Paper-Based Loop Mediated Isothermal Amplification (LAMP) Platforms: Integrating the Versatility of Paper Microfluidics with Accuracy of Nucleic Acid Amplification Tests
Abstract
:1. Introduction
2. Fabrication and Design of Paper-Based LAMP Assays
2.1. Design of the Paper-Based LAMP Platform
2.2. Paper-Based LAMP Assay Preparation
2.3. Paper Membranes
2.4. Incorporation of LAMP Reagents on Paper Matrix
3. Heating Technique
4. Detection Methodologies
5. Sensitivity and Specificity of Paper-Based LAMP Systems
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, H.; Xiong, L.; Huang, Y.; Chen, X.; Yu, Y.; Ye, S.; Dong, H.; Jia, Y.; Zhang, W. AI-aided on-chip nucleic acid assay for smart diagnosis of infectious disease. Fundam. Res. 2021, 2, 476–486. [Google Scholar] [CrossRef]
- Cevik, S. Going Viral: A Gravity Model of Infectious Diseases and Tourism Flows. Open Econ. Rev. 2021, 33, 141–156. [Google Scholar] [CrossRef]
- Nugent, R. Chronic Diseases in Developing Countries. Ann. N. Y. Acad. Sci. 2008, 1136, 70–79. [Google Scholar] [CrossRef]
- Kasperson, J.X.; Kasperson, R.E. Priorities in Profile: Managing Risks in Developing Countries. In The Social Contours of Risk; Routledge: London, UK, 2022; pp. 172–179. [Google Scholar] [CrossRef]
- Christaki, E. New technologies in predicting, preventing and controlling emerging infectious diseases. Virulence 2015, 6, 558–565. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; E Rothman, R. PCR-based diagnostics for infectious diseases: Uses, limitations, and future applications in acute-care settings. Lancet Infect. Dis. 2004, 4, 337–348. [Google Scholar] [CrossRef]
- Shi, A.C.; Ren, P. SARS-CoV-2 serology testing: Progress and challenges. J. Immunol. Methods 2021, 494, 113060. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R.W.; Wedderburn, C.J.; Garcia, P.J.; Boeras, D.; Fongwen, N.; Nkengasong, J.; Sall, A.; Tanuri, A.; Heymann, D.L. Serology testing in the COVID-19 pandemic response. Lancet Infect. Dis. 2020, 20, e245–e249. [Google Scholar] [CrossRef] [PubMed]
- Magro, L.; Escadafal, C.; Garneret, P.; Jacquelin, B.; Kwasiborski, A.; Manuguerra, J.-C.; Monti, F.; Sakuntabhai, A.; Vanhomwegen, J.; Lafaye, P.; et al. Paper microfluidics for nucleic acid amplification testing (NAAT) of infectious diseases. Lab Chip 2017, 17, 2347–2371. [Google Scholar] [CrossRef] [Green Version]
- Craw, P.; Balachandran, W. Isothermal nucleic acid amplification technologies for point-of-care diagnostics: A critical review. Lab a Chip 2012, 12, 2469–2486. [Google Scholar] [CrossRef]
- Liu, D. Molecular Detection of Human Bacterial Pathogens; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Kang, T.; Lu, J.; Yu, T.; Long, Y.; Liu, G. Advances in nucleic acid amplification techniques (NAATs): COVID-19 point-of-care diagnostics as an example. Biosens. Bioelectron. 2022, 206. [Google Scholar] [CrossRef]
- Morisset, D.; Stebih, D.; Cankar, K.; Zel, J.; Gruden, K. Alternative DNA amplification methods to PCR and their application in GMO detection: A review. Eur. Food Res. Technol. 2008, 227, 1287–1297. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, H.-R. A review on continuous-flow microfluidic PCR in droplets: Advances, challenges and future. Anal. Chim. Acta 2016, 914, 7–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.-C.; Chen, C.-C.; Wei, S.-C.; Lu, H.-H.; Liang, Y.-H.; Lin, C.-W. Diagnostic Devices for Isothermal Nucleic Acid Amplification. Sensors 2012, 12, 8319–8337. [Google Scholar] [CrossRef]
