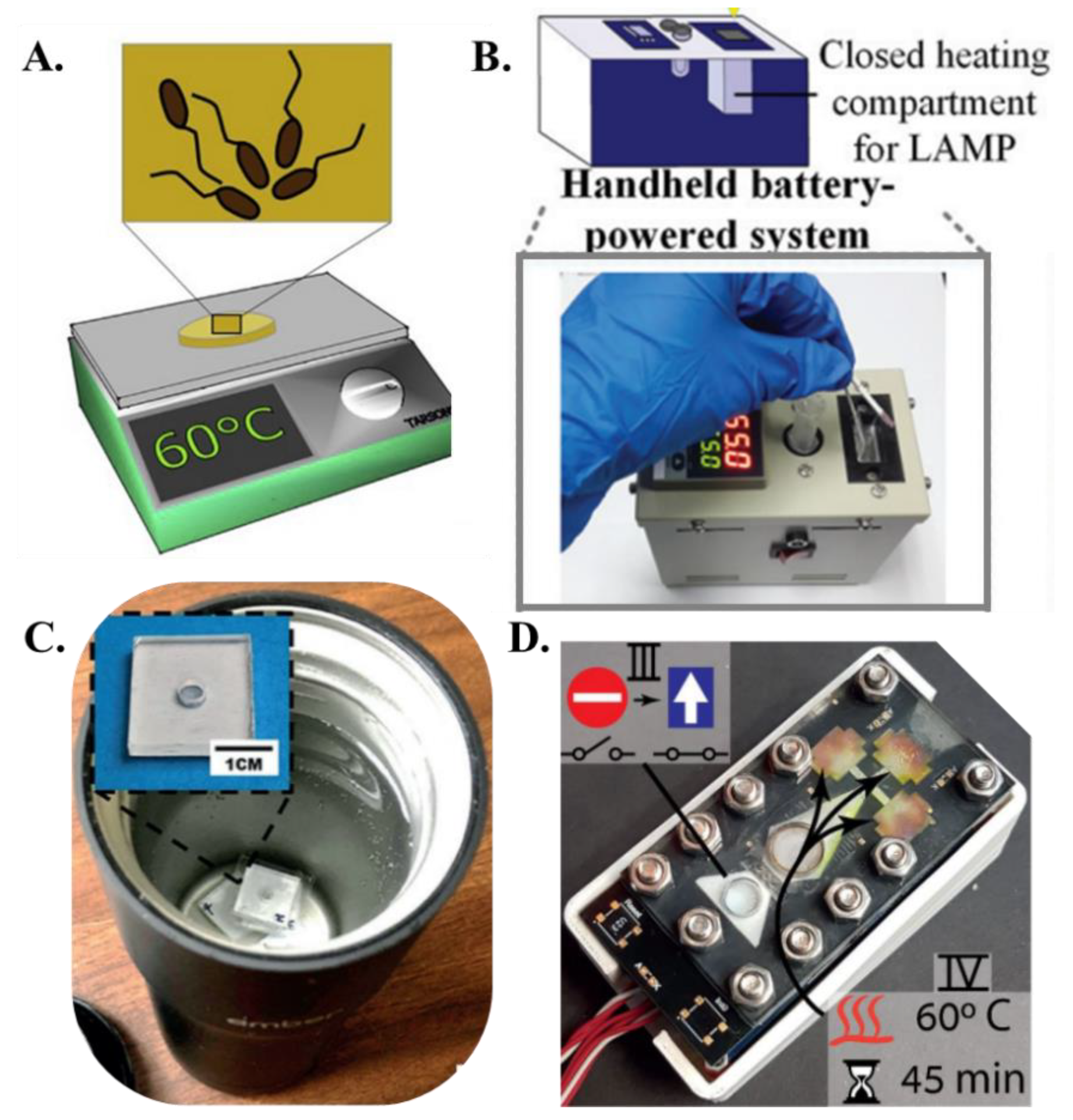
The primary advantage of LAMP over PCR is the simplicity of the heating hardware. PCR thermal cyclers comprise a metal heating block whose temperature is controlled by power-intensive Peltier heaters. Even for heating small fluidic volumes, most of the thermal energy is wasted in heating the metallic blocks with a high heat capacity and do not actively participate in the reaction, resulting in a typical power consumption of anywhere from ~150 W–470 W for PCR protocol. Additionally, cooling fans must be switched on at each thermal cycling step to reduce the reaction temperature to primer annealing temperatures. The repeated heating and cooling steps severely inhibit the PCR-based NAAT devices to be operated with low powered and simple hardware. The isothermal operation of LAMP significantly reduces the thermal management and power consumption of LAMP-based NAATs, making them amenable for portable and off-the-grid use. LAMP reactions require isothermal conditions between 63 °C and 67 °C for optimal amplification. Typically, a temperature of 65 °C is used in most cases as LAMP. In the context of paper-based LAMP platforms, the initial implementation of heating utilized ovens, incubators, and hot plates [68] to maintain the paper-based assay at ~65 °C (Figure 4A). For example, Connelly et al. [45] used a laboratory incubator for heating the paper-based LAMP platform. Rodriguez et al. [57] placed their paper-based chip face-down on a 63 °C heat block or hot plate for 30 min. Seok et al. [46] used a heat block powered by Peltier elements attached to the bottom of the multiplex paper NAAT device. Kaarj et al. [44] used a hot plate for heating the segmented and cut RT-LAMP assays. Apart from this heating apparatus, researchers also used a biological incubator for heating the paper-based LAMP device to the required isothermal temperature. Davidson et al. [52] developed a paper assay for detecting SARS-CoV-2 using RT-LAMP, and this assay was heated to 65 °C in a standard 75 L biological incubator for 60 min. Later, more portable and integrated heating units were integrated with the paper-based LAMP platforms. For example, Choi et al. [58] developed a handheld system consisting of a closed heating compartment for amplification, a non-heating testing compartment for target analyte detection, an integrated battery, an integrated temperature controller, and a charger (Figure 4B). In this setup, the heating compartment was comprised of an aluminum enclosure with external insulation, whereas the testing compartment was comprised of chemical-resistant polyformaldehyde. A battery was integrated into the system with a programmable temperature controller (5 °C to 100 °C), with a resolution of ±0.1 °C. A temperature sensor was installed in the aluminum enclosure, with the temperature displayed externally. Li et al. [63] used a homemade heating device containing a DC power supply, a temperature controller, and a copper heating block in their study for detecting antibiotic resistance genes on paper membranes. Wang et al. [73] designed an integrated thermal and image box to conduct the RT-LAMP reaction to detect prostate cancer biomarkers. Their box included a thermal plate (area: 9 cm2 and thickness: 0.15 cm) powered by a 12 V lithium-ion portable battery capable of generating a heating power of 0.6 w/cm2. Since the operating temperature of LAMP is not very high, researchers have also incorporated inexpensive off-the-shelf kitchen gadgets to carry out the isothermal reaction. For example, Jiang et al. [62] used a battery-powered coffee mug warmer to maintain the water bath temperature at the LAMP operating temperature and detected Zika virus using a paper-based LAMP assay (Figure 4C) whereas Rofman et al. incorporated an extremely low powered thin film heater integrated beneath the paper substrate [65] (Figure 4D). The various methods of heating paper-based LAMP reactions have been summarized in Table 2. While these efforts are bringing us closer to portable operation, there still exists a gap in engineering more efficient and low-powered isothermal heaters to perform LAMP reactions.
