3.1. Application of OTFT in Saliva
With the improvement in living standards, people are becoming more conscious of life and health. Saliva testing has attracted wide attention because of its advantages, such as convenience sampling, no trauma, and continuous testing. Testing for biomarkers in saliva can monitor and assess a person’s physiological state. A large number of clinical studies can also take samples from saliva to test and analyze them in disease diagnosis [
11]. Studies have confirmed that saliva is a complicated matrix containing many biomarkers, such as glucose, hormones, enzymes, nitrogen products, antibodies, etc. As a classical device, OTFTs are receiving increasing attention for measuring biomarkers in complex physiological substrates, including specific analyte substances in saliva [
12,
13].
Diabetes is a kind of metabolic disease characterized by high blood glucose levels. In recent decades, the incidence of diabetes has increased worldwide and there has been a tendency for diabetes to become more widespread and impact people younger. Studies have shown that blood sugar levels are directly related to glucose concentrations in saliva [
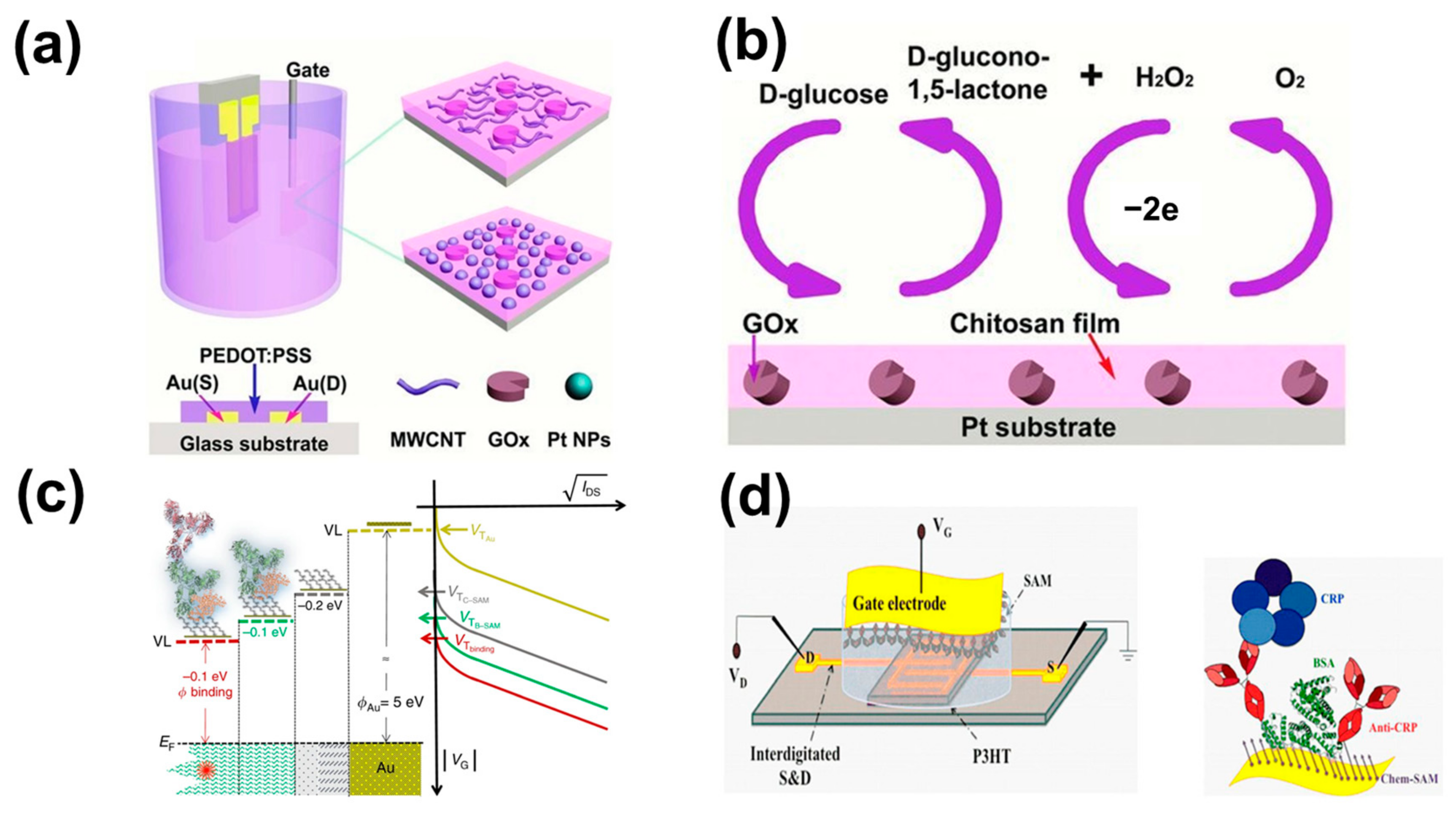
14]. The glucose concentration in blood can be obtained by detecting the glucose concentration in saliva to achieve the purpose of non-invasive, quick, and real-time monitoring of blood glucose levels. OTFTs have become one of the most promising tools for diabetes diagnosis, especially as glucose biosensors. Glucose oxidase (Gox) catalyzes the conversion of D-glucose to D-glucose-1,5-lactone with a reduced process. Vigors are reactivated from the reduced state and produce hydrogen peroxide. Because hydrogen peroxide concentration is directly related to the concentration of glucose, hydrogen peroxide is often used to monitor and quantify glucose concentrations. The Malliaras team first reported the application of a poly (3,4-vinyl dioxythiophene):polystyrene sulfonic acid (PEDOT:PSS) based OECT in glucose sensors [
15]. In their work, the electrolyte was treated with glucose oxidase. The gate and active layers of OECTs have no surface modifications. Source–drain current in relation to effective gate voltage is generally obtained by varying the MOSFET transfer characteristics at different glucose levels. The response of the devices is correlated with the analyte concentration in the OECT-based enzyme biosensor. They discovered a relationship between the glucose concentration and the source leakage current by calculation. As the glucose concentration increases, there is a correlation shift in the offset voltage. The glucose sensor has a detection limit of about a few μM and provides a good measurement of glucose levels in human saliva. Alternatively, no additional glucose oxidase can be added. Tang et al. [
16] developed a new OECT-based glucose sensor (the construction and principle of the device are shown in
Figure 1a,b). In their work, no additional enzymes need to be added to the solution by manipulating the gate with enzymes and nanoparticles. The Pt electrode surface was modified using multi-walled carbon nanotubes (MWCNTs)-chitosan (CHIT) hybridization and electrodeposition of Pt-NPs prior to Gox immobilization. MWCNT-CHIT/Gox/Pt and CHIT/Gox/Pt-NPs/Pt electrodes were applied as OECT gates for PEDOT:PSS, respectively. It is faster and easier to use in practical applications, and the sensitivity and performance of the device are greatly improved. In this experiment, the gate voltage was chosen to be 0.4 V. At this point, the normalized current response (NCR) was maximum. These two kinds of electrodes for the glucose detection limit of 20 μM and 5 μM, respectively, of glucose concentration in saliva detection, have a high sensitivity. The gate electrode can therefore be selectively modified, making OECT more widely available for other enzyme sensors. To further improve the rapid response to low glucose concentrations, Elkington et al. [
17] designed the P3HT/PVP/Nafion:Gox sensor. They used poly (3-hexylthiophene) (P3HT) as the semiconductor layer, and the grid consisted of a thin film of Nafion (a polymer film material) loaded with Gox. The low-voltage OTFTs device can be integrated with Gox without enzyme activity loss and without the chemical modification of the enzyme and polymer matrix, which significantly simplifies the operation process. For this biosensor, the diffusion of glucose is a critical factor in determining the device’s response time. Based on the OTFTs glucose sensor is susceptible to glucose concentration in saliva. It can measure glucose concentrations in saliva ranging from 8 to 200 μM. The feasibility of a low-cost printed saliva glucose biosensor was demonstrated.