- Asiello, P.J.; Baeumner, A. Miniaturized isothermal nucleic acid amplification, a review. Lab a Chip 2011, 11, 1420–1430. [Google Scholar] [CrossRef]
- Deiman, B.; Van Aarle, P.; Sillekens, P. Characteristics and Applications of Nucleic Acid Sequence-Based Amplification (NASBA). Mol. Biotechnol. 2002, 20, 163–180. [Google Scholar] [CrossRef]
- Cook, N. The use of NASBA for the detection of microbial pathogens in food and environmental samples. J. Microbiol. Methods 2003, 53, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Mueller, J.D.; Pütz, B.; Höfler, H. Self-sustained sequence replication (3SR): An alternative to PCR. Histochem. Cell Biol. 1997, 108, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Fahy, E.; Kwoh, D.Y.; Gingeras, T.R. Self-sustained sequence replication (3SR): An isothermal transcription-based amplification system alternative to PCR. Genome Res. 1991, 1, 25–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, C.S. Molecular diagnostic testing for infectious diseases using TMA technology. Expert Rev. Mol. Diagn. 2001, 1, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Simmel, F.C.; Yurke, B.; Singh, H.R. Principles and Applications of Nucleic Acid Strand Displacement Reactions. Chem. Rev. 2019, 119, 6326–6369. [Google Scholar] [CrossRef]
- Hellyer, T.J.; Nadeau, J.G. Strand displacement amplification: A versatile tool for molecular diagnostics. Expert Rev. Mol. Diagn. 2004, 4, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Motamedi, M.H.K.; Saghafinia, M.; Karami, A.; Gill, P. A review of the current isothermal amplification techniques: Applications, advantages and disadvantages. J. Glob. Infect. Dis. 2011, 3, 293. [Google Scholar] [CrossRef]
- Ren, Y.; Cao, L.; You, M.; Ji, J.; Gong, Y.; Ren, H.; Xu, F.; Guo, H.; Hu, J.; Li, Z. “SMART” digital nucleic acid amplification technologies for lung cancer monitoring from early to advanced stages. TrAC Trends Anal. Chem. 2022, 157. [Google Scholar] [CrossRef]
- Andresen, D.; Von Nickisch-Rosenegk, M.; Bier, F.F. Helicase-dependent amplification: Use in OnChip amplification and potential for point-of-care diagnostics. Expert Rev. Mol. Diagn. 2009, 9, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-J.; Park, K.; Kim, D.-E. Isothermal DNA amplification in vitro: The helicase-dependent amplification system. Cell. Mol. Life Sci. 2009, 66, 3325–3336. [Google Scholar] [CrossRef] [PubMed]
- Lobato, I.M.; O’Sullivan, C.K. Recombinase polymerase amplification: Basics, applications and recent advances. TrAC Trends Anal. Chem. 2017, 98, 19–35. [Google Scholar] [CrossRef]
- Daher, R.K.; Stewart, G.; Boissinot, M.; Bergeron, M.G. Recombinase Polymerase Amplification for Diagnostic Applications. Clin. Chem. 2016, 62, 947–958. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.M.; Li, F.; Zhang, Z.; Zhang, K.; Kang, D.-K.; Ankrum, J.A.; Le, X.C.; Zhao, W. Rolling circle amplification: A versatile tool for chemical biology, materials science and medicine. Chem. Soc. Rev. 2014, 43, 3324–3341. [Google Scholar] [CrossRef]
- Zhao, W.; Ali, M.M.; Brook, M.A.; Li, Y. Rolling Circle Amplification: Applications in Nanotechnology and Biodetection with Functional Nucleic Acids. Angew. Chem. Int. Ed. 2008, 47, 6330–6337. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Brandwein, M.; Hsuih, T.; Li, H.B. Ramification Amplification: A Novel Isothermal DNA Amplification Method. Mol. Diagn. 2001, 6, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Dean, F.B.; Hosono, S.; Fang, L.; Wu, X.; Faruqi, A.F.; Bray-Ward, P.; Sun, Z.; Zong, Q.; Du, Y.; Du, J.; et al. Comprehensive human genome amplification using multiple displacement amplification. Proc. Natl. Acad. Sci. USA 2002, 99, 5261–5266. [Google Scholar] [CrossRef] [Green Version]