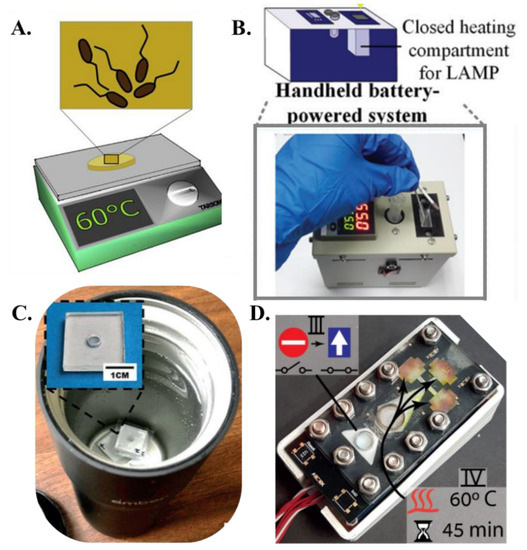
Figure 4.
Heating methods (A). A hot plate incubator [from [68], copyright 2019 Elsevier]. (B). A handheld battery-powered system for paper-based amplification and detection of nucleic acid [from [58], copyright 2016 American Chemical Society]. (C). ZIKV detection using VLEAD where RT-LAMP is carried out in a water bath inside an Ember coffee mug [from [62], copyright 2018 Wiley-VCH GmbH]. (D). Paper-based lab-on-a-chip device allowing implementation of multistep assays. The lower stacks serve for power and logic and the top is the functional PCB containing heating elements and driving electrodes [from [65], copyright 2022 Royal Society of Chemistry].

Table 2.
Heating methods for paper-based LAMP platforms.
4. Detection Methodologies
The detection in paper-based LAMP assays/devices has been mostly carried out using optical readouts (colorimetric or fluorescence-based) [9,37]. Visual detection methods of LAMP are simple to use, easy to read, and do not require specialized equipment, making it a cost-effective detection method. One common method is the use of a color-changing indicator, such as a pH indicator, that changes color in the presence of amplified DNA, suggesting a positive LAMP reaction. The user can quickly interpret a positive or negative assay readout with the naked eye or use an optical detector for more quantitative analysis. Many researchers have used color-changing dyes on paper-LAMP assays. Seok et al. [46], Hongwarittorrn et al. [74], and Batule et al. [48] utilized the color change by hydroxynapthol blue (HNB) dye for identifying positive LAMP reaction, which generally depends on the concentration of magnesium ions and the resulting pyrophosphate generation. Kaarj et al. used phenol red colorimetric pH indicator along with their RT-LAMP master mix to detect positive ZIKV samples [44] (Figure 5A). Tung Trieu and Lee [64] used methylene blue (MB) dye for qualitatively detecting the LAMP amplification in a paper-based assay containing sodium sulphite. In the absence of an amplified target, colored MB completely reacts with excessive sodium sulphite to produce colorless leuco-methylene blue (LMB). Phenol red was used as a colorimetric dye for the detection of DNA amplicons by Davidson et al. [52]. Trinh et al. [75] used a unique colorimetric dye named Chemosensor L, ((Z)-4-((2,3 dihydroxybenzylidene)amino)benzenesulfonamide) for amplicon detection. Overall, colorimetric assays can be used with paper-based LAMP assays to provide simple visual detection but fail to yield high signal to noise in positive and negative samples for robust discrimination.
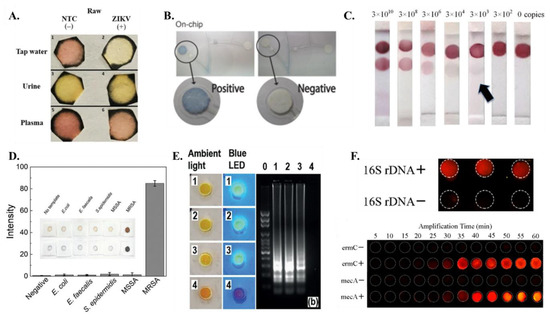
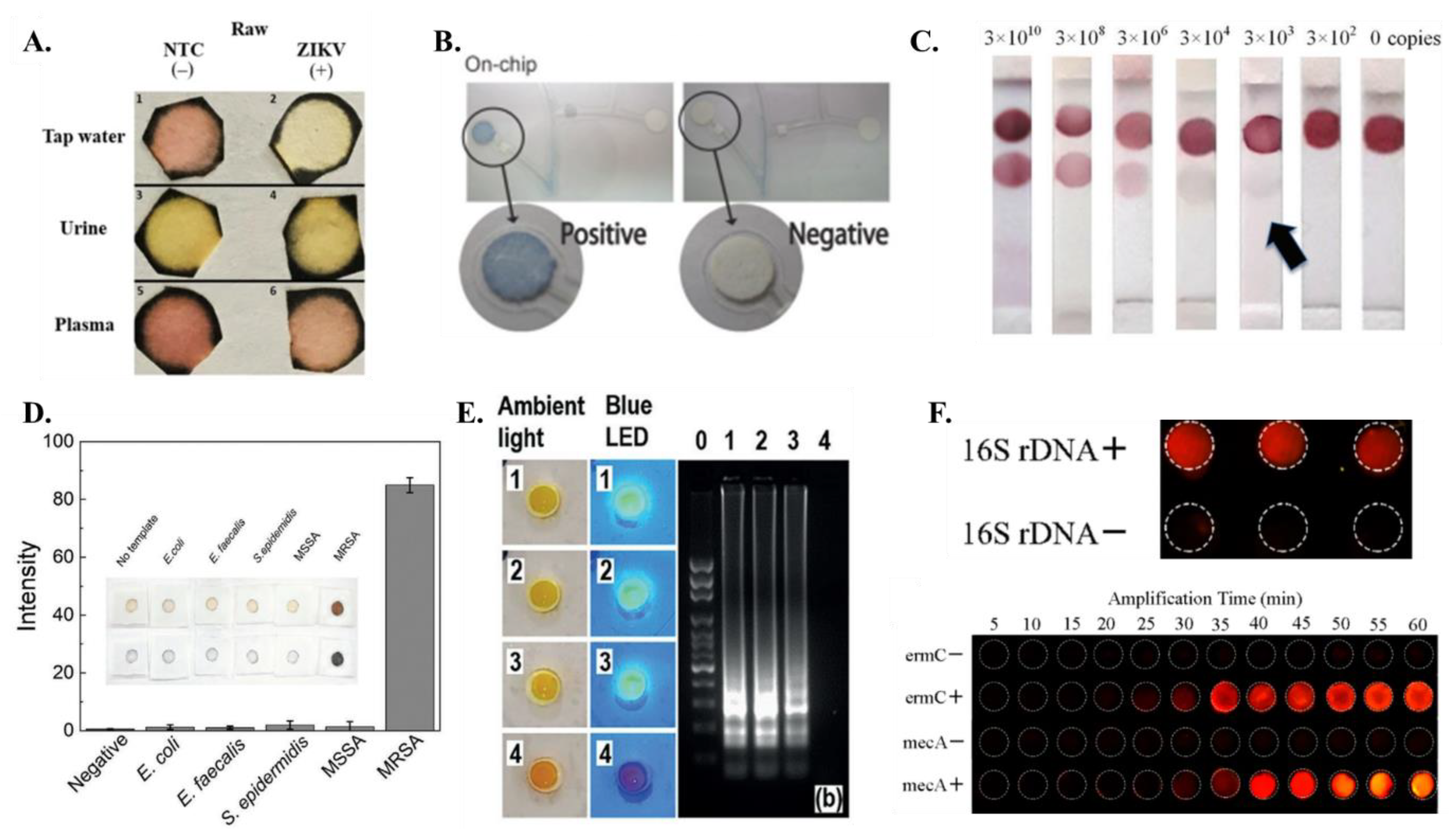
Figure 5.