OTFTs-based biosensors also hold good promise for monitoring proteins. In total, 25–30% of the proteins in saliva are the same as those contained in blood, and many proteins can be used for clinical disease diagnosis [
18]. Macchia et al. [
19] reported an electrolyte-gated OFET (EGOFET) fixed-based ~10
12 immunoglobulin-resistant G (IgG) resistance of a single molecule detection platform without a label (in
Figure 1c). The antigen is captured on its millimeter-sized gated plate. Mixed in with saliva approved the selectivity and the capture of the single molecule IgG, without a label, in all 15 IgGs detected in serum. No labels based on the FET of the single-molecule transistor (SiMoT) platform contain a highly filling capture antibody self-assembled monolayer (SAM), covalent attachment on the golden surface gate. The fewer defects in the SAM, the more pronounced the change in the power function caused by a single binding event, the larger the measurable correlation signal, and the steeper the response of the dose profile. To improve the selectivity and reliability of equipment, Macchia et al. further discussed the biosensor based on EGOTFT (in
Figure 1d) to measure the trace of the concentration of C-reactive protein (CRP) in human saliva [
20]. CRP is an important biomarker of the body’s inflammatory response and is involved in non-specific immunity in the human body. Usually, surgery, accidental trauma, heart attack, rheumatic diseases, and malignant tumors cause an increase in CRP [
21]. In their work, P3HT is used as an OSCto form a conductive path between the source and drain contact points, with aqueous as the electrolyte medium. A monolayer functionalized by the self-assembled gate is used to capture the anti-CRP protein. Saliva stock solution was obtained by diluting human saliva with PBS at a ratio of 1:50. The biosensor platform is combined with low-cost manufacturing technologies for the sensitive detection of biomarkers of clinical relevance. Measurement of CRP in the dilution of saliva can be no labels in the saliva of CRP detection selectivity, low LOD to 13 ± 4 protein. This EGOTFT-based biosensor is not only highly sensitive but also has good selectivity. It is not only limited to the detection of markers in saliva but also in tears and urine. For early diagnosis of protein biomarkers detection and no labels of new revolution laid a foundation. The EGOTFT was successfully used as a biosensor by Palazzo et al. [
22]. They proved that the EGOFET-based biosensor (in
Figure 2a) works in high concentration in the solution (λ = 0.7 nm) and can sensitively detect the distance of the transistor channel protein events of more than 20 nanometers. The bio-EGOFET sensing platform contains a biological layer in the middle of the electrolyte-OSC interface. The bio-layer consists of phospholipid bilayers that are fixed to the surface of the OSC covalently by plasma depositing a functionalized thin layer. This capacitively tuned EGOFETs reaction is almost not sensitive to the Debye length.
Figure 2b shows the change in fraction after
Ids formation.
Figure 1.
(
a) The schematic representation of the component construction of PEDOT:PSS. (
b) The reaction cycle of glucose in OECTs. (
c) Variation in
Ids of two different systems as they form a new surface. (
d) The schematic diagram of the SiMoT biosensing platform based on OTFTs. (
a) Reproduced with permission [
16]. Copyright 2014, John Wiley and Sons. (
b) Reproduced with permission [
16]. Copyright 2014, John Wiley and Sons. (
c) Reproduced with permission [
19]. Copyright 2018, Springer Nature. (
d) Reproduced with permission [
20]. Copyright 2019, Springer Nature.
Figure 1.
(
a) The schematic representation of the component construction of PEDOT:PSS. (
b) The reaction cycle of glucose in OECTs. (
c) Variation in
Ids of two different systems as they form a new surface. (
d) The schematic diagram of the SiMoT biosensing platform based on OTFTs. (
a) Reproduced with permission [
16]. Copyright 2014, John Wiley and Sons. (
b) Reproduced with permission [
16]. Copyright 2014, John Wiley and Sons. (
c) Reproduced with permission [
19]. Copyright 2018, Springer Nature. (
d) Reproduced with permission [
20]. Copyright 2019, Springer Nature.
Figure 2.