- Lasken, R.S. Genomic DNA amplification by the multiple displacement amplification (MDA) method. Biochem. Soc. Trans. 2009, 37, 450–453. [Google Scholar] [CrossRef]
- Notomi, T.; Mori, Y.; Tomita, N.; Kanda, H. Loop-mediated isothermal amplification (LAMP): Principle, features, and future prospects. J. Microbiol. 2015, 53, 1–5. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, E63. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Yang, D.; Liu, G. Signal amplification strategies for paper-based analytical devices. Biosens. Bioelectron. 2019, 136, 60–75. [Google Scholar] [CrossRef]
- McCalla, S.E.; Ong, C.; Sarma, A.; Opal, S.M.; Artenstein, A.W.; Tripathi, A. A Simple Method for Amplifying RNA Targets (SMART). J. Mol. Diagn. 2012, 14, 328–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meagher, R.J.; Priye, A.; Light, Y.K.; Huang, C.; Wang, E. Impact of primer dimers and self-amplifying hairpins on reverse transcription loop-mediated isothermal amplification detection of viral RNA. Analyst 2018, 143, 1924–1933. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Y.; Fohlerova, Z.; Chang, H.; Iliescu, C.; Neuzil, P. LAMP-on-a-chip: Revising microfluidic platforms for loop-mediated DNA amplification. TrAC Trends Anal. Chem. 2019, 113, 44–53. [Google Scholar] [CrossRef]
- Gong, M.M.; Sinton, D. Turning the Page: Advancing Paper-Based Microfluidics for Broad Diagnostic Application. Chem. Rev. 2017, 117, 8447–8480. [Google Scholar] [CrossRef]
- Carrell, C.; Kava, A.; Nguyen, M.; Menger, R.; Munshi, Z.; Call, Z.; Nussbaum, M.; Henry, C. Beyond the lateral flow assay: A review of paper-based microfluidics. Microelectron. Eng. 2019, 206, 45–54. [Google Scholar] [CrossRef]
- Fu, E.; Ramsey, S.A.; Kauffman, P.; Lutz, B.; Yager, P. Transport in two-dimensional paper networks. Microfluid. Nanofluid. 2010, 10, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Kaarj, K.; Akarapipad, P.; Yoon, J.-Y. Simpler, Faster, and Sensitive Zika Virus Assay Using Smartphone Detection of Loop-mediated Isothermal Amplification on Paper Microfluidic Chips. Sci. Rep. 2018, 8, 12438. [Google Scholar] [CrossRef]
- Connelly, J.T.; Rolland, J.P.; Whitesides, G.M. “Paper Machine” for Molecular Diagnostics. Anal. Chem. 2015, 87, 7595–7601. [Google Scholar] [CrossRef]
- Seok, Y.; Joung, H.-A.; Byun, J.-Y.; Jeon, H.-S.; Shin, S.J.; Kim, S.; Shin, Y.-B.; Han, H.S.; Kim, M.-G. A Paper-Based Device for Performing Loop-Mediated Isothermal Amplification with Real-Time Simultaneous Detection of Multiple DNA Targets. Theranostics 2017, 7, 2220–2230. [Google Scholar] [CrossRef]
- Das, D.; Namboodiri, S. Selection of a suitable paper membrane for Loop Mediated Isothermal DNA amplification reaction (LAMP) in a point-of-care diagnostic kit—Experimental and CFD analysis. Chem. Eng. Sci. 2020, 229, 116130. [Google Scholar] [CrossRef]
- Batule, B.S.; Seok, Y.; Kim, M.-G. Paper-based nucleic acid testing system for simple and early diagnosis of mosquito-borne RNA viruses from human serum. Biosens. Bioelectron. 2019, 151, 111998. [Google Scholar] [CrossRef]
- Choi, J.R.; Yong, K.W.; Tang, R.; Gong, Y.; Wen, T.; Li, F.; Pingguan-Murphy, B.; Bai, D.; Xu, F. Advances and challenges of fully integrated paper-based point-of-care nucleic acid testing. TrAC Trends Anal. Chem. 2017, 93, 37–50. [Google Scholar] [CrossRef]