Detection methods (A) Colorimetric detection of ZIKV RT-LAMP assay on samples with tap water, urine and plasma [from [44], copyright 2018 Springer Nature]. (B) Colorimetric signal detection on On-chip [from [60] copyright 2016 Wiley-VCH GmbH]. (C) The integrated paper-based LFA device can achieve the colorimetric detection limit of as low as 3 × 103 copies at the optimum incubation time of 60 min [from [58], copyright 2016 Royal Society of Chemistry]. (D) The application of gold nanoparticles (AgNPIs) with paper pad enable quantitative assay readout [from [69], copyright 2021 Wiley-VCH GmbH]. (E) Smartphone photos of the detection units under the ambient light and blue LED flashlight to detect 1, 0.5, and 0.1 PFU of ZIKV spiked in human urine samples while device 4 is a negative control. [from [62], copyright 2018 Wiley-VCH GmbH]. (F) (top) Fluorescence image of the paper-based chip for 16S rDNA detection; (bottom) Real-time amplification of multiple LAMP for mecA and ermC. Fluorescence intensity of amplification changes with time. [from [63], copyright 2018 American Chemical Society].
Due to their operational simplicity, the paper-based LAMP platforms have often combined colorimetric detection with lateral flow assay (LFA). These systems frequently use nanoparticles which can be used for colorimetric detection by exploiting the unique optical properties of their small size. By functionalizing the nanoparticles with specific biomolecules, they can be used to detect the presence of specific analytes through color changes. The on-chip signal detection in the paper-LAMP device designed by Champlauk et al. [60] used a paper-based LAMP assay in conjunction with silver nanoparticles (AgNP) to facilitate a colorimetric readout of amplified LAMP products (Figure 5B). Choi et al. [58,59] used a coupled detector probe (DP)-gold nanoparticle (AuNP) for capturing the nucleic acid target (Figure 5C). The streptavidin lining at the test line effectively captures the biotinylated amplicons, while the remaining DP-AuNP gets captured at the control line. A slightly different approach was adopted by Rodriquez et al. [57], where they used streptavidin-conjugated gold nanoparticles bound to the FITC (fluorescein isothiocyanate) probe-amplicons, biotin-based control line, and anti-FITC test line. A positive detection will result in the binding of the FITC bound amplicons on the anti-FITC line while excess streptavidin-conjugated gold nanoparticles will be captured at the biotin control line. Ru Choi et al. [61] also used gold nanoparticle conjugates for detection on lateral flow assay. Suea-Ngam et al. [69] used silver nanoparticles for detection in their paper-LAMP device (Figure 5D). It was found that free primers produced orange-yellow color, whereas silver nanoparticles were less etched and red in color. Similar to Rodriquez et al. [57], FITC-labeled probe was also used for detection purposes by Zhu et al. [76]. Jawla et al. [77] demonstrated a lateral flow assay where the amplified product was tagged with biotin and FITC, which combined streptavidin-gold nanoparticles in the conjugate pad downstream. Both Linnes et al. [66] and Saxena et al. [78] have used FAM signals to detect LAMP products on paper.
Fluorescence dyes can be used to detect LAMP products by binding to the double-stranded DNA produced during the amplification process. This binding results in a change in the fluorescence intensity of the dye, which can be detected using a fluorometer or even a smartphone [79,80]. The use of fluorescence dyes (such as SYBR Green, EvaGreen, Rox, etc.,) allows for real-time monitoring of the LAMP reaction and can provide a rapid and sensitive method for detecting specific nucleic acid sequences. This allows for early detection of LAMP products and can provide quantitative information about the amount of amplified DNA present. In the context of paper-based LAMP, SYBR Green fluorescent intercalating dye has been used by Connelly et al. [45] for visualizing the amplicons. Hiltunen et al. [81] used EvaGreen fluorophore for detection on an aluminum-coated paper substrate and PMMA. EvaGreen has been found to be fluorescent at ambient temperature, and it was effective in the detection of positive LAMP reactions on paper substrates. Jiang et al. [62] and Liu et al. [82] incorporated SYBR Green 1 dye in the paper-LAMP chambers for visualizing the products generated from LAMP (Figure 5E). Li et al. [63] introduced a new fluorescent detection agent in the form of a transition metal complex [Ru(phen)2dppz](PF6)2 in their paper-based LAMP assay. Their fluorescent detection method enabled them to achieve high signal to noise ratio enabling them to not only robustly discriminate between positive and negative samples but also enable real time and quantification amplification on paper (Figure 5F). DNA binding Picogreen dye was used to generate fluorescence on paper by Naik et al. [68] to detect LAMP amplicons. Calcein has been used by Wang et al. [73] and Zhou et al. [83] since the presence of manganese ions initially inhibits the fluorescence of calcein. However, LAMP results in the formation of pyrophosphate, which further reacts with manganese, and eventually, it separates out from calcein generating bright fluorescence. Garneret et al. [51] and Rofman et al. [65] used SYTO82 in their paper-based assay for amplicon detection and visualization. While several researchers have incorporated fluorescent dyes in their paper-based LAMP assays, they are known to inhibit the LAMP reaction to some extent, and their use is not optimal for resource-limited settings.
