Dual-Response Photofunctional Covalent Organic Framework for Acid Detection in Various Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentations
2.2. The Solid-State UV-Vis Absorption Test of TPBD-TA COF on Detecting Acid
2.3. The Fluorescence Test of TPBD-TA COF on Detecting Acid
2.4. The Recyclability Test of the COF
2.5. The Preparation of COF Filter Paper
3. Results and Discussion
3.1. Synthesis and Characterization
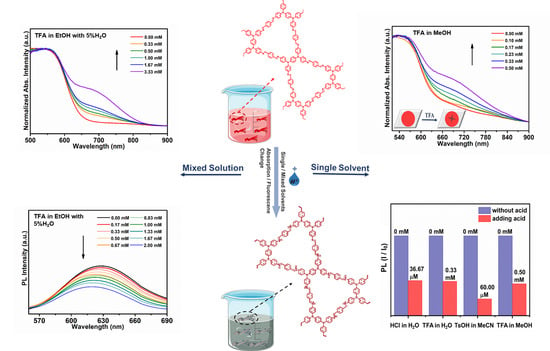
3.2. Acid Sensing
3.3. Acid Sensing Mechanism
3.4. Acid Sensing Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, S.; Meng, L.C.; Ma, W.Y.; Pan, G.C.; Zhang, W.; Zou, Y.C.; Liu, L.J.; Xu, B.; Tian, W.J. Dual-functional two-dimensional covalent organic frameworks for water sensing and harvesting. Mater. Chem. Front. 2021, 5, 4193–4201. [Google Scholar] [CrossRef]
- Wang, P.; Xu, Q.; Li, Z.P.; Jiang, W.M.; Jiang, Q.H.; Jiang, D.L. Exceptional Iodine Capture in 2D Covalent Organic Frameworks. Adv. Mater. 2018, 30, 1801991. [Google Scholar] [CrossRef] [PubMed]
- Spitler, E.L.; Koo, B.T.; Novotney, J.L.; Colson, J.W.; Uribe-Romo, F.J.; Gutierrez, G.D.; Clancy, P.; Dichtel, W.R. A 2D Covalent Organic Framework with 4.7-nm Pores and Insight into Its Interlayer Stacking. J. Am. Chem. Soc. 2011, 133, 19416–19421. [Google Scholar] [CrossRef] [PubMed]
- Colson, J.W.; Woll, A.R.; Mukherjee, A.; Levendorf, M.P.; Spitler, E.L.; Shields, V.B.; Spencer, M.G.; Park, J.; Dichtel, W.R. Oriented 2D Covalent Organic Framework Thin Films on Single-Layer Graphene. Science 2011, 332, 228–231. [Google Scholar] [CrossRef]
- Evans, A.M.; Parent, L.R.; Flanders, N.C.; Bisbey, R.P.; Vitaku, E.; Kirschner, M.S.; Schaller, R.D.; Chen, L.X.; Gianneschi, N.C.; Dichtel, W.R. Seeded growth of single-crystal two-dimensional covalent organic frameworks. Science 2018, 361, 53–57. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Lee, C.C.; El-Mahdy, A.F.M.; Luder, J.; Yu, M.H.; Li, Z.; Zhu, Z.L.; Chueh, C.C.; Kuo, S.W. Exploitation of two-dimensional conjugated covalent organic frameworks based on tetraphenylethylene with bicarbazole and pyrene units and applications in perovskite solar cells. J. Mater. Chem. A 2020, 8, 11448–11459. [Google Scholar] [CrossRef]
- Lv, Y.K.; Li, Y.S.; Zhang, G.; Peng, Z.X.; Ye, L.; Chen, Y.; Zhang, T.; Xing, G.L.; Chen, L. An In Situ Film to Film Transformation Approach toward Highly Crystalline Covalent Organic Framework Films. Ccs Chem. 2022, 4, 1519–1525. [Google Scholar] [CrossRef]
- Fang, Q.R.; Gu, S.; Zheng, J.; Zhuang, Z.B.; Qiu, S.L.; Yan, Y.S. 3D Microporous Base-Functionalized Covalent Organic Frameworks for Size-Selective Catalysis. Angew. Chem. Int. Edit. 2014, 53, 2878–2882. [Google Scholar] [CrossRef]
- Lin, G.Q.; Ding, H.M.; Chen, R.F.; Peng, Z.K.; Wang, B.S.; Wang, C. 3D Porphyrin-Based Covalent Organic Frameworks. J. Am. Chem. Soc. 2017, 139, 8705–8709. [Google Scholar] [CrossRef]
- El-Kaderi, H.M.; Hunt, J.R.; Mendoza-Cortes, J.L.; Cote, A.P.; Taylor, R.E.; O’Keeffe, M.; Yaghi, O.M. Designed synthesis of 3D covalent organic frameworks. Science 2007, 316, 268–272. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, X.; Clowes, R.; Cui, P.; Chen, L.J.; Little, M.A.; Cooper, A.I. 3D Cage COFs: A Dynamic Three-Dimensional Covalent Organic Framework with High-Connectivity Organic Cage Nodes. J. Am. Chem. Soc. 2020, 142, 16842–16848. [Google Scholar] [CrossRef]
- Cote, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, crystalline, covalent organic frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.R.; Doonan, C.J.; LeVangie, J.D.; Cote, A.P.; Yaghi, O.M. Reticular synthesis of covalent organic borosilicate frameworks. J. Am. Chem. Soc. 2008, 130, 11872–11873. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.; Chen, W.B.; Xing, G.L.; Jiang, D.L.; Chen, L. New synthetic strategies toward covalent organic frameworks. Chem. Soc. Rev. 2020, 49, 2852–2868. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-San-Miguel, D.; Montoro, C.; Zamora, F. Covalent organic framework nanosheets: Preparation, properties and applications. Chem. Soc. Rev. 2020, 49, 2291–2302. [Google Scholar] [CrossRef] [PubMed]