- Tian, T.; Bi, Y.; Xu, X.; Zhu, Z.; Yang, C. Integrated paper-based microfluidic devices for point-of-care testing. Anal. Methods 2018, 10, 3567–3581. [Google Scholar] [CrossRef]
- Garneret, P.; Coz, E.; Martin, E.; Manuguerra, J.-C.; Brient-Litzler, E.; Enouf, V.; Obando, D.F.G.; Olivo-Marin, J.-C.; Monti, F.; Van Der Werf, S.; et al. Performing point-of-care molecular testing for SARS-CoV-2 with RNA extraction and isothermal amplification. PLoS ONE 2021, 16, e0243712. [Google Scholar] [CrossRef]
- Davidson, J.L.; Wang, J.; Maruthamuthu, M.K.; Dextre, A.; Pascual-Garrigos, A.; Mohan, S.; Putikam, S.V.S.; Osman, F.O.I.; McChesney, D.; Seville, J.; et al. A paper-based colorimetric molecular test for SARS-CoV-2 in saliva. Biosens. Bioelectron. X 2021, 9, 100076. [Google Scholar] [CrossRef]
- Chowdury, M.A.; Khalid, F. Application of microfluidic paper-based analytical device ( μPAD ) to detect COVID-19 in energy deprived countries. Int. J. Energy Res. 2021, 45, 18275–18280. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dextre, A.; Pascual-Garrigos, A.; Davidson, J.L.; Maruthamuthu, M.K.; McChesney, D.; Seville, J.; Verma, M.S. Fabrication of a paper-based colorimetric molecular test for SARS-CoV-2. MethodsX 2021, 8, 101586. [Google Scholar] [CrossRef]
- Liu, C.; Geva, E.; Mauk, M.; Qiu, X.; Abrams, W.R.; Malamud, D.; Curtis, K.; Owen, S.M.; Bau, H.H. An isothermal amplification reactor with an integrated isolation membrane for point-of-care detection of infectious diseases. Analyst 2011, 136, 2069–2076. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhang, Y.; Wang, C.; Feng, Q.; Fan, F.; Zhang, G.; Kang, X.; Qin, X.; Sun, J.; Li, Y.; et al. Integrated Microcapillary for Sample-to-Answer Nucleic Acid Pretreatment, Amplification, and Detection. Anal. Chem. 2014, 86, 10461–10466. [Google Scholar] [CrossRef]
- Rodriguez, N.M.; Wong, W.S.; Liu, L.; Dewar, R.; Klapperich, C.M. A fully integrated paperfluidic molecular diagnostic chip for the extraction, amplification, and detection of nucleic acids from clinical samples. Lab a Chip 2016, 16, 753–763. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.R.; Hu, J.; Gong, Y.; Feng, S.; Abas, W.A.B.W.; Pingguan-Murphy, B.; Xu, F. An integrated lateral flow assay for effective DNA amplification and detection at the point of care. Analyst 2016, 141, 2930–2939. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.R.; Liu, Z.; Hu, J.; Tang, R.; Gong, Y.; Feng, S.; Ren, H.; Wen, T.; Yang, H.; Qu, Z.; et al. Polydimethylsiloxane-Paper Hybrid Lateral Flow Assay for Highly Sensitive Point-of-Care Nucleic Acid Testing. Anal. Chem. 2016, 88, 6254–6264. [Google Scholar] [CrossRef] [PubMed]
- Chaumpluk, P.; Plubcharoensook, P.; Prasongsuk, S. Rapid detection of aflatoxigenic Aspergillus sp. in herbal specimens by a simple, bendable, paper-based lab-on-a-chip. Biotechnol. J. 2016, 11, 768–779. [Google Scholar] [CrossRef]
- Choi, J.R.; Hu, J.; Tang, R.; Gong, Y.; Feng, S.; Ren, H.; Wen, T.; Li, X.; Abas, W.A.B.W.; Pingguan-Murphy, B.; et al. An integrated paper-based sample-to-answer biosensor for nucleic acid testing at the point of care. Lab a Chip 2015, 16, 611–621. [Google Scholar] [CrossRef]
- Jiang, X.; Loeb, J.C.; Manzanas, C.; Lednicky, J.A.; Fan, Z.H. Valve-Enabled Sample Preparation and RNA Amplification in a Coffee Mug for Zika Virus Detection. Angew. Chem. Int. Ed. 2018, 57, 17211–17214. [Google Scholar] [CrossRef]
- Li, B.; Zhou, X.; Liu, H.; Deng, H.; Huang, R.; Xing, D. Simultaneous Detection of Antibiotic Resistance Genes on Paper-Based Chip Using [Ru(phen)2dppz]2+ Turn-on Fluorescence Probe. ACS Appl. Mater. Interfaces 2018, 10, 4494–4501. [Google Scholar] [CrossRef]