5. Sensitivity and Specificity of Paper-Based LAMP Systems
The sensitivity and specificity of LAMP reactions depend on several factors, including the initial concentration of target nucleic acid, the specificity of the primers, and the reaction conditions. Generally, LAMP is considered to have similar sensitivity as traditional PCR and can detect as low as a few copies of target DNA. In some studies, LAMP has been shown to detect as low as ten copies of target DNA, and can even detect single copies of a viral genome in clinical samples. Ideally, a molecular diagnostic assay should have high sensitivity and high specificity [84]; however, paper-based assays generally exhibit lower sensitivity than their liquid assay counterparts. Sensitivity is often expressed in terms of limit of detection (LOD) with lower LOD assays corresponding to higher sensitivity [84].
The first paper-based LAMP assay developed by Connelly et al. [45] demonstrated a LOD corresponding to one copy of dsDNA and 5 E. coli cells. Champlauk et al. [60] targeted six areas of the aflR gene in the Aspergillus strain for amplification and demonstrated a LOD of 100 aflR copies with 94.47% specificity. At the same time, 43.75% of contamination was identified in 14 of 32 herbal samples tested. In the integrated LAMP-LFA paper-based platform, Choi et al. [58] demonstrated a LOD of 3 × 103 copies of dengue viral RNA. Later, in their PDMS drop-infused modified design of LFA, Choi et al. [59] demonstrated a LOD of 20 pM, with a 2.5-fold signal enhancement for the case involving 5 PDMS drops. Also, these assays also demonstrated good specificity in detecting the Hepatitis B virus. Rodriguez et al. [57] demonstrated a LOD of 104 HPV (human papilloma virus) copies in their combined paper-based LAMP-LFA device. Ru Choi et al. [61] achieved a LOD of 10 CFU/mL E. coli copies in milk and 103 CFU/mL E. coli copies in spinach from the paper-LAMP biosensor device resulting in a higher sensitivity in comparison to the existing paper-based assays. For specificity analysis, S. pneumonia was targeted, and this was only detected when the other HBV and E. coli samples showed negative results. Seok et al. [46] demonstrated that the sensitivity of their paper-based LAMP assay for detecting S. pneumonia was in the range 0.7 pg–700 pg of DNA mass with an LOD of 0.7 pg. Jiang et al. [62] demonstrated that the detection limit of ZIKA virus on their paper-device is 0.5 PFU of 140 mL of ZIKA spiked urine sample. In the same year, Kaarj et al. [44] found the detection limit as 1 genome ZIKA sample per ml for their paper-based LAMP assay. Li et al. [63] showed an LOD of 100 copies for mecA gene and 285 copies for ermC gene from MRSA (methicillin resistant Staphylococcus aureus). Liu et al. [82] demonstrated a LOD of 10−4 dilution for their proposed LAMP-LFA device. A detection sensitivity of almost 100 CFU/mL was found in the paper substrate used for LAMP by Naik et al. [68]. An E. coli bacterial concentration as low as 1000 CFU/mL was found as the detection limit on paper substrates by Tung Trieu and Lee [64]. Zhu et al. [76] displayed the LOD of Prymnesium parvum g DNA as 0.03 ng/μL in their LAMP-LFA device. Batule et al. [48] showed that the detection limit of ZIKA virus was 1 and 10 copies from 1×PBS and 100% human serum sample, respectively, utilizing the paper-based setup used by Seok et al. [46]. Wang et al. [73] found the detection sensitivity of Prostate cancer antigen 3 (PCA 3) to be around 0.34 fg/μL in the PVA-paper-LAMP-based setup. Suea-Ngam et al. [69] concluded that when FIP primers were immobilized on the paper surface, two fold sensitivity increased as compared to the solution-based LAMP. Davidson et al. [52] found 100% sensitivity with a detection limit of 200 copies of SARS-CoV-2 virus particles per μL. In addition, the specificity of SARS-CoV-2 detection was found to be around 76%. Jawla et al. [77] found that pig DNA template concentration of 10 fg produced an observable amplification signal in their paper LAMP-LFA device. Nearly a detection limit of 100 CFU/mL of vancomycin-resistant Enterococcus in milk was achieved by Trinh et al. [71]. Wang et al. [54] achieved a sensitivity of 3 SARS-CoV-2 RNA copies in their paper-LAMP-ELISA assay whereas 1 SARS-CoV-2 copy per ml was found to be the LOD in the paper-based device demonstrated by Garneret et al. [51]. Saxena et al. [78] found the limit of detection to be around 10 agμL−1 for the N-gene (corresponds to 1.61 × 102 copies of customized DNA fragment), ORF1ab-gene (2.13 × 102 copies of customized DNA fragment) and E-gene (8.69 × 102 copies of customized DNA fragment), and 100 agμL−1 (7.44 × 103 copies of customized DNA fragment) for the S-gene. Zhou et al. [83] displayed a pathogen (E. coli, Salmonella sp., and Staphylococcus aureus) detection sensitivity of 2.8 × 10−5 ng per μL and 10 CFU/mL in spiked milk samples. The detection methods employed along with the limit of detection values for various paper-based LAMP platforms have been summarized in Table 3. Overall, almost all the paper-based LAMP assay systems demonstrated high enough sensitivity and specificity to be applicable for clinical diagnostics.

Table 3.
Paper-based LAMP platforms, targets, detection methods and limit of detection.
6. Conclusions and Future Perspectives
While a direct comparison of paper-based LAMP and microfluidic LAMP platforms for point-of-care diagnostics cannot be made without defining precise comparison criteria, generally, microfluidic LAMP platforms offer improved assay performance including higher sensitivity, specificity, and reproducibility. This may be due to several factors, such as the ability to control and optimize reaction conditions in microfluidic platforms, the use of specialized materials and fabrication methods to reduce variability and increase sensitivity, and the integration of additional diagnostic features such as on-chip sample preparation and detection. However, microfluidic LAMP platforms can be more complex and costly to develop and manufacture compared to paper-based platforms. Previously, paper membranes have been used in microfluidic-based systems only for extracting nucleic acids [55,56]. Additionally, paper membranes, specifically lateral flow assays, have been widely used for detection after carrying out tube/solution-based LAMP [89]. The present review article discusses emerging trends in developing various paper-based LAMP assays/devices in recent years. While still in its infancy, the future prospects of paper-based LAMP are promising as it offers cost-effective, rapid, and reliable detection capabilities for a wide range of applications. It can be used as point-of-care diagnostics, allowing for rapid and accurate diagnosis at the patient’s bedside or in remote or field settings. The ability to multiplex the assay helps detecting multiple targets at the same time and increases its diagnostic utility. In addition, paper-based LAMP can be used for environmental monitoring, detecting microorganisms in water, soil, and other environmental samples. It can also be used for food safety, providing a rapid and specific detection of food-borne pathogens in food products. Additionally, it can be used in research applications, such as monitoring the presence of specific microorganisms in a sample or for genotyping. Due to its relatively low cost, it is an attractive option for large-scale testing in low- and middle-income countries, where the cost of traditional diagnostic tests can be prohibitive. Overall, paper-based LAMP has a wide range of applications and great potential to improve global health, food safety, and environmental monitoring.
