A Broad Spectral Photodetector Using Organic Bisindolo Quinoxaline on ZnO Nanorods
Abstract
:1. Introduction
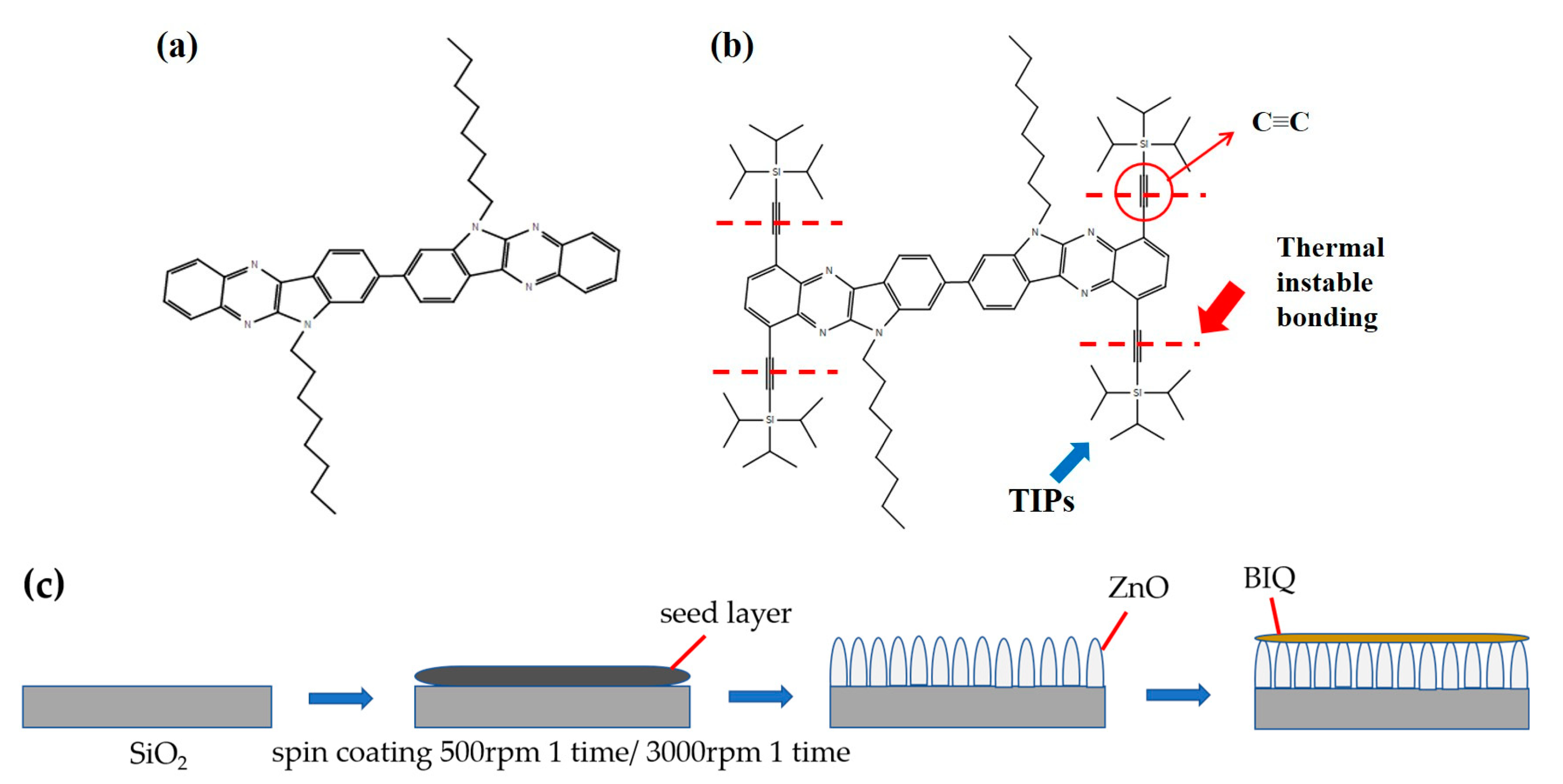
2. Materials and Methods
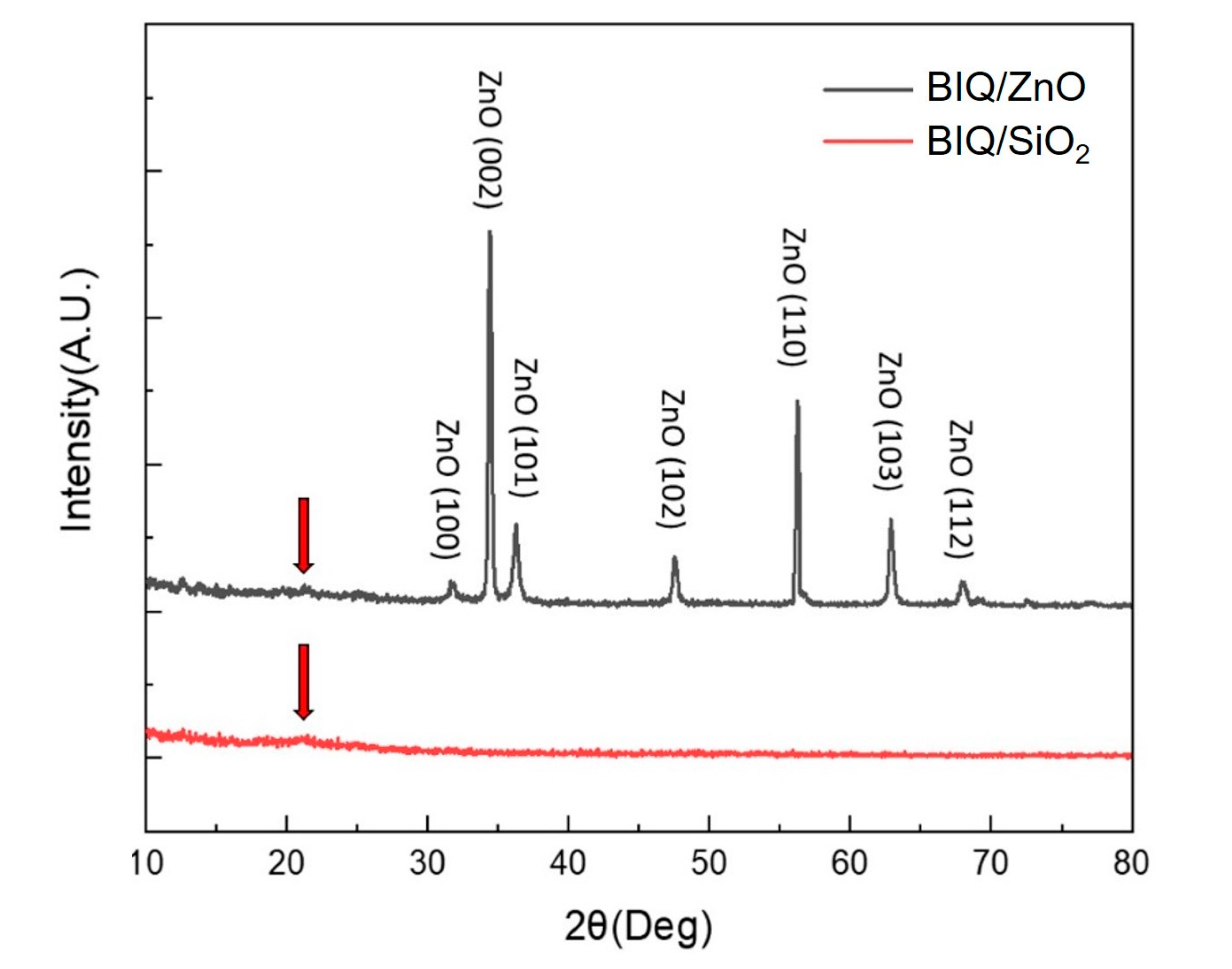
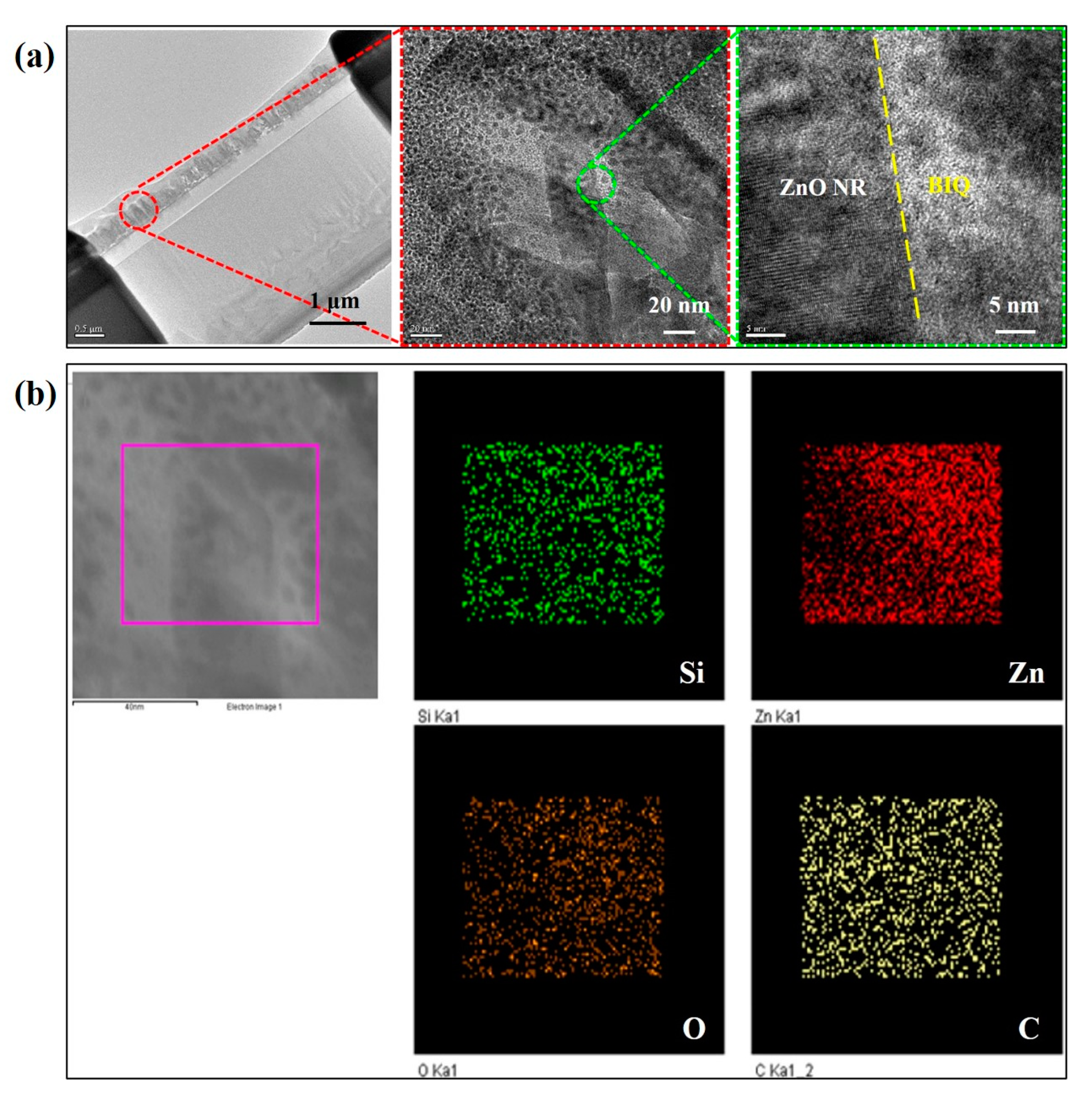
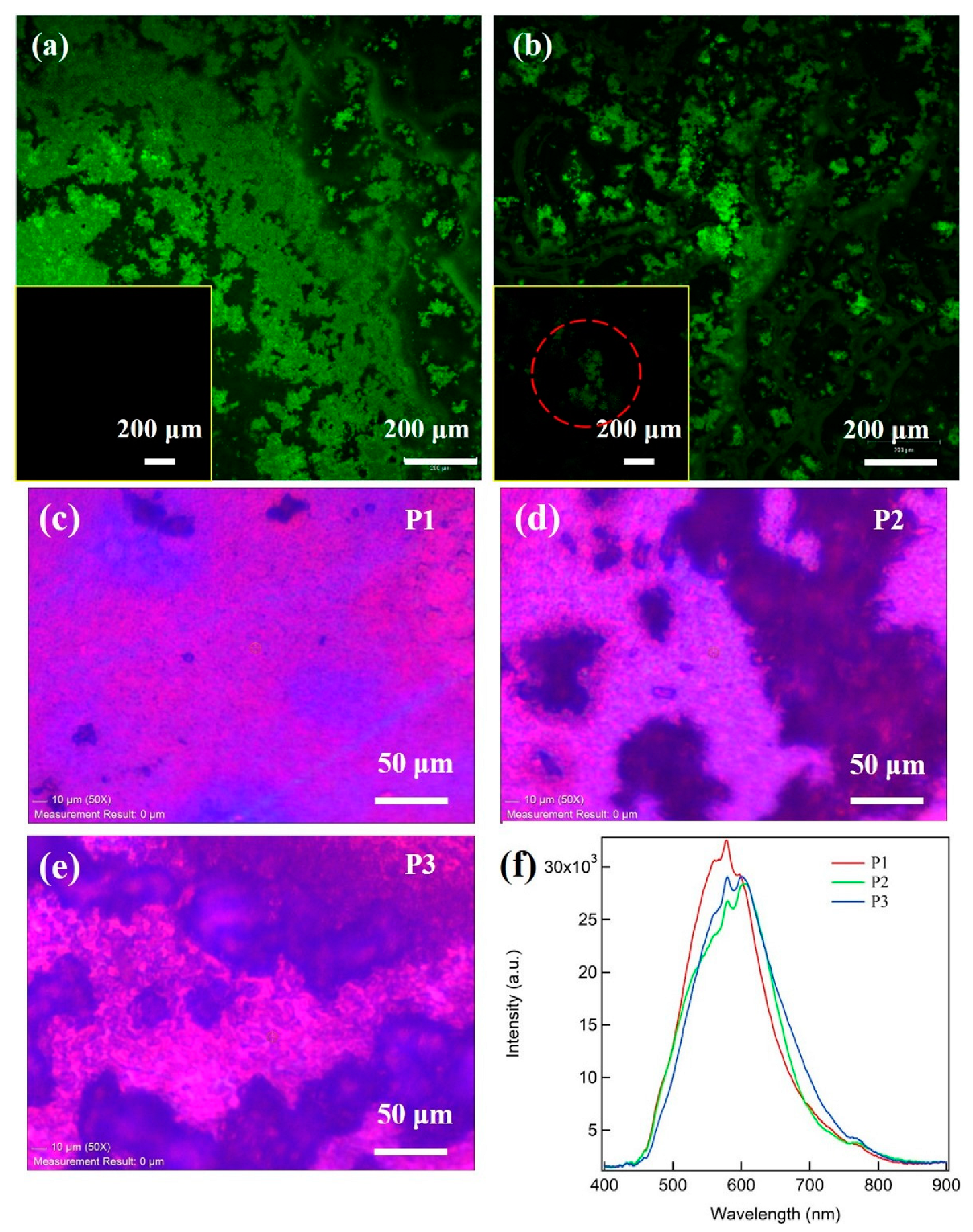
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, J.; Ouyang, W.; Yang, W.; He, H., Jr.; Fang, X. Recent progress of heterojunction ultraviolet photodetectors: Materials, integrations, and applications. Adv. Funct. Mater. 2020, 30, 1909909. [Google Scholar]
- Singh, J.; Kaur, S.; Kaur, G.; Basu, S.; Rawat, M. Biogenic ZnO nanoparticles: A study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight. Green Process. Synth. 2019, 8, 272–280. [Google Scholar]
- Liu, K.; Sakurai, M.; Aono, M. ZnO-based ultraviolet photodetectors. Sensors 2010, 10, 8604–8634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, W.; Chen, J.; Shi, Z.; Fang, X. Self-powered UV photodetectors based on ZnO nanomaterials. Appl. Phys. Rev. 2021, 8, 031315. [Google Scholar]