- Trieu, P.T.; Lee, N.Y. Paper-Based All-in-One Origami Microdevice for Nucleic Acid Amplification Testing for Rapid Colorimetric Identification of Live Cells for Point-of-Care Testing. Anal. Chem. 2019, 91, 11013–11022. [Google Scholar] [CrossRef] [PubMed]
- Rofman, B.; Naddaf, R.; Bar-Dolev, M.; Gefen, T.; Ben-Assa, N.; Geva-Zatorsky, N.; Bercovici, M. Automated device for multi-stage paper-based assays enabled by an electroosmotic pumping valve. Lab a Chip 2022, 22, 4511–4520. [Google Scholar] [CrossRef]
- Linnes, J.C.; Rodriguez, N.M.; Liu, L.; Klapperich, C.M. Polyethersulfone improves isothermal nucleic acid amplification compared to current paper-based diagnostics. Biomed. Microdevices 2016, 18, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, R.; Xie, M.Y.; Li, M.; Cao, L.; Feng, S.; Li, Z.; Xu, F. Nitrocellulose Membrane for Paper-based Biosensor. Appl. Mater. Today 2021, 26, 101305. [Google Scholar] [CrossRef]
- Naik, P.; Jaitpal, S.; Shetty, P.; Paul, D. An integrated one-step assay combining thermal lysis and loop-mediated isothermal DNA amplification (LAMP) in 30 min from E. coli and M. smegmatis cells on a paper substrate. Sens. Actuators B Chem. 2019, 291, 74–80. [Google Scholar] [CrossRef]
- Suea-Ngam, A.; Choopara, I.; Li, S.; Schmelcher, M.; Somboonna, N.; Howes, P.D.; Demello, A.J. In Situ Nucleic Acid Amplification and Ultrasensitive Colorimetric Readout in a Paper-Based Analytical Device Using Silver Nanoplates. Adv. Healthc. Mater. 2020, 10, e2001755. [Google Scholar] [CrossRef]
- Choopara, I.; Suea-Ngam, A.; Teethaisong, Y.; Howes, P.D.; Schmelcher, M.; Leelahavanichkul, A.; Thunyaharn, S.; Wongsawaeng, D.; Demello, A.J.; Dean, D.; et al. Fluorometric Paper-Based, Loop-Mediated Isothermal Amplification Devices for Quantitative Point-of-Care Detection of Methicillin-Resistant Staphylococcus aureus (MRSA). ACS Sens. 2021, 6, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Trinh, T.N.D.; Thai, D.A.; Lee, N.Y. Pop-up paper-based and fully integrated microdevice for point-of-care testing of vancomycin-resistant Enterococcus. Sens. Actuators B Chem. 2021, 345, 130362. [Google Scholar] [CrossRef]
- Das, D.; Singh, T.; Ahmed, I.; Masetty, M.; Priye, A. Effects of Relative Humidity and Paper Geometry on the Imbibition Dynamics and Reactions in Lateral Flow Assays. Langmuir 2022. [CrossRef]
- Wang, L.-X.; Fu, J.-J.; Zhou, Y.; Chen, G.; Fang, C.; Lu, Z.S.; Yu, L. On-chip RT-LAMP and colorimetric detection of the prostate cancer 3 biomarker with an integrated thermal and imaging box. Talanta 2019, 208, 120407. [Google Scholar] [CrossRef] [PubMed]
- Hongwarittorrn, I.; Chaichanawongsaroj, N.; Laiwattanapaisal, W. Semi-quantitative visual detection of loop mediated isothermal amplification (LAMP)-generated DNA by distance-based measurement on a paper device. Talanta 2017, 175, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Trinh, K.T.L.; Chae, W.R.; Lee, N.Y. Recent advances in the fabrication strategies of paper-based microfluidic devices for rapid detection of bacteria and viruses. Microchem. J. 2022, 180. [Google Scholar] [CrossRef]
- Zhu, P.; Huang, H.-L.; Zhou, C.-X.; Xu, J.; Qiao, L.-L.; Dang, C.-Y.; Pang, J.-H.; Gao, W.-F.; Yan, X.-J. Sensitive and rapid detection of Prymnesium parvum (Haptophyceae) by loop-mediated isothermal amplification combined with a lateral flow dipstick. Aquaculture 2019, 505, 199–205. [Google Scholar] [CrossRef]