(
a) The schematic representation of an EGOFET based on CRP detection and the reaction principle. (
b) Transport properties of SiMoT after functionalization. (
c) Test plan for ISFET-based microsystem design and fabrication. (
d) The schematic representation of the construction of an ISFET for detecting ion concentrations in saliva. (
a) Reproduced with permission [
22]. Copyright 2015, John Wiley and Sons. (
b) Reproduced with permission [
22]. Copyright 2015, John Wiley and Sons. (
c) Reproduced with permission [
23]. Copyright 2005, Elsevier (
d) Reproduced with permission [
24]. Copyright 2019, Elsevier.
Figure 2.
(
a) The schematic representation of an EGOFET based on CRP detection and the reaction principle. (
b) Transport properties of SiMoT after functionalization. (
c) Test plan for ISFET-based microsystem design and fabrication. (
d) The schematic representation of the construction of an ISFET for detecting ion concentrations in saliva. (
a) Reproduced with permission [
22]. Copyright 2015, John Wiley and Sons. (
b) Reproduced with permission [
22]. Copyright 2015, John Wiley and Sons. (
c) Reproduced with permission [
23]. Copyright 2005, Elsevier (
d) Reproduced with permission [
24]. Copyright 2019, Elsevier.
The electrolyte ions in saliva are also an essential indicator of the health of the body. Ion selective field effect transistors (ISFETs) are OTFTs that can detect saliva pH and ions [
23].
Figure 2c shows a test plan for ISFETs-based microsystem design and manufacturing. Many electrolyte ions, such as calcium, potassium, and sodium ions, are present in human body fluids (including saliva). Electrolytes in the body maintain the body’s pH balance, osmotic pressure balance, and cells’ structural and functional integrity. They are also involved in nerve conduction and metabolism in the body. Bao et al. [
24] put forward printing the organic and inorganic ion selective electrode transistor hybrid to prepare flexible ISFET (as shown in
Figure 2d). They were used to detect potassium (K
+), calcium (Ca
2+) and ammonium (NH
4+) ions in saliva. The presented serpentine trench design proves that the transistor has good uniform trench reproducibility. The prepared ISFET is highly reliable with the slope of the potential versus ion concentration curve with negligible value variation. During the operation of the device, the change of ion concentration in the movement of the ion-selective membrane potential, as well as the changes between the source and drain current. The ISFET can detect K
+, NH
4+, and Ca
2+ at 10
−6~1 M, 10
−6~1 M, and 10
−4~1 M, which exhibits good selectivity and immunity without interference by other ions. In addition to the biomarkers mentioned above, tests for peptides, such as oxytocin, have also been investigated. Oxytocin simple detection can contribute to the basic research of diagnosis, maternal care, pharmacology, and molecular biology.
Ohshiro et al. [
25] reported an extended gate type OFET-based sensor for oxytocin. This OFET-based sensor successfully detected PPT-level oxytocin in human saliva. When an OFET-based sensor catches a charged chemical, the electrical potential at the interface between the separating grid electrode and the target analyte solution changes, and the conductance of the OFET is altered. With the addition of oxytocin, the OFET functionalized with anti-Oxytocin antibodies is negatively shifted. At the same time, the corresponding titration isotherm is nonlinear. By uniformly attaching the anti-oxytocin antibody to the extended grid electrode, the OFET can respond to oxytocin quickly, rapidly, and quantitatively, even without many interfering substances. The sensor applied a mixture of 3,9-dihexyldinaphtho[2,3-b:2,3-d]thiophene (C6-DNT-VW) and polystyrene to achieve a reproducible and homogeneous semiconductor layer. It can detect human saliva in the normal range of physiology (0~10 pg mL
−1). In addition, they used eight chemicals contained in saliva for selective testing. The OFET-based sensor successfully detected oxytocin selectively. It will make a path toward implementing portable detectors for healthcare monitoring. It is essential to understand that while the number of OTFTs for saliva identification is increasing, the number of intelligent biosensors that can independently analyze biomarkers in saliva reliably, efficiently, and consistently still needs to be increased [
26]. Most sensors need to be further optimized and evaluated regarding human trials and sensor reliability before being developed on a large scale and used daily.
3.2. Application of OTFT in Sweat
Sweat has gained much attention as a potential source of body fluids due to its close connection to health and disease. It is readily obtained from the skin surface in humans, including separated from the blood of the specific biomarkers [
27,
28]. The main component of human sweat is water (99%). However, it also contains various trace components, such as protein, fatty acids, electrolytes, metabolites, etc., which can indicate fatigue, body diseases, dehydration, and emotional stress [
29]. Thus, sweat may be a possibility to be a biomarker of a non-invasive nature. With the biosensor field’s rapid development, sweat biomarkers analysis has become a trend, oriented toward monitoring disease and managing health. This is because the biomarker of human sweat is at a significantly lower concentration than the biomarker of blood [
30], the sensor requires high sensitivity and selectivity. Illustration of IoT technologies and typical structures of biosensors is in
Figure 3a,b.
Glucose in sweat is one of the most attractive markers for researchers. Sweat and the correlation between blood glucose levels are used to diagnose diabetes. The OECT obtained by screen printing by Scheiblin et al. [
31] detects glucose and lactic acid. The transistor uses a solid electrolyte containing reagent and is capable of detecting glucose and lactate in a small amount of media. They selected an organically modified sol-gel electrolyte in which the enzyme is encapsulated. The stability of the equipment and the capability to perform a long period of storage, and the influence of other interfering substances in the medium, are issues that need to be resolved in the future. However, the sensor has a high detection limit. The thinner PB membrane has a solid and stable electrocatalytic activity at low operating potentials with high susceptibility and a high degree of selectivity, thus facilitating the assay of glucose in sweat at low H
2O
2 concentrations [
32]. To further improve sensitivity and stability in liquid phase detection, Mano et al. [
33] developed an enzyme sensor based on OFET (in
Figure 3c,d). Its low cost, high sensitivity, and biocompatibility were used to detect glucose accurately. D-glucose and Gox undergo an enzymatic reaction to produce hydrogen peroxide, and the extended grid electrode continuously monitors glucose levels. The reducing medium PB is then oxidized by hydrogen peroxide from divalent to trivalent. The potentiometric redox difference between the electrodes induces the redox cycle of PB. Therefore, expanding the grid potential changes is based on the Nernst equation. This leads to a change in the threshold voltage (
Vth) or source-drain current (
Ids) of the OFET. The sensor of D-glucose displayed a gradually stable and reversible reaction. Glucose concentrations of 0.01~1.11 mM in sweat can be detected without adding other chemical reagents. The response to glucose levels in tears and saliva is equally good. OTFTs-based biosensors have come a long way, and rapid detection of target analytes with low-cost devices in complex environments is no longer a fantasy. In the future, OTFTs sensors in wearable smart devices, real-time health monitoring, and artificial electronic skin and organs will have critical applications.