- Kao, C.H.-L.; Nguyen, B.; Roseway, A.; Dickey, M. Earthtones: Chemical sensing powders to detect and display environmental hazards through color variation. In Proceedings of the 2017 CHI Conference Extended Abstracts on Human Factors in Computing Systems (CHI EA’17). Association for Computing Machinery, New York, NY, USA, 6–11 May 2017; pp. 872–883. [Google Scholar]
- Fortunato, E.M.C.; Barquinha, P.M.C.; Pimentel, A.C.M.B.G.; Gonçalves, A.M.F.; Marques, A.J.S.; Martins, R.F.P.; Pereira, L.M. Wide-bandgap high-mobility ZnO thin-film transistors produced at room temperature. Appl. Phys. Lett. 2004, 85, 2541–2543. [Google Scholar] [CrossRef]
- Panwar, V.; Nandi, S.; Majumder, M.; Misra, A. Self-powered ZnO based pyro-phototronic photodetectors: Impact of heterointerfaces and parametric studies. J. Mater. Chem. C 2022, 10, 12487–12510. [Google Scholar]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Yuan, Z.; Gao, H.; Zhang, R.; Meng, F. Perovskite-structured LaCoO3 modified ZnO gas sensor and investigation on its gas sensing mechanism by first principle. Sens. Actuators B Chem. 2021, 341, 130015. [Google Scholar] [CrossRef]