- Jawla, J.; Kumar, R.R.; Mendiratta, S.; Agarwal, R.; Singh, P.; Saxena, V.; Kumari, S.; Boby, N.; Kumar, D.; Rana, P. On-site paper-based Loop-Mediated Isothermal Amplification coupled Lateral Flow Assay for pig tissue identification targeting mitochondrial CO I gene. J. Food Compos. Analyst 2021, 102, 104036. [Google Scholar] [CrossRef]
- Saxena, A.; Rai, P.; Mehrotra, S.; Baby, S.; Singh, S.; Srivastava, V.; Priya, S.; Sharma, S.K. Development and Clinical Validation of RT-LAMP-Based Lateral-Flow Devices and Electrochemical Sensor for Detecting Multigene Targets in SARS-CoV-2. Int. J. Mol. Sci. 2022, 23, 13105. [Google Scholar] [CrossRef]
- Priye, A.; Ball, C.S.; Meagher, R.J. Colorimetric-Luminance Readout for Quantitative Analysis of Fluorescence Signals with a Smartphone CMOS Sensor. Anal. Chem. 2018, 90, 12385–12389. [Google Scholar] [CrossRef]
- Priye, A.; Ugaz, V. DNA-to-Go: A Portable Smartphone-Enabled PCR Assay Platform. arXiv 2016, arXiv:1606.02252. [Google Scholar]
- Hiltunen, J.; Liedert, C.; Hiltunen, M.; Huttunen, O.-H.; Hiitola-Keinänen, J.; Aikio, S.; Harjanne, M.; Kurkinen, M.; Hakalahti, L.; Lee, L.P. Roll-to-roll fabrication of integrated PDMS–paper microfluidics for nucleic acid amplification. Lab a Chip 2018, 18, 1552–1559. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Xu, Y.; Zhong, W.; Li, L.; Li, W.; Xiao, Q. Comparison of three terminal detection methods based on loop mediated isothermal amplification (LAMP) assay for spring viremia of carp virus (SVCV). Turk. J. Fish. Aquat. Sci. 2019, 19, 805–816. [Google Scholar] [CrossRef]
- Zhou, Q.; Pan, J.; Mo, L.; Luo, Z.; Qin, Z.; Dai, Z.; Yi, C. Fluorescent on-site detection of multiple pathogens using smartphone-based portable device with paper-based isothermal amplification chip. Microchim. Acta 2022, 189, 1–10. [Google Scholar] [CrossRef]
- Lalkhen, A.; McCluskey, A. Clinical tests: Sensitivity and specificity. Contin. Educ. Anaesth. Crit. Care Pain 2008, 8, 221–223. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Mohd-Naim, N.F.; Safavieh, M.; Ahmed, M.U. Colorimetric Nucleic Acid Detection on Paper Microchip Using Loop Mediated Isothermal Amplification and Crystal Violet Dye. ACS Sens. 2017, 2, 1713–1720. [Google Scholar] [CrossRef]
- Kim, J.-H.; Yoo, I.S.; An, J.H.; Kim, S. A novel paper-plastic hybrid device for the simultaneous loop-mediated isothermal amplification and detection of DNA. Mater. Lett. 2018, 214, 243–246. [Google Scholar] [CrossRef]
- Lin, X.; Huang, X.; Urmann, K.; Xie, X.; Hoffmann, M.R. Digital Loop-Mediated Isothermal Amplification on a Commercial Membrane. ACS Sens. 2019, 4, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Varsha, V.; Aishwarya, S.; Murchana, S.; Naveen, G.; Ramya, M.; Rathinasabapathi, P. Correction pen based paper fluidic device for the detection of multiple gene targets of Leptospira using Loop Mediated Isothermal Amplification. J. Microbiol. Methods 2020, 174, 105962. [Google Scholar] [CrossRef]
- Kaewphinit, T.; Arunrut, N.; Kiatpathomchai, W.; Santiwatanakul, S.; Jaratsing, P.; Chansiri, K. Detection of Mycobacterium tuberculosis by Using Loop-Mediated Isothermal Amplification Combined with a Lateral Flow Dipstick in Clinical Samples. BioMed Res. Int. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [Green Version]

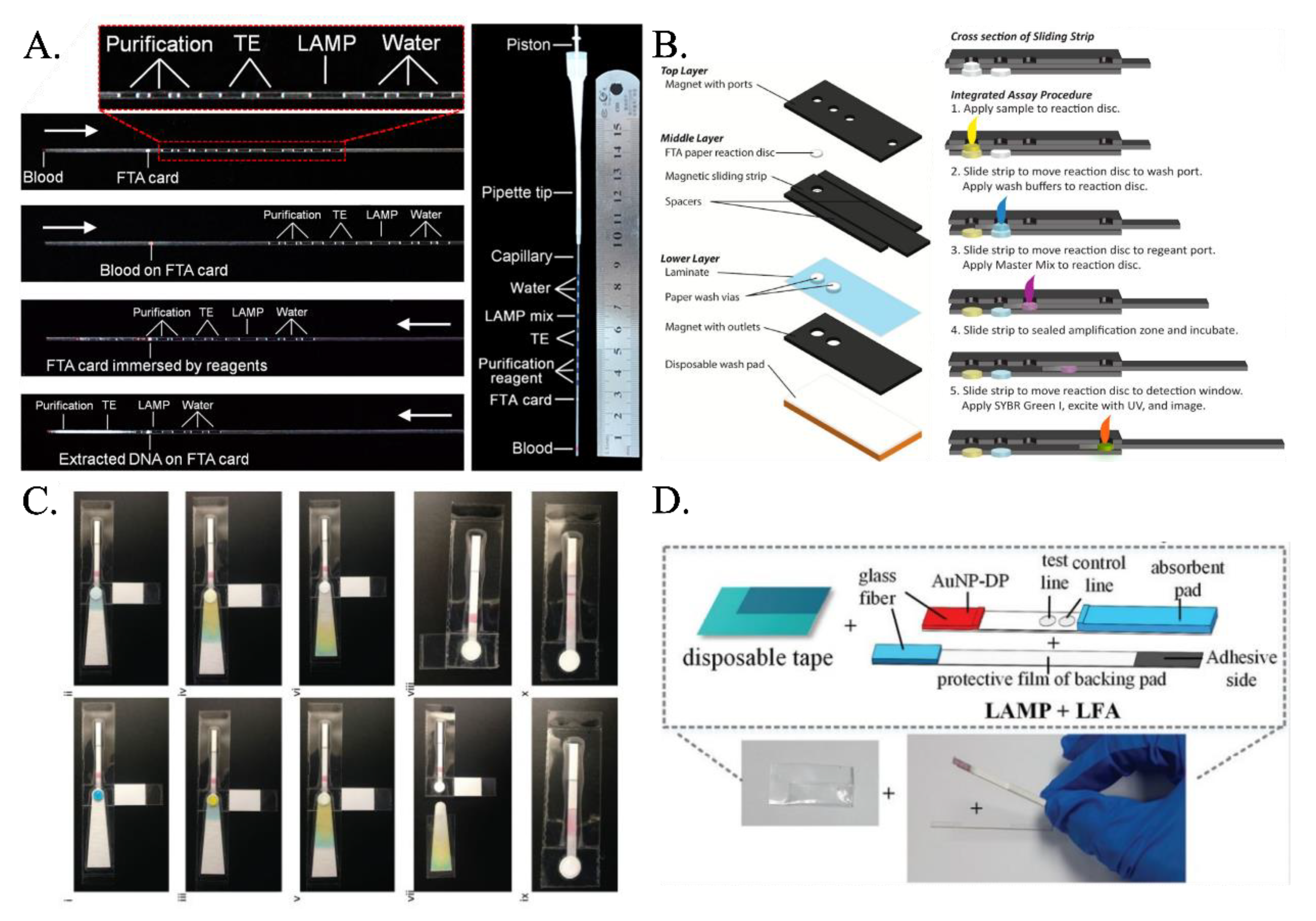
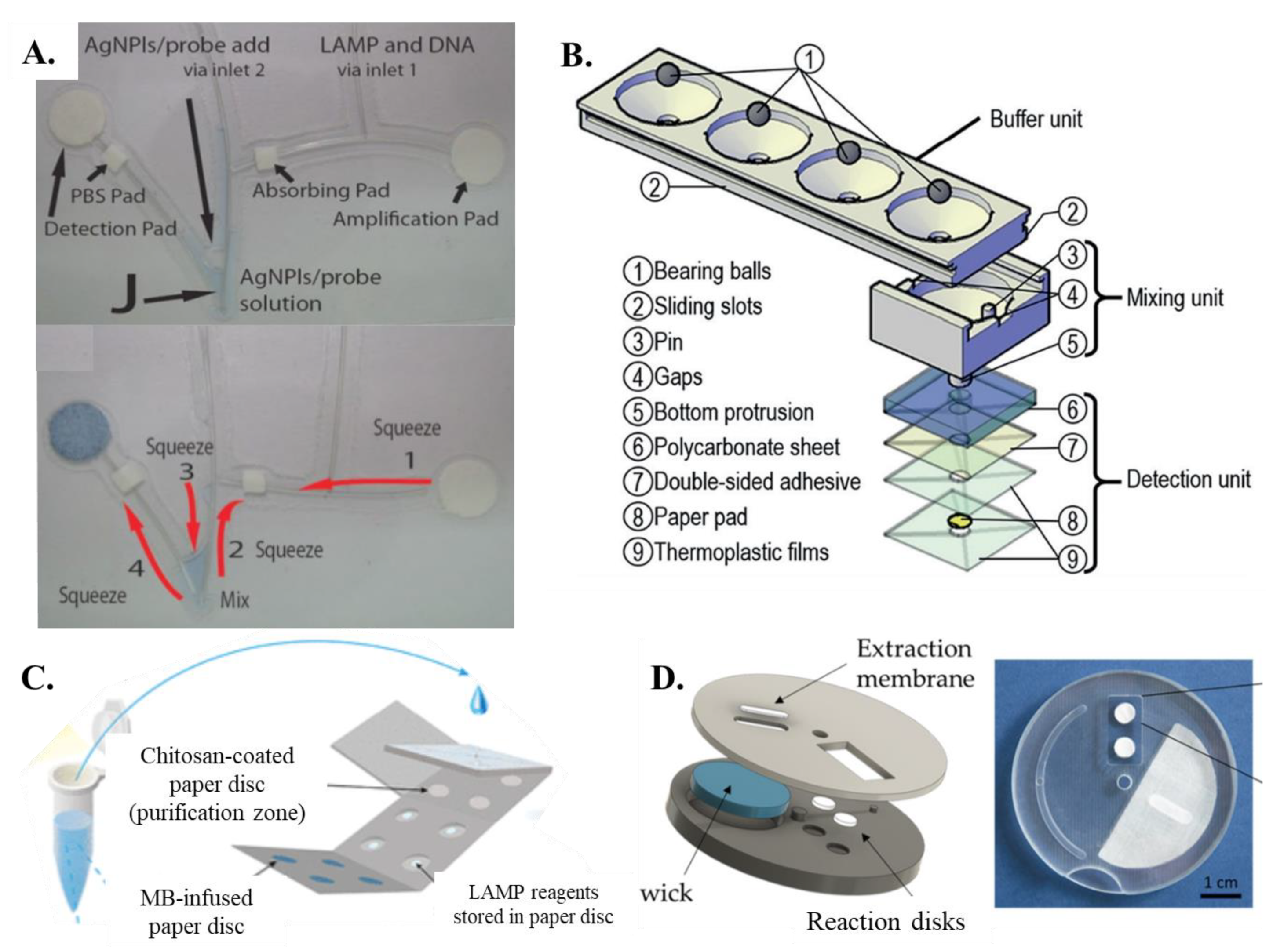
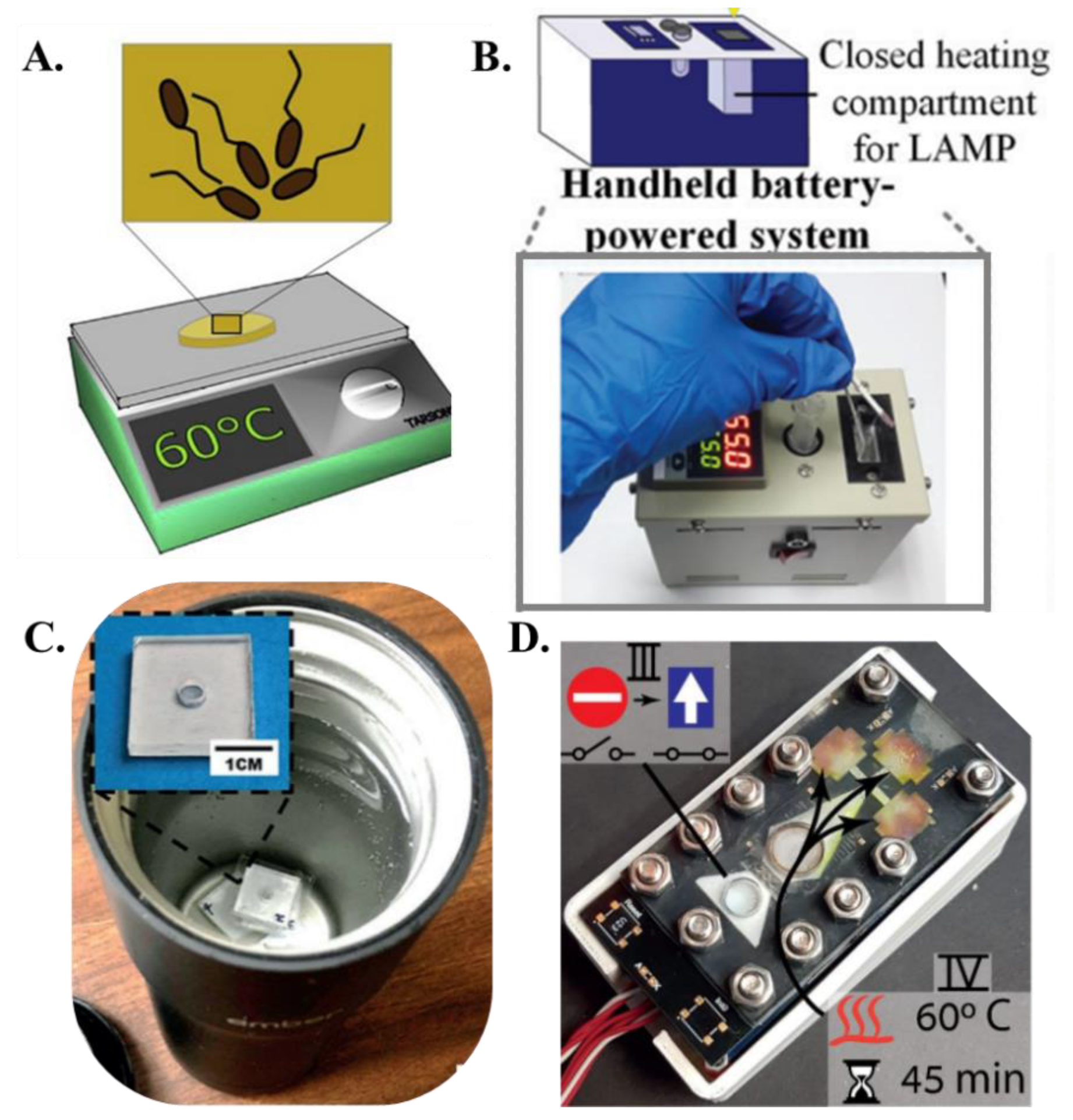
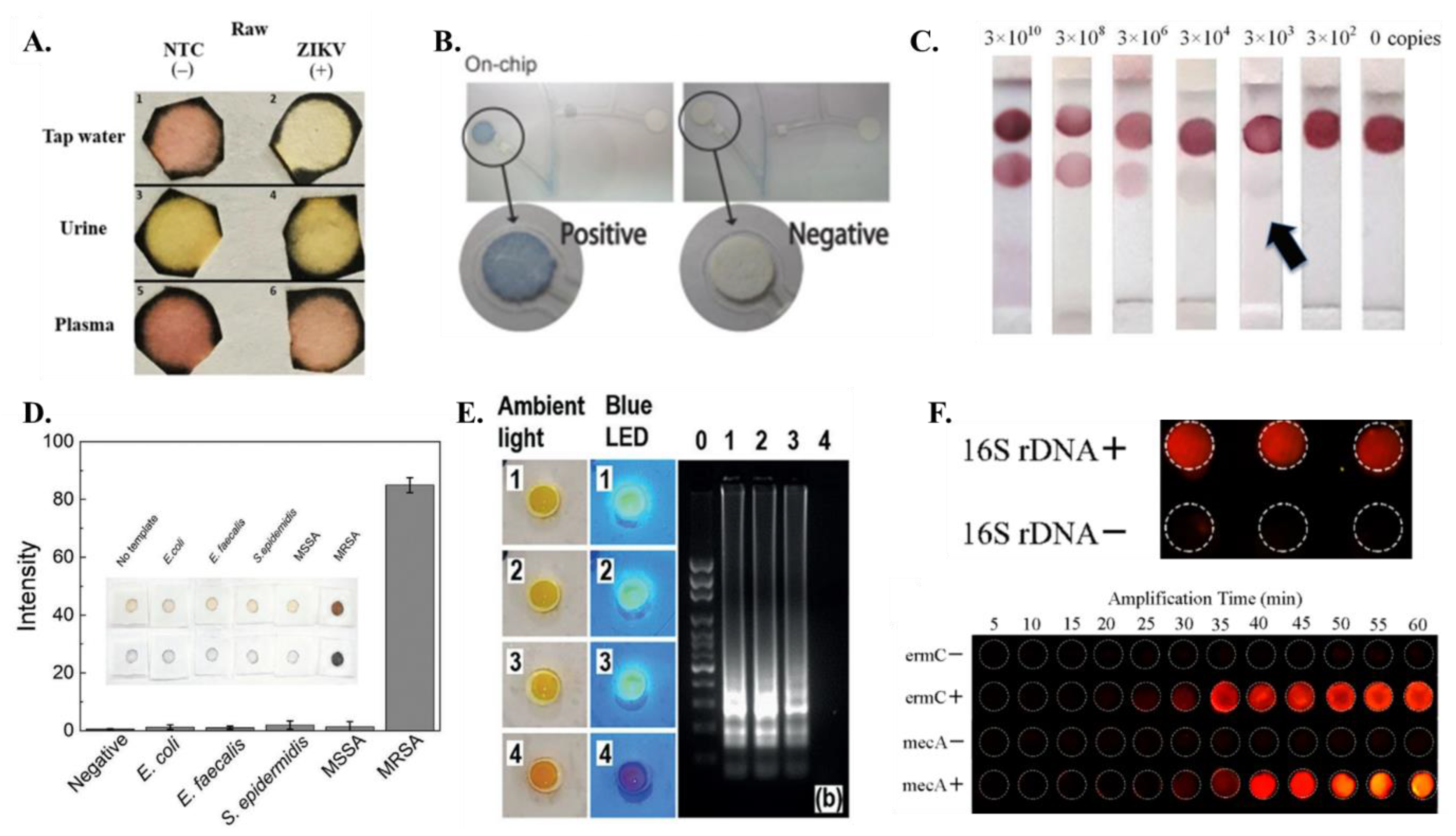
| Reference | Paper Matrix | LAMP Reagent Integration Method |
|---|---|---|