However, several bottlenecks should be addressed to enable wider adoption of this technology. The heating setup to actuate paper-based LAMP assays should be optimally engineered so that the heat source is miniaturized, low-powered, and ideally—electricity free. The detection technique should be revised to incorporate inexpensive dyes (possibly carbon dots) as opposed to costly fluorophores. Smartphone-based readout coupled with improved image analysis should be developed to enable quantitative analysis with paper-based LAMP assays in contrast to the traditional yes/no result. Paper-based assays should be adapted and optimized to incorporate multiplexed LAMP to enable differential diagnostics. This will be essential in detecting multiple pathogens in a clinical sample. Paper-based sample preparation protocols should be further analyzed to incorporate a wider range of clinical samples. Storage of LAMP reagents in their dried form on the paper substrate should be explored in more detail with a range of drying and lyophilizing protocols. This should greatly make paper-based assay platforms more robust and increase their shelf life. Even with these challenges, researchers are pouring in efforts from several directions to make the next generation of paper-based NAAT devices more robust and scalable so that they can address the global diagnostic demands in a cost-effective manner.
Author Contributions
Conceptualization, D.D. and A.P.; formal analysis, D.D., M.M. and A.P.; data curation, D.D., M.M. and A.P.; writing—original draft preparation, D.D. and A.P.; writing—review and editing, D.D. and A.P.; supervision, A.P.; funding acquisition, A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded partly by a grant from Department of Defense (CDMRP grant number: W81XWH2210071; PI: Aashish Priye) and University of Cincinnati (Faculty scholar research award, grant number: 1018268; PI: Aashish Priye). This was an invited publication with a waiver on the article processing charges.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data was generated.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sun, H.; Xiong, L.; Huang, Y.; Chen, X.; Yu, Y.; Ye, S.; Dong, H.; Jia, Y.; Zhang, W. AI-aided on-chip nucleic acid assay for smart diagnosis of infectious disease. Fundam. Res. 2021, 2, 476–486. [Google Scholar] [CrossRef]
- Cevik, S. Going Viral: A Gravity Model of Infectious Diseases and Tourism Flows. Open Econ. Rev. 2021, 33, 141–156. [Google Scholar] [CrossRef]
- Nugent, R. Chronic Diseases in Developing Countries. Ann. N. Y. Acad. Sci. 2008, 1136, 70–79. [Google Scholar] [CrossRef]
- Kasperson, J.X.; Kasperson, R.E. Priorities in Profile: Managing Risks in Developing Countries. In The Social Contours of Risk; Routledge: London, UK, 2022; pp. 172–179. [Google Scholar] [CrossRef]
- Christaki, E. New technologies in predicting, preventing and controlling emerging infectious diseases. Virulence 2015, 6, 558–565. [Google Scholar] [CrossRef]
- Yang, S.; E Rothman, R. PCR-based diagnostics for infectious diseases: Uses, limitations, and future applications in acute-care settings. Lancet Infect. Dis. 2004, 4, 337–348. [Google Scholar] [CrossRef]
- Shi, A.C.; Ren, P. SARS-CoV-2 serology testing: Progress and challenges. J. Immunol. Methods 2021, 494, 113060. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R.W.; Wedderburn, C.J.; Garcia, P.J.; Boeras, D.; Fongwen, N.; Nkengasong, J.; Sall, A.; Tanuri, A.; Heymann, D.L. Serology testing in the COVID-19 pandemic response. Lancet Infect. Dis. 2020, 20, e245–e249. [Google Scholar] [CrossRef] [PubMed]
- Magro, L.; Escadafal, C.; Garneret, P.; Jacquelin, B.; Kwasiborski, A.; Manuguerra, J.-C.; Monti, F.; Sakuntabhai, A.; Vanhomwegen, J.; Lafaye, P.; et al. Paper microfluidics for nucleic acid amplification testing (NAAT) of infectious diseases. Lab Chip 2017, 17, 2347–2371. [Google Scholar] [CrossRef]
- Craw, P.; Balachandran, W. Isothermal nucleic acid amplification technologies for point-of-care diagnostics: A critical review. Lab a Chip 2012, 12, 2469–2486. [Google Scholar] [CrossRef]
- Liu, D. Molecular Detection of Human Bacterial Pathogens; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Kang, T.; Lu, J.; Yu, T.; Long, Y.; Liu, G. Advances in nucleic acid amplification techniques (NAATs): COVID-19 point-of-care diagnostics as an example. Biosens. Bioelectron. 2022, 206. [Google Scholar] [CrossRef]
- Morisset, D.; Stebih, D.; Cankar, K.; Zel, J.; Gruden, K. Alternative DNA amplification methods to PCR and their application in GMO detection: A review. Eur. Food Res. Technol. 2008, 227, 1287–1297. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, H.-R. A review on continuous-flow microfluidic PCR in droplets: Advances, challenges and future. Anal. Chim. Acta 2016, 914, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Chen, C.-C.; Wei, S.-C.; Lu, H.-H.; Liang, Y.-H.; Lin, C.-W. Diagnostic Devices for Isothermal Nucleic Acid Amplification. Sensors 2012, 12, 8319–8337. [Google Scholar] [CrossRef]
- Asiello, P.J.; Baeumner, A. Miniaturized isothermal nucleic acid amplification, a review. Lab a Chip 2011, 11, 1420–1430. [Google Scholar] [CrossRef]
- Deiman, B.; Van Aarle, P.; Sillekens, P. Characteristics and Applications of Nucleic Acid Sequence-Based Amplification (NASBA). Mol. Biotechnol. 2002, 20, 163–180. [Google Scholar] [CrossRef]