- de Arquer, F.P.G.; Talapin, D.V.; Klimov, V.I.; Arakawa, Y.; Bayer, M.; Sargent, E.H.; Baggiolini, A.; Callahan, S.J.; Luo, L.; Zuko, A.; et al. Semiconductor quantum dots: Technological progress and future challenges. Science 2021, 373, eaaz8541. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Han, E.; Meng, F.; Zuo, K. Detection and identification of volatile organic compounds based on temperature-modulated ZnO sensors. IEEE Trans. Instrum. Meas. 2019, 69, 4533–4544. [Google Scholar] [CrossRef]
- Manjari, G.; Saran, S.; Radhakrishanan, S.; Rameshkumar, P.; Pandikumar, A.; Devipriya, S.P. Facile green synthesis of Ag–Cu decorated ZnO nanocomposite for effective removal of toxic organic compounds and an efficient detection of nitrite ions. J. Environ. Manag. 2020, 262, 110282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-S.; Liu, J.-X.; Cheng, X.-F.; He, J.-H.; Li, H.; Xu, Q.-F.; Li, N.-J.; Chen, D.-Y.; Lu, J.-M. Ultrasensitive humidity sensing using one-dimensional π-d conjugated coordination polymers for breath monitoring. Sens. Actuators B Chem. 2020, 330, 129353. [Google Scholar] [CrossRef]