Figure 3.
(
a) The illustration of IoT technologies. (
b) Based on the top of the grid and bottom gate transistor, the typical structure of biosensors. (
c) Schematic representation of the structure of the enzyme sensor based on an extended-gate organic transistor. (
d) The schematic diagram of the transistor array and the enzymatic reaction at the extended gate electrode. (
a) Reproduced with permission [
27]. Copyright 2022, Elsevier. (
b) Reproduced with permission [
27]. Copyright 2022, Elsevier. (
c) Reproduced with permission [
33]. Copyright 2018, John Wiley and Sons. (
d) Reproduced with permission [
33]. Copyright 2018, John Wiley and Sons.
Figure 3.
(
a) The illustration of IoT technologies. (
b) Based on the top of the grid and bottom gate transistor, the typical structure of biosensors. (
c) Schematic representation of the structure of the enzyme sensor based on an extended-gate organic transistor. (
d) The schematic diagram of the transistor array and the enzymatic reaction at the extended gate electrode. (
a) Reproduced with permission [
27]. Copyright 2022, Elsevier. (
b) Reproduced with permission [
27]. Copyright 2022, Elsevier. (
c) Reproduced with permission [
33]. Copyright 2018, John Wiley and Sons. (
d) Reproduced with permission [
33]. Copyright 2018, John Wiley and Sons.
In addition to detecting glucose in sweat, OTFT can also detect cortisol levels. The optimal concentration of cortisol in sweat is between 0.02 and 0.5 μM [
34]. Cortisol is an adrenal corticosteroid hormone, often referred to as the “stress hormone” [
35]. Elevated levels of cortisol can have a negative impact on a person’s health. Persistent stress can impair the balance of the heart, kidneys, bones, and internal secretion system, resulting in the progressive progression of chronic diseases. It increases the likelihood of depression, suicide, and anxiety [
36]. Therefore, we can measure cortisol levels in sweat as a biomarker to monitor physical health. Jang et al. [
34] showed a cortisol sensor based on a field-effect transistor (in
Figure 4a). The approach is to embed cortisol antibodies in polystyrene-methacrylic acid (PSMA) to form membranes that are selective and sensitive to cortisol. Receptors in the polymer-anchored structure enable cortisol atoms in the film matrix near the interface combination. They designed a sensor with high sensitivity and low detection limits (down to 1 pg/mL). The OECT sensor enables cortisol detection by functionalizing the molecularly imprinted membrane (MIM) only. The technology of increased use in clinical environment transistor biological sensors detect the possibility of cortisol in saliva or sweat. Parlak et al. [
37] subsequently developed an artificially recognizable molecularly printed polymers-based membrane that was inserted between the PEDOT:PSS channel layer and the analyte (sweat) to manage and regulate the direct segregative molecular transport of cortisol from the surface of the skin to the OECT sensor sensing channel (in
Figure 4b). Cortisol concentration in 0.01~10.0 μM is a logarithmic, linear response range. Electrochemical crystals and integration of bionic polymer membrane, with high sensitivity, promote stability and selectivity of cortisol molecular recognition. The design principle of selective detection can also be applied to other molecules, such as electrically neutral biological molecules. They have demonstrated the integration of a biomimetic polymer membrane that performs stable, rapid, and specific molecular recognition through the OECTs for real-time response to changes in cortisol concentration. This wearable sensor, by conducting polymers channel and the flexible can choose membrane tensile elastic substrate to produce cortisol functionalization. This biosensor can collect discharge sweat when people move, similar to the motion of normal human skin under the condition of tensile and bending. In addition, they designed a system of passive fluid manipulation. The system is composed of the design of a laser micro-capillary channel array. Sweat can be quickly and accurately to directly to the sensor interface. Wearable sweat diagnosis platforms of miniaturization and non-invasive sweat induction are the future development direction.
To reduce the detection limits of the sensor, Janardhanan et al. [
38] designed a novel OECT cortisol immune sensor (in
Figure 4c). The sensor in the activity of the OECT device channel area using poly (EDOT-COOH-co-EDOT-EG3) nanotubes and PEDOT:PSS base enhances the sweat of cortisol in response to the human body. The OECT cortisol immunosensor can detect cortisol linearly in the concentration range of 1 fg/mL to 1 μg/mL, the limit of detection (LOD) is 0.0088 fg/mL, and the linearity is excellent (R
2 = 0.9566). Due to the sensor showing good stability and repeatability, the author thinks it has clinical application in future healthcare monitoring application potential. OTFTs also respond well to changes in the pH of sweat. Chronically high-stress levels can affect a person’s physical health and psychological condition, such as high blood pressure and depression. pH can provide appropriate information about emotional and bodily stress [
40]. Ching-Mei et al. [
41] proposed a new method of stress state monitoring using disposable flexible sensors. The electrode based on OTFT was fabricated by full vacuum printing technology. Polymer thin films were deposited rapidly by flash evaporation and combined with semiconductor dinaphtho[2,3-b:2′,3′-f]thieno[3,2-b] thiophene (DNTT) to form devices with high reproductivity and high yield. A signal amplification front-end circuit based on an OTFT was used to capture biological signals using the piezoelectric response of polyvinylidene difluoride (PVDF) combined with the pH evaluation of sweat. The sensor’s response time is stable, repeatable, and fast (less than 20 s), with a sensitivity of 62.8 mV per unit pH. Similarly, Mariani et al. [
39] showed an OECT sensor for pH monitoring (in
Figure 4d,e). To pH signature transmission, PEDOT:dye composites by photochemical pH dye-doped (MO and BTB, methyl orange and bromothymol blue) composite. They have evaluated the two kinds of materials to the pH change of electrochemical reaction. The sensitivity of PEDOT:BTB was 62 ± 2 mV per pH unit, and that of PEDOT:MO was 31 ± 2 mV per pH unit. Based on pH monitoring, OTFT sensors applied in health care and portable sensing technology will have a good prospect in the future.