- Cook, N. The use of NASBA for the detection of microbial pathogens in food and environmental samples. J. Microbiol. Methods 2003, 53, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Mueller, J.D.; Pütz, B.; Höfler, H. Self-sustained sequence replication (3SR): An alternative to PCR. Histochem. Cell Biol. 1997, 108, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Fahy, E.; Kwoh, D.Y.; Gingeras, T.R. Self-sustained sequence replication (3SR): An isothermal transcription-based amplification system alternative to PCR. Genome Res. 1991, 1, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.S. Molecular diagnostic testing for infectious diseases using TMA technology. Expert Rev. Mol. Diagn. 2001, 1, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Simmel, F.C.; Yurke, B.; Singh, H.R. Principles and Applications of Nucleic Acid Strand Displacement Reactions. Chem. Rev. 2019, 119, 6326–6369. [Google Scholar] [CrossRef]
- Hellyer, T.J.; Nadeau, J.G. Strand displacement amplification: A versatile tool for molecular diagnostics. Expert Rev. Mol. Diagn. 2004, 4, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Motamedi, M.H.K.; Saghafinia, M.; Karami, A.; Gill, P. A review of the current isothermal amplification techniques: Applications, advantages and disadvantages. J. Glob. Infect. Dis. 2011, 3, 293. [Google Scholar] [CrossRef]
- Ren, Y.; Cao, L.; You, M.; Ji, J.; Gong, Y.; Ren, H.; Xu, F.; Guo, H.; Hu, J.; Li, Z. “SMART” digital nucleic acid amplification technologies for lung cancer monitoring from early to advanced stages. TrAC Trends Anal. Chem. 2022, 157. [Google Scholar] [CrossRef]
- Andresen, D.; Von Nickisch-Rosenegk, M.; Bier, F.F. Helicase-dependent amplification: Use in OnChip amplification and potential for point-of-care diagnostics. Expert Rev. Mol. Diagn. 2009, 9, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-J.; Park, K.; Kim, D.-E. Isothermal DNA amplification in vitro: The helicase-dependent amplification system. Cell. Mol. Life Sci. 2009, 66, 3325–3336. [Google Scholar] [CrossRef] [PubMed]
- Lobato, I.M.; O’Sullivan, C.K. Recombinase polymerase amplification: Basics, applications and recent advances. TrAC Trends Anal. Chem. 2017, 98, 19–35. [Google Scholar] [CrossRef]
- Daher, R.K.; Stewart, G.; Boissinot, M.; Bergeron, M.G. Recombinase Polymerase Amplification for Diagnostic Applications. Clin. Chem. 2016, 62, 947–958. [Google Scholar] [CrossRef]
- Ali, M.M.; Li, F.; Zhang, Z.; Zhang, K.; Kang, D.-K.; Ankrum, J.A.; Le, X.C.; Zhao, W. Rolling circle amplification: A versatile tool for chemical biology, materials science and medicine. Chem. Soc. Rev. 2014, 43, 3324–3341. [Google Scholar] [CrossRef]
- Zhao, W.; Ali, M.M.; Brook, M.A.; Li, Y. Rolling Circle Amplification: Applications in Nanotechnology and Biodetection with Functional Nucleic Acids. Angew. Chem. Int. Ed. 2008, 47, 6330–6337. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Brandwein, M.; Hsuih, T.; Li, H.B. Ramification Amplification: A Novel Isothermal DNA Amplification Method. Mol. Diagn. 2001, 6, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Dean, F.B.; Hosono, S.; Fang, L.; Wu, X.; Faruqi, A.F.; Bray-Ward, P.; Sun, Z.; Zong, Q.; Du, Y.; Du, J.; et al. Comprehensive human genome amplification using multiple displacement amplification. Proc. Natl. Acad. Sci. USA 2002, 99, 5261–5266. [Google Scholar] [CrossRef]
- Lasken, R.S. Genomic DNA amplification by the multiple displacement amplification (MDA) method. Biochem. Soc. Trans. 2009, 37, 450–453. [Google Scholar] [CrossRef]
- Notomi, T.; Mori, Y.; Tomita, N.; Kanda, H. Loop-mediated isothermal amplification (LAMP): Principle, features, and future prospects. J. Microbiol. 2015, 53, 1–5. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, E63. [Google Scholar] [CrossRef]
- Liu, L.; Yang, D.; Liu, G. Signal amplification strategies for paper-based analytical devices. Biosens. Bioelectron. 2019, 136, 60–75. [Google Scholar] [CrossRef]
- McCalla, S.E.; Ong, C.; Sarma, A.; Opal, S.M.; Artenstein, A.W.; Tripathi, A. A Simple Method for Amplifying RNA Targets (SMART). J. Mol. Diagn. 2012, 14, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Meagher, R.J.; Priye, A.; Light, Y.K.; Huang, C.; Wang, E. Impact of primer dimers and self-amplifying hairpins on reverse transcription loop-mediated isothermal amplification detection of viral RNA. Analyst 2018, 143, 1924–1933. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Y.; Fohlerova, Z.; Chang, H.; Iliescu, C.; Neuzil, P. LAMP-on-a-chip: Revising microfluidic platforms for loop-mediated DNA amplification. TrAC Trends Anal. Chem. 2019, 113, 44–53. [Google Scholar] [CrossRef]
- Gong, M.M.; Sinton, D. Turning the Page: Advancing Paper-Based Microfluidics for Broad Diagnostic Application. Chem. Rev. 2017, 117, 8447–8480. [Google Scholar] [CrossRef]
- Carrell, C.; Kava, A.; Nguyen, M.; Menger, R.; Munshi, Z.; Call, Z.; Nussbaum, M.; Henry, C. Beyond the lateral flow assay: A review of paper-based microfluidics. Microelectron. Eng. 2019, 206, 45–54. [Google Scholar] [CrossRef]
- Fu, E.; Ramsey, S.A.; Kauffman, P.; Lutz, B.; Yager, P. Transport in two-dimensional paper networks. Microfluid. Nanofluid. 2010, 10, 29–35. [Google Scholar] [CrossRef]
- Kaarj, K.; Akarapipad, P.; Yoon, J.-Y. Simpler, Faster, and Sensitive Zika Virus Assay Using Smartphone Detection of Loop-mediated Isothermal Amplification on Paper Microfluidic Chips. Sci. Rep. 2018, 8, 12438. [Google Scholar] [CrossRef]
- Connelly, J.T.; Rolland, J.P.; Whitesides, G.M. “Paper Machine” for Molecular Diagnostics. Anal. Chem. 2015, 87, 7595–7601. [Google Scholar] [CrossRef]
- Seok, Y.; Joung, H.-A.; Byun, J.-Y.; Jeon, H.-S.; Shin, S.J.; Kim, S.; Shin, Y.-B.; Han, H.S.; Kim, M.-G. A Paper-Based Device for Performing Loop-Mediated Isothermal Amplification with Real-Time Simultaneous Detection of Multiple DNA Targets. Theranostics 2017, 7, 2220–2230. [Google Scholar] [CrossRef]