- Li, M.-H.; Liang, K.-T.; Wu, Z.-H.; Lin, S.-H.; Chiu, Y.-S.; Lee, C.-H.; Kuo, M.-Y.; Lin, C.-F.; Chen, H.; Wuu, D.-S.; et al. Synergetic Effects of ZnS Core Shell and Bisindolo Quinoxaline-Tips (BIQ-TIPs) Spreading on ZnO Nanorod-Based Light/Gas Dual Sensors. IEEE Sens. J. 2022, 22, 23821–23828. [Google Scholar] [CrossRef]
- Ytsai, Y.-S.; Chen, C.-J.; Huang, Y.-T.; Liang, K.-T.; Jhang, J.-J.; Huang, S.-H.; Li, M.-H.; Wu, Y.S.; Kuo, M.-Y.; Chen, H.; et al. Incorporation of hydrophobic-like bisindolo quinoxaline-tips (BIQ-TIPs) aggregation on ZnO nanorods for spectral broadening photodetection. Results Phys. 2022, 34, 105318. [Google Scholar]
- Liu, X.; Chen, C. Mxene enhanced the photocatalytic activity of ZnO nanorods under visible light. Mater. Lett. 2019, 261, 127127. [Google Scholar] [CrossRef]
- Yang, X.; Liu, H.; Li, T.; Huang, B.; Hu, W.; Jiang, Z.; Chen, J.; Niu, Q. Preparation of flower-like ZnO@ ZnS core-shell structure enhances photocatalytic hydrogen production. Int. J. Hydrogen Energy 2020, 45, 26967–26978. [Google Scholar] [CrossRef]
- Zhao, X.; Feng, J.; Liu, J.; Lu, J.; Shi, W.; Yang, G.; Wang, G.; Feng, P.; Cheng, P. Metal–Organic Framework-Derived ZnO/ZnS Heteronanostructures for Efficient Visible-Light-Driven Photocatalytic Hydrogen Production. Adv. Sci. 2018, 5, 1700590. [Google Scholar] [CrossRef] [PubMed]