| Connelly et al. [45] | FTA | Purification buffer and nuclease-free water applied on detection pad, followed by drying and application of LAMP Master Mix |
| Chaumpluk et al. [60] | Whatman filter paper | LAMP reagents and DNA sample added through inlet tube to amplification pad |
| Choi et al. [58,59] | Glass fiber pad | Mixture of samples and LAMP reagents pipetted onto pad, protected by disposable tape |
| Linnes et al. [66] | Circular paper discs | Nucleic acid sample added followed by LAMP reagents |
| Zhang et al. [56] | FTA card in icLAMP microcapillary system | Segments of purification reagent, TE buffer, LAMP reaction mix, and water droplets added in sequence, with no reagent directly added to FTA card |
| Rodriguez et al. [57] | PES membrane | Liquid LAMP reaction mix added directly onto sample port of amplification pad |
| Seok et al. [46], Batule et al. [48] | Glass pad | Dried LAMP reagent pre-imbibed on pad, followed by pipetting of reaction buffer without primer, polymerase, and HNB, and heating |
| Jiang et al. [62] | Coffee mug-paper-based unit | Fluid-control ball valve used to trigger release of reagent from buffer to mixing unit |
| Kaarj et al. [44] | Excised paper | 15 μL of RT-LAMP reaction mixture inserted directly onto paper, covered with glass slide, sealed with parafilm M |
| Li et al. [63] | Paper-based chip | Isothermal amplification buffer and bacteria genome specimen added, followed by application of mineral oil to each hole to prevent evaporation |
| Naik et al. [68] | Paper disc | LAMP reaction mixture containing bacterial culture added, heat-sealed into plastic pouch, and incubated at 60 °C for varying durations |
| Trieu and Lee [64] | Origami all-in-one paper structure | Chitosan-based DNA purification pad overlapped on LAMP reaction pad to initiate reaction |
| Wang et al. [54] | Sponge-like PVA pad | Amplification mixtures loaded onto pad, sealed with transparent pressure-sensitive adhesive tape, and placed on thermal plate |
| Suea-Ngam et al. [69] | Paper disc | LAMP primer solution added first, followed by addition of polymerase, target DNA, and LAMP buffer |
| Choopara et al. [70] | Reaction pad | LAMP mixture inserted onto pad, dried, and stored at low temperatures, then DNA sample and sterile water pipetted onto pad and covered by clear top seal for LAMP incubation |
| Trinh et al. [71] | Agarose gel | Agarose gel and LAMP reagents mixed and deposited on surface, solidified at room temperature, followed by addition of DNA solution |
| Reference | Heating Method |
|---|---|
| Connelly et al. [45] | Laboratory incubator |
| Rodriguez et al. [57] | Heat block or hot plate |
| Seok et al. [46] | Heat block powered by Peltier elements |
| Kaarj et al. [44] | Hot plate |
| Davidson et al. [52] | Biological incubator |
| Choi et al. [58] | Handheld system with closed heating compartment |
| Li et al. [63] | Homemade heating device |
| Wang et al. [73] | Integrated thermal and image box |
| Jiang et al. [62] | Battery-powered coffee mug warmer |
| Rofman et al. [65] | Thin film heater |
| Reference | LAMP Platform | Detection Method | Targets | Limit of Detection |
|---|---|---|---|---|
| Connelly et. al. [45] | A paper microfluidic device that enables a central patterned paper strip to slide in and out of fluidic path and allows sample preparation, isothermal amplification and detection. | Fluorescence detection using SYBR Green I | Whole, live E. coli cells (malB gene) in human plasma | 5 cells |