- Das, D.; Namboodiri, S. Selection of a suitable paper membrane for Loop Mediated Isothermal DNA amplification reaction (LAMP) in a point-of-care diagnostic kit—Experimental and CFD analysis. Chem. Eng. Sci. 2020, 229, 116130. [Google Scholar] [CrossRef]
- Batule, B.S.; Seok, Y.; Kim, M.-G. Paper-based nucleic acid testing system for simple and early diagnosis of mosquito-borne RNA viruses from human serum. Biosens. Bioelectron. 2019, 151, 111998. [Google Scholar] [CrossRef]
- Choi, J.R.; Yong, K.W.; Tang, R.; Gong, Y.; Wen, T.; Li, F.; Pingguan-Murphy, B.; Bai, D.; Xu, F. Advances and challenges of fully integrated paper-based point-of-care nucleic acid testing. TrAC Trends Anal. Chem. 2017, 93, 37–50. [Google Scholar] [CrossRef]
- Tian, T.; Bi, Y.; Xu, X.; Zhu, Z.; Yang, C. Integrated paper-based microfluidic devices for point-of-care testing. Anal. Methods 2018, 10, 3567–3581. [Google Scholar] [CrossRef]
- Garneret, P.; Coz, E.; Martin, E.; Manuguerra, J.-C.; Brient-Litzler, E.; Enouf, V.; Obando, D.F.G.; Olivo-Marin, J.-C.; Monti, F.; Van Der Werf, S.; et al. Performing point-of-care molecular testing for SARS-CoV-2 with RNA extraction and isothermal amplification. PLoS ONE 2021, 16, e0243712. [Google Scholar] [CrossRef]
- Davidson, J.L.; Wang, J.; Maruthamuthu, M.K.; Dextre, A.; Pascual-Garrigos, A.; Mohan, S.; Putikam, S.V.S.; Osman, F.O.I.; McChesney, D.; Seville, J.; et al. A paper-based colorimetric molecular test for SARS-CoV-2 in saliva. Biosens. Bioelectron. X 2021, 9, 100076. [Google Scholar] [CrossRef]
- Chowdury, M.A.; Khalid, F. Application of microfluidic paper-based analytical device ( μPAD ) to detect COVID-19 in energy deprived countries. Int. J. Energy Res. 2021, 45, 18275–18280. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dextre, A.; Pascual-Garrigos, A.; Davidson, J.L.; Maruthamuthu, M.K.; McChesney, D.; Seville, J.; Verma, M.S. Fabrication of a paper-based colorimetric molecular test for SARS-CoV-2. MethodsX 2021, 8, 101586. [Google Scholar] [CrossRef]
- Liu, C.; Geva, E.; Mauk, M.; Qiu, X.; Abrams, W.R.; Malamud, D.; Curtis, K.; Owen, S.M.; Bau, H.H. An isothermal amplification reactor with an integrated isolation membrane for point-of-care detection of infectious diseases. Analyst 2011, 136, 2069–2076. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Wang, C.; Feng, Q.; Fan, F.; Zhang, G.; Kang, X.; Qin, X.; Sun, J.; Li, Y.; et al. Integrated Microcapillary for Sample-to-Answer Nucleic Acid Pretreatment, Amplification, and Detection. Anal. Chem. 2014, 86, 10461–10466. [Google Scholar] [CrossRef]
- Rodriguez, N.M.; Wong, W.S.; Liu, L.; Dewar, R.; Klapperich, C.M. A fully integrated paperfluidic molecular diagnostic chip for the extraction, amplification, and detection of nucleic acids from clinical samples. Lab a Chip 2016, 16, 753–763. [Google Scholar] [CrossRef]
- Choi, J.R.; Hu, J.; Gong, Y.; Feng, S.; Abas, W.A.B.W.; Pingguan-Murphy, B.; Xu, F. An integrated lateral flow assay for effective DNA amplification and detection at the point of care. Analyst 2016, 141, 2930–2939. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.R.; Liu, Z.; Hu, J.; Tang, R.; Gong, Y.; Feng, S.; Ren, H.; Wen, T.; Yang, H.; Qu, Z.; et al. Polydimethylsiloxane-Paper Hybrid Lateral Flow Assay for Highly Sensitive Point-of-Care Nucleic Acid Testing. Anal. Chem. 2016, 88, 6254–6264. [Google Scholar] [CrossRef] [PubMed]
- Chaumpluk, P.; Plubcharoensook, P.; Prasongsuk, S. Rapid detection of aflatoxigenic Aspergillus sp. in herbal specimens by a simple, bendable, paper-based lab-on-a-chip. Biotechnol. J. 2016, 11, 768–779. [Google Scholar] [CrossRef]
- Choi, J.R.; Hu, J.; Tang, R.; Gong, Y.; Feng, S.; Ren, H.; Wen, T.; Li, X.; Abas, W.A.B.W.; Pingguan-Murphy, B.; et al. An integrated paper-based sample-to-answer biosensor for nucleic acid testing at the point of care. Lab a Chip 2015, 16, 611–621. [Google Scholar] [CrossRef]
- Jiang, X.; Loeb, J.C.; Manzanas, C.; Lednicky, J.A.; Fan, Z.H. Valve-Enabled Sample Preparation and RNA Amplification in a Coffee Mug for Zika Virus Detection. Angew. Chem. Int. Ed. 2018, 57, 17211–17214. [Google Scholar] [CrossRef]
- Li, B.; Zhou, X.; Liu, H.; Deng, H.; Huang, R.; Xing, D. Simultaneous Detection of Antibiotic Resistance Genes on Paper-Based Chip Using [Ru(phen)2dppz]2+ Turn-on Fluorescence Probe. ACS Appl. Mater. Interfaces 2018, 10, 4494–4501. [Google Scholar] [CrossRef]
- Trieu, P.T.; Lee, N.Y. Paper-Based All-in-One Origami Microdevice for Nucleic Acid Amplification Testing for Rapid Colorimetric Identification of Live Cells for Point-of-Care Testing. Anal. Chem. 2019, 91, 11013–11022. [Google Scholar] [CrossRef] [PubMed]
- Rofman, B.; Naddaf, R.; Bar-Dolev, M.; Gefen, T.; Ben-Assa, N.; Geva-Zatorsky, N.; Bercovici, M. Automated device for multi-stage paper-based assays enabled by an electroosmotic pumping valve. Lab a Chip 2022, 22, 4511–4520. [Google Scholar] [CrossRef]
- Linnes, J.C.; Rodriguez, N.M.; Liu, L.; Klapperich, C.M. Polyethersulfone improves isothermal nucleic acid amplification compared to current paper-based diagnostics. Biomed. Microdevices 2016, 18, 30. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Xie, M.Y.; Li, M.; Cao, L.; Feng, S.; Li, Z.; Xu, F. Nitrocellulose Membrane for Paper-based Biosensor. Appl. Mater. Today 2021, 26, 101305. [Google Scholar] [CrossRef]
- Naik, P.; Jaitpal, S.; Shetty, P.; Paul, D. An integrated one-step assay combining thermal lysis and loop-mediated isothermal DNA amplification (LAMP) in 30 min from E. coli and M. smegmatis cells on a paper substrate. Sens. Actuators B Chem. 2019, 291, 74–80. [Google Scholar] [CrossRef]