The testing of dopamine has likewise attracted a substantial amount of attention. Dopamine is an essential neurotransmitter that is widely present in the central nervous system, helping nerve cells to transmit a variety of physiological signals and maintain normal body functions. When its concentration is abnormal, it may lead to various diseases, such as Tourette’s disease, Schizophrenia, Parkinson’s disease, and Pituitary tumors [
42]. Tang H et al. [
43] suggested using PEDOT:PSS (in
Figure 5a).
Figure 5b shows that this OECT has excellent transmission performance. By comparing different types of grids, including graphite (GE), gold, and platinum electrodes, and GE modified using a mixture of multi-walled carbon nanotube (MWCNT)-chitosan (CHIT) and Pt electrodes, they found that the apparatus with Pt grids exhibited the best limit of detection (less than 5 nM). However, the sensor was not selective for dopamine. Gualandi et al. [
44] prepared a sensor based on PEDOT:PSS OECTs (in
Figure 5c,d) to selectively detect dopamine with a 6 μM detection limit. Xing Q et al. [
45] designed and fabricated a novel polypyrrole/nanofibers/polyamide 6 (PPy/NFs/PA6) filamentous electrochemical transistor (in
Figure 6a). The magnification ratio and current response rate were significantly better than those of conventional OECTs, with excellent sensitivity even at dopamine concentrations as low as 1 nM.
Figure 6b shows the transistor’s capability to be responsive to dopamine and in the presence of a variety of interference, have excellent selectivity. Finally, Shiwaku et al. [
46] demonstrated a novel potentiometric electrochemical sensing system in terms of electrolyte ions (in
Figure 6c–e). Lack of potassium ions in the human body results in symptoms such as muscle weakness, body fatigue, loss of appetite, and tachycardia. They used two negative feedback inverters based on OTFTs. The inverters used a low molecule p-type semiconductor, 2,7-dihexyl-dithieno[2,3-d; 2′,3′-d′]benzo[1,2-b; 4,5-b′]dithiophene (DTBDT-C
6), and polystyrene (PS) as the reactive layer. The ion concentration sensitivity sensor for K
+ of 34 mV/dec was prepared and scaled up to 160 mV/dec with high linearity, enabling the measurement of K
+ levels by in situ human sweat analysis. The results show that potential galvanic sensors based on organic circuit printed devices are achievable.
3.3. Application of OTFT in Urine
There are a variety of biomarkers in urine, so people think of effective real-time detection of human health by monitoring abnormal components in urine. The are now several urine sample detection methods, such as gas chromatography/mass spectrometry (GC/MS) [
47], liquid chromatography/mass spectrometry (LC/MS), and so on [
48]. However, such as the need for professional operation, long response time and high production cost, the requirements for professional venues are all problems that have yet to be solved in this industry. Sensors based on OTFT have the following advantages: high sensitivity, simple manufacturing, short reaction time, and so on, which have a good application prospect in the current industry [
49,
50]. In recent years, many applications for the OTFT principle of biosensors in actual diagnoses have been found, such as pulse oximetry [
51], temperature/pressure signals, and electrophysiology arrays [
52]. Although these biosensors are widely used, the stability and sensitivity of practical applications are challenging in this field. Hereupon, to mitigate this challenge, Hu et al. [
53] studied the preparation of an OECT sensor using PEDOT:PSS as channel material and gold nanoparticles were mixed on the carbon tube to modify OECT, which improved the sensitivity of the sensor very efficiently and could effectively measure the content of dopamine in urine in a short time. It is a sensor with broad prospects [
54].
In 2017, Kimet et al. [
48] reported on a biofunctional OFET-based biosensor for the detection of amphetamine-type stimulants (ATS) in the real world (in
Figure 7a,b). They designed the biosensor to show high sensitivity and accuracy to ATS in water and urine [
49,
55,
56,
57]. In addition, they also designed wireless sensors connected to mobile phone applications based on this principle. In practical situations, such portable devices can play a decisive role in field detection. Wang et al. [
58] developed a portable sensor for detecting human urine to detect ectopic pregnancy. The sensor mainly recognizes β-human chorionic gonadotropin (β-hCG). The transistor sensor is a biosensor with an extended gate electrode, and the signal transmission is still sound even at shallow sample concentrations (in
Figure 7c–e). It provides a good model for later researchers to learn from. In 2021, Alan [
59] improved the sensitivity on this basis. They showed us an ultra-accurate tungsten diselenide (WSe
2) FET biosensor that can be applied to the early diagnosis of prostate cancer. First, they modified the FET channel with a monoclonal antibody to prostate-specific antigen (anti-PSA). Then, they treated it with bovine serum albumin to ensure the modification was complete. Their biosensor had a low detection limit, with a subthreshold swing of 235 mV/dec, a 70% increase in drain current in the FET compared to the original device, resulting in high efferent amplification performance. The above summarized several transistor sensors not only in the past traditional methods to be innovative but also in the improved sensitivity and accuracy. With the development of modern society, health monitoring real-time measurement awareness gradually increased. The several above biosensors in the portability of the results have also been very significant.