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-like malleable materials from permanent organic networks. Science 2011, 334, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Ohe, T.; Kuribayashi, M.; Yasuda, R.; Tsuboi, A.; Nomoto, K.; Satori, K.; Itabashi, M.; Kasahara, J. Solution-processed organic thin-film transistors with vertical nanophase separation. Appl. Phys. Lett. 2008, 93, 286. [Google Scholar] [CrossRef]
- Cheng, C.-C.; Weng, W.C.; Lin, H.; Chiu, J.L.; Jhao, H.-Y.; Liao, Y.T.A.; Yu, C.T.R.; Chen, H. Fabrication and characterization of distinctive ZnO/ZnS core–shell structures on silicon substrates via a hydrothermal method. RSC Adv. 2018, 8, 26341–26348. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| UV LED | White LED | Green LED | Red LED |
|---|---|---|---|
| 22.2 W/m2 | 46.67 W/m2 | 41.67 W/m2 | 33.3 W/m2 |
| UV LED | Green LED | |
|---|---|---|
| BIQ/ZnO NRs PDs | 800.3% | 12.3% |
| BIQ-TIPs/ZnO NRs PDs | 169.4% | 5.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.-H.; Huang, Y.-H.; Chuang, C.-C.; Lin, S.-H.; Huang, Y.-H.; Lin, C.-F.; Lin, Y.-S.; Kuo, M.-Y.; Chen, H. A Broad Spectral Photodetector Using Organic Bisindolo Quinoxaline on ZnO Nanorods. Chemosensors 2023, 11, 199. https://doi.org/10.3390/chemosensors11030199
Li M-H, Huang Y-H, Chuang C-C, Lin S-H, Huang Y-H, Lin C-F, Lin Y-S, Kuo M-Y, Chen H. A Broad Spectral Photodetector Using Organic Bisindolo Quinoxaline on ZnO Nanorods. Chemosensors. 2023; 11(3):199. https://doi.org/10.3390/chemosensors11030199
Chicago/Turabian StyleLi, Ming-Hsien, Yao-Hong Huang, Chi-Chih Chuang, Sang-Hao Lin, Yi-Hsuan Huang, Chia-Feng Lin, Yung-Sen Lin, Ming-Yu Kuo, and Hsiang Chen. 2023. "A Broad Spectral Photodetector Using Organic Bisindolo Quinoxaline on ZnO Nanorods" Chemosensors 11, no. 3: 199. https://doi.org/10.3390/chemosensors11030199
APA StyleLi, M.-H., Huang, Y.-H., Chuang, C.-C., Lin, S.-H., Huang, Y.-H., Lin, C.-F., Lin, Y.-S., Kuo, M.-Y., & Chen, H. (2023). A Broad Spectral Photodetector Using Organic Bisindolo Quinoxaline on ZnO Nanorods. Chemosensors, 11(3), 199. https://doi.org/10.3390/chemosensors11030199