- Suea-Ngam, A.; Choopara, I.; Li, S.; Schmelcher, M.; Somboonna, N.; Howes, P.D.; Demello, A.J. In Situ Nucleic Acid Amplification and Ultrasensitive Colorimetric Readout in a Paper-Based Analytical Device Using Silver Nanoplates. Adv. Healthc. Mater. 2020, 10, e2001755. [Google Scholar] [CrossRef]
- Choopara, I.; Suea-Ngam, A.; Teethaisong, Y.; Howes, P.D.; Schmelcher, M.; Leelahavanichkul, A.; Thunyaharn, S.; Wongsawaeng, D.; Demello, A.J.; Dean, D.; et al. Fluorometric Paper-Based, Loop-Mediated Isothermal Amplification Devices for Quantitative Point-of-Care Detection of Methicillin-Resistant Staphylococcus aureus (MRSA). ACS Sens. 2021, 6, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Trinh, T.N.D.; Thai, D.A.; Lee, N.Y. Pop-up paper-based and fully integrated microdevice for point-of-care testing of vancomycin-resistant Enterococcus. Sens. Actuators B Chem. 2021, 345, 130362. [Google Scholar] [CrossRef]
- Das, D.; Singh, T.; Ahmed, I.; Masetty, M.; Priye, A. Effects of Relative Humidity and Paper Geometry on the Imbibition Dynamics and Reactions in Lateral Flow Assays. Langmuir 2022. [CrossRef]
- Wang, L.-X.; Fu, J.-J.; Zhou, Y.; Chen, G.; Fang, C.; Lu, Z.S.; Yu, L. On-chip RT-LAMP and colorimetric detection of the prostate cancer 3 biomarker with an integrated thermal and imaging box. Talanta 2019, 208, 120407. [Google Scholar] [CrossRef] [PubMed]
- Hongwarittorrn, I.; Chaichanawongsaroj, N.; Laiwattanapaisal, W. Semi-quantitative visual detection of loop mediated isothermal amplification (LAMP)-generated DNA by distance-based measurement on a paper device. Talanta 2017, 175, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Trinh, K.T.L.; Chae, W.R.; Lee, N.Y. Recent advances in the fabrication strategies of paper-based microfluidic devices for rapid detection of bacteria and viruses. Microchem. J. 2022, 180. [Google Scholar] [CrossRef]
- Zhu, P.; Huang, H.-L.; Zhou, C.-X.; Xu, J.; Qiao, L.-L.; Dang, C.-Y.; Pang, J.-H.; Gao, W.-F.; Yan, X.-J. Sensitive and rapid detection of Prymnesium parvum (Haptophyceae) by loop-mediated isothermal amplification combined with a lateral flow dipstick. Aquaculture 2019, 505, 199–205. [Google Scholar] [CrossRef]
- Jawla, J.; Kumar, R.R.; Mendiratta, S.; Agarwal, R.; Singh, P.; Saxena, V.; Kumari, S.; Boby, N.; Kumar, D.; Rana, P. On-site paper-based Loop-Mediated Isothermal Amplification coupled Lateral Flow Assay for pig tissue identification targeting mitochondrial CO I gene. J. Food Compos. Analyst 2021, 102, 104036. [Google Scholar] [CrossRef]
- Saxena, A.; Rai, P.; Mehrotra, S.; Baby, S.; Singh, S.; Srivastava, V.; Priya, S.; Sharma, S.K. Development and Clinical Validation of RT-LAMP-Based Lateral-Flow Devices and Electrochemical Sensor for Detecting Multigene Targets in SARS-CoV-2. Int. J. Mol. Sci. 2022, 23, 13105. [Google Scholar] [CrossRef]
- Priye, A.; Ball, C.S.; Meagher, R.J. Colorimetric-Luminance Readout for Quantitative Analysis of Fluorescence Signals with a Smartphone CMOS Sensor. Anal. Chem. 2018, 90, 12385–12389. [Google Scholar] [CrossRef]
- Priye, A.; Ugaz, V. DNA-to-Go: A Portable Smartphone-Enabled PCR Assay Platform. arXiv 2016, arXiv:1606.02252. [Google Scholar]
- Hiltunen, J.; Liedert, C.; Hiltunen, M.; Huttunen, O.-H.; Hiitola-Keinänen, J.; Aikio, S.; Harjanne, M.; Kurkinen, M.; Hakalahti, L.; Lee, L.P. Roll-to-roll fabrication of integrated PDMS–paper microfluidics for nucleic acid amplification. Lab a Chip 2018, 18, 1552–1559. [Google Scholar] [CrossRef]
- Liu, L.; Xu, Y.; Zhong, W.; Li, L.; Li, W.; Xiao, Q. Comparison of three terminal detection methods based on loop mediated isothermal amplification (LAMP) assay for spring viremia of carp virus (SVCV). Turk. J. Fish. Aquat. Sci. 2019, 19, 805–816. [Google Scholar] [CrossRef]
- Zhou, Q.; Pan, J.; Mo, L.; Luo, Z.; Qin, Z.; Dai, Z.; Yi, C. Fluorescent on-site detection of multiple pathogens using smartphone-based portable device with paper-based isothermal amplification chip. Microchim. Acta 2022, 189, 1–10. [Google Scholar] [CrossRef]
- Lalkhen, A.; McCluskey, A. Clinical tests: Sensitivity and specificity. Contin. Educ. Anaesth. Crit. Care Pain 2008, 8, 221–223. [Google Scholar] [CrossRef]
- Roy, S.; Mohd-Naim, N.F.; Safavieh, M.; Ahmed, M.U. Colorimetric Nucleic Acid Detection on Paper Microchip Using Loop Mediated Isothermal Amplification and Crystal Violet Dye. ACS Sens. 2017, 2, 1713–1720. [Google Scholar] [CrossRef]
- Kim, J.-H.; Yoo, I.S.; An, J.H.; Kim, S. A novel paper-plastic hybrid device for the simultaneous loop-mediated isothermal amplification and detection of DNA. Mater. Lett. 2018, 214, 243–246. [Google Scholar] [CrossRef]
- Lin, X.; Huang, X.; Urmann, K.; Xie, X.; Hoffmann, M.R. Digital Loop-Mediated Isothermal Amplification on a Commercial Membrane. ACS Sens. 2019, 4, 242–249. [Google Scholar] [CrossRef]
- Varsha, V.; Aishwarya, S.; Murchana, S.; Naveen, G.; Ramya, M.; Rathinasabapathi, P. Correction pen based paper fluidic device for the detection of multiple gene targets of Leptospira using Loop Mediated Isothermal Amplification. J. Microbiol. Methods 2020, 174, 105962. [Google Scholar] [CrossRef]
- Kaewphinit, T.; Arunrut, N.; Kiatpathomchai, W.; Santiwatanakul, S.; Jaratsing, P.; Chansiri, K. Detection of Mycobacterium tuberculosis by Using Loop-Mediated Isothermal Amplification Combined with a Lateral Flow Dipstick in Clinical Samples. BioMed Res. Int. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).