To further optimize portability and sensitivity, Y. Pan et al. [
60] designed a graphene polymer-functionalized FETportable biomarker sensor, mainly used in real-time glucose monitoring (in
Figure 8a,b). The biosensor polymer is synthesized from acrylamide/3-acrylamide phenylboronic acid (AAPBA)/N,N-dimethylamino-acrylamide. The biosensor mechanism is that when glucose is present, polymers appear on graphene, and the covalent bonds produced by the reaction of glucose and AAPBA lead to Dirac point shift and current change in the polymer functionalized graphene field-effect transistor (P-GFET) [
61].
There have also been significant advances in the detection of transitioned urine. The kidneys play a crucial role in the circulation system. Gundlach et al. [
62] developed a new device design method. They measured the magnetoconductance (MC) by manipulating the spin process (in
Figure 8c). The authors manipulated the carrier migration by regulating the gate voltage, and they successfully observed the fission process of MC. Hamzah et al. [
63] have developed and designed an extremely accurate transistor sensor modified with antidiuretic hormone (ADH) antibodies on graphene. They also demonstrated in later experiments that the sensor could detect not only human urine but also proteins in the blood. Subsequently, OTFT-based biosensors could also play an essential role in detecting other diseases. For many years, the complex urine environment and low levels of cancer-related markers have been challenging in this field. Yang et al. [
64] developed a sensor for detecting malignant bladder tumors, which can detect single-stranded nucleic acid in urine and has good repeatability and functional stability (in
Figure 8d). In addition, there has been some progress in detecting trace elements in the human body. In the middle of 2022, Patolsky et al. [
65] developed an ultra-sensitive biosensor which modified the uranyl junction fit body into a nano silicon transistor (in
Figure 9a,b). This biosensor is easy to operate and can directly detect the content of uranyl ions in human urine. This research will have important implications for human health in dangerous occupational fields. The research achievements of the above researchers are very significant and have certain application value, which opens up the way for the later development and progress.
Figure 8.
(
a) For the detection of glucose concentration polymer functionalization graphene sensors and functionalization of graphene sensor. (
b) The difference in surface charge between N-dimethylaminopropyl Acrylamide (DMAAPA) and acrylamide/3-acrylamidophenylboronic acid (AAPBA), with yellow representing charge accumulation and blue representing charge loss. (
c) Device structure diagram based on OFET principle and corresponding transmission output curve. (
d) Schematic diagram of nucleic acid detection in a biosensor. (
a) Reproduced with permission [
60]. Copyright 2022, Elsevier. (
b) Reproduced with permission [
60]. Copyright 2022, Elsevier. (
c) Reproduced with permission [
62]. Copyright 2019, American Chemical Society. (
d) Reproduced with permission [
64]. Copyright 2021, Elsevier.
Figure 8.
(
a) For the detection of glucose concentration polymer functionalization graphene sensors and functionalization of graphene sensor. (
b) The difference in surface charge between N-dimethylaminopropyl Acrylamide (DMAAPA) and acrylamide/3-acrylamidophenylboronic acid (AAPBA), with yellow representing charge accumulation and blue representing charge loss. (
c) Device structure diagram based on OFET principle and corresponding transmission output curve. (
d) Schematic diagram of nucleic acid detection in a biosensor. (
a) Reproduced with permission [
60]. Copyright 2022, Elsevier. (
b) Reproduced with permission [
60]. Copyright 2022, Elsevier. (
c) Reproduced with permission [
62]. Copyright 2019, American Chemical Society. (
d) Reproduced with permission [
64]. Copyright 2021, Elsevier.
In terms of early detection of cancer, the urine diagnostic method for bladder cancer was developed by Quan et al. [
66]. In 2020, low accuracy appeared in terms of detection sensitivity. A high-precision molybdenum disulfide (MoS
2) nanosheet FET sensor array was developed for real-time monitoring of bladder cancer biomarkers in human urine. Quan et al. [
67] designed an indium gallium zinc oxide field effect transistor (IGZO FET) biosensor array last year, which can be connected to Internet terminals to make a human urine analysis assay device for detecting biomarkers of bladder cancer in urine, and realized clinical detection (in
Figure 9c,d). The FEThas a good application prospect in urine detection [
6]. Their IGZO FET-based biosensor, which can be connected to an Internet terminal, provides high sensitivity and selectivity in the actual complex urine test. These works highlight the wide range of applications of OTFT-based biosensors for the non-invasive, ultra-sensitive biological detection of urine. These designs, which can be summarized as the features of man-carried, penny-a-line, non-invasive, automated data processing and analysis, and no need for the professional operation of professionals, are expected to be translated into standard clinical practice in the diagnosis and prognosis of serious diseases.
Figure 9.
(
a) Schematic diagram of the finished product after the final experiment. (
b) A physical image of the device. (
c) Urine sample detection device, which is connected to the Internet terminals. (
d) Sensor surface modification sketches, including five channels for bladder cancer protein function, three channels for urine test background signal, a total of eight sensor channels. (
a) Reproduced with permission [
65]. Copyright 2020, American Chemical Society. (
b) Reproduced with permission [
65]. Copyright 2020, American Chemical Society. (
c) Reproduced with permission [
67]. Copyright 2022, John Wiley and Sons. (
d) Reproduced with permission [
67]. Copyright 2022, John Wiley and Sons.
Figure 9.
(
a) Schematic diagram of the finished product after the final experiment. (
b) A physical image of the device. (
c) Urine sample detection device, which is connected to the Internet terminals. (
d) Sensor surface modification sketches, including five channels for bladder cancer protein function, three channels for urine test background signal, a total of eight sensor channels. (
a) Reproduced with permission [
65]. Copyright 2020, American Chemical Society. (
b) Reproduced with permission [
65]. Copyright 2020, American Chemical Society. (
c) Reproduced with permission [
67]. Copyright 2022, John Wiley and Sons. (
d) Reproduced with permission [
67]. Copyright 2022, John Wiley and Sons.