| Linnes et. al. [66] | Lateral flow detection strip with polyethersulphone membrane | Colorimetric-Biotin Streptavidin chemistry | Four separate DNA and RNA targets (Bordetella pertussis, Chlamydia trachomatis, Neisseria, gonorrhoeae, and Influenza A H1N1) | NA |
| Choi et. al. [59] | Lateral flow assay strip which incorporates a piece of nitro cellulose paper-based shunt and a polydimethylsiloxane barrier to the strip | Gold Nano Particle Streptavidin chemistry | Hepatitis B Virus | 102 IU/mL (International units per milliliter) |
| Choi et. al. [58] | An integrated paper-based biosensor incorporating nucleic acid extraction, amplification and visual detection | Gold Nano Particle Streptavidin chemistry | Escherichia coli and Streptococcus pneumonia | 10–1000 CFU/mL |
| Roy et. al. [85] | Paper microchip fabricated in a cellulose paper and a small wax chamber | Colorimetric- Leuco crystal violet | Sus scrofa (porcine) and Bacillus subtilis (bacteria) DNA | 1 picogram/μL and 10 picogram/μL respectively |
| Kaarj et. al. [44] | Paper microfluidic chip | Colorimetric- Phenol red | ZIKV RNA | 1 copy/uL |
| Kim et. al. [86] | Polyethersulfone (PES) paper embedded with a polymethyl methacrylate (PMMA) platform for simultaneous DNA amplification and colorimetric detection | Colorimetric- eriochrome black T (EBT) | DNA of Staphylococcus aureus | 1 femtogram/mL |
| Lin et. al. [87] | Digital LAMP directly on polycarbonate membrane | Primer-probe-primer-quencher fluorescence chemistry | E. coli, E. faecalis, and Salmonella Typhi DNA; also MS2 virus in wastewater | 11 to 1.1 × 105 copies/μL |
| Naik et. al. [68] | Paper-based LAMP | Fluorescence detection using PicoGreen | Escherichia coli (MG1655) and Mycobacterium smegmatis (mc2155) cells | NA |
| Varsha et. al. [88] | Penta- cloverleaf modelled paper-based (Whatman filter paper) LAMP device | Colorimetric- Leuco crystal violet | Multiple gene targets in Leptospira | 50 attogram/μL |
| Jawla et. al. [77] | Paper-based LAMP lateral flow assay strip with nitro cellulose membrane | Colorimetric-Biotin Streptavidin chemistry | Cattle DNA | 0.1 picogram |
| Choopara et. al. [70] | Paper-based (cellulose membrane) LAMP device consisting of a sandwich-like bottom base, a reaction pad in the center, and a clear top seal | Fluorescence detection using SYBR Green I | MRSA (gene mecA) | 10 attogram—equivalent to 1 copy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, D.; Masetty, M.; Priye, A. Paper-Based Loop Mediated Isothermal Amplification (LAMP) Platforms: Integrating the Versatility of Paper Microfluidics with Accuracy of Nucleic Acid Amplification Tests. Chemosensors 2023, 11, 163. https://doi.org/10.3390/chemosensors11030163
Das D, Masetty M, Priye A. Paper-Based Loop Mediated Isothermal Amplification (LAMP) Platforms: Integrating the Versatility of Paper Microfluidics with Accuracy of Nucleic Acid Amplification Tests. Chemosensors. 2023; 11(3):163. https://doi.org/10.3390/chemosensors11030163
Chicago/Turabian StyleDas, Debayan, Manaswini Masetty, and Aashish Priye. 2023. "Paper-Based Loop Mediated Isothermal Amplification (LAMP) Platforms: Integrating the Versatility of Paper Microfluidics with Accuracy of Nucleic Acid Amplification Tests" Chemosensors 11, no. 3: 163. https://doi.org/10.3390/chemosensors11030163
APA StyleDas, D., Masetty, M., & Priye, A. (2023). Paper-Based Loop Mediated Isothermal Amplification (LAMP) Platforms: Integrating the Versatility of Paper Microfluidics with Accuracy of Nucleic Acid Amplification Tests. Chemosensors, 11(3), 163. https://doi.org/10.3390/chemosensors11030163