3.4. Application of OTFT in Blood
There are some charged biomarkers in blood which can be used for real-time monitoring of human physiological conditions, and also have essential application prospects in the early detection of various diseases [
68]. Most current blood tests are usually combined with blood pressure measurement, albuminuria, and biochemical and hematological assessment. However, in modern centralized laboratories, this usually takes 4–6 h, and comprehensive albuminuria assessment can take up to 24 h [
69,
70]. Most importantly, clinical manifestations vary so much that it is difficult to objectively determine which patients need urgent medical attention and which patients can continue to use before testing some data for subsequent diagnosis [
71,
72]. However, most existing traditional detection methods require that the experimental analysis be carried out by professional technical personnel and professional analysis sites, which leads to the diagnosis in some special cases and limits the application in the actual situation. In the past, the Triage-Alere placental growth factor (PLGF) test has been considered a very timely and effective medical treatment [
73,
74]. However, the actual situation is that this method requires ethylene diamine tetraacetic acid (EDTA) clotting vein puncture blood, and the following steps require professionally trained personnel for blood drawing and condensation, as well as the problematic operation of daily calibration and routine quality control inspection of the fluorescent reader [
75]. This has led to the use of the device in remote areas, and the disease is characterized by higher demand for the technology in remote areas [
76].
The blood test is an important parameter of biological detection therapy. Because of this, OTFT-based biomarker sensors for blood testing were well developed over the next few decades. For example, Abdollahi et al. [
77] summarized some aspects of early diagnosis of diabetes and the management of different biomarkers, including fluorescence, nanotechnology, electrochemical detection, and FET biosensors, and they made early diagnosis of diabetes a research focus. This research project lays a foundation for further development in the later period. Torsi et al. [
19] have made some progress in detecting transistor-based biomarkers. In the past, traditional methods that used nano sensors were not effective in complex environments. To alleviate this challenge, they modified the surface of the sensor; capturing the anti-immunoglobulin and modifying a hydrogen bond network onto the surface of the sensor resulted in a good recognition effect in serum detection. Elnathan et al. [
78] further discussed the field effect performance of single-molecule and multi-molecule components on the basis of the former, and they believed that the practicability of the device was the top priority (in
Figure 10a,b). They believed that the implementation of grid voltage regulation could effectively promote the gating of single-molecule components. Although they only put forward a theory, they provide more possibilities for subsequent development. According to the literature summary above, Zhang et al. [
79] developed a sensor based on the FET principle modified with gold nanoparticles and two ligands for the detection of specific cell derivatives (in
Figure 10c,d). They made a portable platform for the detection of serum proteins related to liver cancer, as well as for the differentiation of other cancers.
Huang et al. [
80,
81] designed a new method of mercury ion (Hg
2+) FET sensor using (ZnO-NB) nanoribbon as thin film channel material. The Langmuir-Blodgett (L-B) assembly technique was used to prepare different types of FET chips by taking advantage of the different concentrations of ZnO-NB. Afterward, Chen et al. [
82] designed a new type of glycosylated hemoglobin-specific nucleic acid ligand (Apt
GP). The sensor-measured ligand phylogenetic assays by exponential enrichment (SELEX) showed strong associations with GP or HbA1c but negligible binding to glycosylated hemoglobin A1c (HbA1c) and non-glycosylated polypeptides (in
Figure 11a). In addition, molecular modeling of GP-Apt
GP compounds and previous studies have shown that hydrogen bonding dominates the binding of GP-Apt
GP, and non-covalent bonds of GP ligands are first bound to the front end of Apt
GP, thus affecting the amino acid arrangement of the peptide chain. The sensor described above is less invasive and more timely than previously reported sensors, but it lacks some accuracy. So, to solve the problem of accuracy and sensitivity, Lee et al. [
83] developed electrolyte-gating graphene FET sensors (in
Figure 11b). This device can effectively detect tau protein through an optimized, connectionless antibody fixation process. In their paper, they show that two-dimensional graphene is classified into eight different types, modified FET sensors are connected to them, and their performance is tested. Experiments have shown that the sensor they designed has antibodies modified on the edges and dope-like behavior on the graphene. When the tau protein in the electrolyte increases to a particular concentration, compared with the original graphene sensor connected by PSE, the graphene sensor they designed and developed, which does not use a linker, shows greater sensitivity.
In 2021, with further improvements having been made on the basis of the previous ones, Thierry et al. [
84] designed and developed a nanoscale indium oxide FET sensor that is completely based on a biological diagnostic platform. Later, they developed a portable sample processing device that integrates blood, thus greatly reducing the need for professional operation. In addition, the device can measure PLGF in about 30 µL of blood samples in just 40 min, with a dynamic range of five orders of magnitude. In 2022, Kordrostami et al. [
85] further improved the sensitivity. Their research and development were on the principle of FET detecting glucose sensors with high sensitivity and accuracy (in
Figure 11c,d). Their paper shows that the flexible FET biosensor can directly detect glucose in the blood and make a portable dipstick device. The reduced graphene oxide (rGO)-modified CuO nanostructured hollow micro-spheres (NHS) synthesized in this way are deposited on a flexible polyethylene terephthalate (PET) substrate as a channel for the back gate transistor. Furthermore, they have improved both the concentration and the sensor to bring a higher level of accuracy and precision. As reported in the above two articles, both OFET and OECT prototype sensors have greatly improved the sensitivity and accuracy of biometric identification samples, and both have good stability, but there is still a lack of portability. On this basis, Wang et al. [
86] made further improvements in the simplicity and sensitivity of use. Because of the great harm of lead ions to the human body, they modified the glutathione gate and showed us a solution-gated graphene transistor for detecting lead ions (in
Figure 12a,b). Blood-based detection of early Alzheimer’s disease (AD) is vital for patients, but the complex blood environment and the low content of specifically related proteins have been difficult to overcome. Zhang et al. [
87] designed a sensor based on the principle of OFET to provide a solution to this situation (in
Figure 12c,d).
Figure 11.
(
a) Surface modification of multiple-parallel-connected (MPC) SiNW-FET requires the modification of mercap topropyltrimethoxy-silane (MPTMS), peg-silane and PTMS on the surface of SiNW, and then a silyl molecular layer with sulfhydryl, hydroxyl and methyl groups is formed, and then Apt
GP is modified on MPTMS through the action of disulfide bonds. (The source is denoted by S and the drain by D). (
b) The initial graphene state and the patterned graphene state with the pyrenebutanoic acid, succinimidyl ester (PSE) connector. (
c) A step diagram of a silver electrode was modified on a shadow mask plate with PET as the base. (
d) The process diagram of making the material and the schematic diagram of the reduced graphene oxide (rGO)/CuO-NHS sensor. (
a) Reproduced with permission [
82]. Copyright 2021, John Wiley and Sons. (
b) Reproduced with permission [
83]. Copyright 2021, Elsevier. (
c) Reproduced with permission [
85]. Copyright 2022, Springer Nature. (
d) Reproduced with permission [
85]. Copyright 2022, Springer Nature.
Figure 11.
(
a) Surface modification of multiple-parallel-connected (MPC) SiNW-FET requires the modification of mercap topropyltrimethoxy-silane (MPTMS), peg-silane and PTMS on the surface of SiNW, and then a silyl molecular layer with sulfhydryl, hydroxyl and methyl groups is formed, and then Apt
GP is modified on MPTMS through the action of disulfide bonds. (The source is denoted by S and the drain by D). (
b) The initial graphene state and the patterned graphene state with the pyrenebutanoic acid, succinimidyl ester (PSE) connector. (
c) A step diagram of a silver electrode was modified on a shadow mask plate with PET as the base. (
d) The process diagram of making the material and the schematic diagram of the reduced graphene oxide (rGO)/CuO-NHS sensor. (
a) Reproduced with permission [
82]. Copyright 2021, John Wiley and Sons. (
b) Reproduced with permission [
83]. Copyright 2021, Elsevier. (
c) Reproduced with permission [
85]. Copyright 2022, Springer Nature. (
d) Reproduced with permission [
85]. Copyright 2022, Springer Nature.
![Chemosensors 11 00202 g011 Chemosensors 11 00202 g011]()
Later, to alleviate this challenge, Rajeswari et al. developed graphene field effect transistors that can detect a variety of myoglobin biomarkers at low concentrations [
88], and graphene itself is an excellent material to use as a grid because of its sensitivity to a variety of other biomarkers. When the graphene FET they designed was built to detect myoglobin antigen, there was a significant change in the current. With a 30 fg/mL strategy level, they have also made a lab-on-a-chip portable device that provides a strong foundation for cardiac care and more. In addition, Sun et al. [
89] further optimized the sensitivity of the sensor on this basis. They developed a new organic material, 2,6-bis(4-formyl phenyl)-anthracene (BFPA), and used it as the channel of OFET biosensor (in
Figure 13a,b). It achieves the ultra-sensitive detection of alpha-fetoprotein (AFP) in human blood samples and shows that the changes in source leakage current and threshold voltage electrical signals fluctuate significantly with the change of concentration. This device can distinguish liver cancer patients and healthy people very efficiently. It lays a foundation for follow-up research on blood detection.
Furthermore, Das et al. [
90] synthesized a useful FET that uses layered transition metal disulfide compounds to detect hydrogen sulfide concentrations in blood. In their study, they showed that they used multiple layers of the compound as the channel material for the FET sensor. With the change in hydrogen sulfide concentration, the dynamic response of the sensor is very good, and the dynamic linear range is 1 μM~1 mM. In addition, it has a very low detection limit and can quickly detect the content of hydrogen sulfide in blood in a very short time, which lays an excellent foundation for subsequent diagnosis. In a recent paper, Huang et al. [
91] produced a dual ion-selective membrane deposited ion-sensitive field-effect transistor (DISM-ISFET) sensor with two ion-selective depositions, and for portability in practical applications, they developed a microchamber at a later stage, which can effectively realize the sensing and response of different ions in serum (in
Figure 13c,d). In order to prove this point, they tested different concentrations of sodium chloride solution and potassium chloride solution, respectively, on the sensor’s surface and successfully found the highest ion sensitivity and selectivity. As shown in this document, their design allowed all sample processing and measurement to be performed in a single chip in less than 10 min. This greatly improves the sensitivity of the blood test and shortens the time required. Therefore, we can conclude that the sensor can realize ultra-high speed and ultra-sensitive detection of biomarkers. Compared with traditional methods in the past, this device has excellent selectivity and ultra-high sensitivity, and the detection method does not require a professional operation, shorter analysis time, and low sample size requirements, which will contribute to the further development of blood detection and diagnosis.
Figure 13.
(
a) Schematic diagram of a bottom-top contact gated organic transistor sensor based on OTFT. (
b) Organic molecules BFPA and PDVT-8 structure diagram. (
c) Cutaway view of transistorized organic sensor based on OTFT. (
d) Continuous steps of the
Ids profile. (
a) Reproduced with permission [
89]. Copyright 2021, American Chemical Society. (
b) Reproduced with permission [
89]. Copyright 2021, American Chemical Society. (
c) Reproduced with permission [
91]. Copyright 2023, American Chemical Society. (
d) Reproduced with permission [
91]. Copyright 2023, American Chemical Society.
Figure 13.
(
a) Schematic diagram of a bottom-top contact gated organic transistor sensor based on OTFT. (
b) Organic molecules BFPA and PDVT-8 structure diagram. (
c) Cutaway view of transistorized organic sensor based on OTFT. (
d) Continuous steps of the
Ids profile. (
a) Reproduced with permission [
89]. Copyright 2021, American Chemical Society. (
b) Reproduced with permission [
89]. Copyright 2021, American Chemical Society. (
c) Reproduced with permission [
91]. Copyright 2023, American Chemical Society. (
d) Reproduced with permission [
91]. Copyright 2023, American Chemical Society.
As mentioned above, the design parameters of many OTFT biosensors have a considerable influence on device performance. Through the continuous efforts of subsequent researchers to optimize the parameters, the sensitivity of the transistor sensor gradually adapts to the experimental requirements of blood molecular analysis [
92]. In particular, one can continue to expand the field of OTFT biosensors from the continuous development of transistor sensors, so as to have greater progress, improving the long-term stability of devices, and comparisons with other types of sensing technologies.