- Segura, J.L.; Mancheno, M.J.; Zamora, F. Covalent organic frameworks based on Schiff-base chemistry: Synthesis, properties and potential applications. Chem. Soc. Rev. 2016, 45, 5635–5671. [Google Scholar] [CrossRef]
- Huang, N.; Wang, P.; Jiang, D.L. Covalent organic frameworks: A materials platform for structural and functional designs. Nat. Rev. Mater. 2016, 1, 1–19. [Google Scholar] [CrossRef]
- Hu, Y.M.; Dunlap, N.; Wan, S.; Lu, S.L.; Huang, S.F.; Sellinger, I.; Ortiz, M.; Jin, Y.H.; Lee, S.H.; Zhang, W. Crystalline Lithium Imidazolate Covalent Organic Frameworks with High Li-Ion Conductivity. J. Am. Chem. Soc. 2019, 141, 7518–7525. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Atayde, E.C.; Matsagar, B.M.; Na, J.; Yamauchi, Y.; Wu, K.C.W.; Kuo, S.W. Construction Hierarchically Mesoporous/Microporous Materials Based on Block Copolymer and Covalent Organic Framework. J. Taiwan Inst. Chem. Eng. 2020, 112, 180–192. [Google Scholar] [CrossRef]
- Shan, M.X.; Liu, X.L.; Wang, X.R.; Yarulina, I.; Seoane, B.; Kapteijn, F.; Gascon, J. Facile manufacture of porous organic framework membranes for precombustion CO2 capture. Sci. Adv. 2018, 4, eaau1698. [Google Scholar] [CrossRef]
- Khan, N.A.; Zhang, R.N.; Wu, H.; Shen, J.L.; Yuan, J.Q.; Fan, C.Y.; Cao, L.; Olson, M.A.; Jiang, Z.Y. Solid-Vapor Interface Engineered Covalent Organic Framework Membranes for Molecular Separation. J. Am. Chem. Soc. 2020, 142, 13450–13458. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.M.; Li, C.Z.; Kang, W.C.; Li, H.; Ta, N.; Ye, S.; Hu, L.Y.; Wang, X.L.; Li, C.; Yang, Q.H. Enormous Promotion of Photocatalytic Activity through the Use of Near-Single Layer Covalent Organic Frameworks. CCS Chem. 2022, 4, 2429–2439. [Google Scholar] [CrossRef]
- Kang, Z.X.; Peng, Y.W.; Qian, Y.H.; Yuan, D.Q.; Addicoat, M.A.; Heine, T.; Hu, Z.G.; Tee, L.; Guo, Z.G.; Zhao, D. Mixed Matrix Membranes (MMMs) Comprising Exfoliated 2D Covalent Organic Frameworks (COFs) for Efficient CO2 Separation. Chem. Mater. 2016, 28, 1277–1285. [Google Scholar] [CrossRef]
- Fan, H.W.; Mundstock, A.; Feldhoff, A.; Knebel, A.; Gu, J.H.; Meng, H.; Caro, J. Covalent Organic Framework-Covalent Organic Framework Bilayer Membranes for Highly Selective Gas Separation. J. Am. Chem. Soc. 2018, 140, 10094–10098. [Google Scholar] [CrossRef] [PubMed]
- Rice, A.M.; Dolgopolova, E.A.; Yarbrough, B.J.; Leith, G.A.; Martin, C.R.; Stephenson, K.S.; Heugh, R.A.; Brandt, A.J.; Chen, D.A.; Karakalos, S.G.; et al. Stack the Bowls: Tailoring the Electronic Structure of Corannulene-Integrated Crystalline Materials. Angew. Chem. Int. Edit. 2018, 57, 11310–11315. [Google Scholar] [CrossRef]
- Zhang, L.; Yi, L.; Sun, Z.J.; Deng, H.X. Covalent organic frameworks for optical applications. Aggregate 2021, 2, 13011. [Google Scholar] [CrossRef]
- Diercks, C.S.; Yaghi, O.M. The atom, the molecule, and the covalent organic framework. Science 2017, 355, eaal1585. [Google Scholar] [CrossRef]
- Feng, L.; Wang, K.Y.; Day, G.S.; Zhou, H.C. The chemistry of multi-component and hierarchical framework compounds. Chem. Soc. Rev. 2019, 48, 4823–4853. [Google Scholar] [CrossRef]
- Gilmanova, L.; Bon, V.; Shupletsov, L.; Pohl, D.; Rauche, M.; Brunner, E.; Kaskel, S. Chemically Stable Carbazole-Based Imine Covalent Organic Frameworks with Acidochromic Response for Humidity Control Applications. J. Am. Chem. Soc. 2021, 143, 18368–18373. [Google Scholar] [CrossRef]
- Luo, Y.C.; Zhang, S.; Wang, H.; Luo, Q.; Xie, Z.G.; Xu, B.; Tian, W.J. Precise Detection and Visualization of Cyclooxygenase-2 for Golgi Imaging by a Light-Up Aggregation-Induced Emission Based Probe. CCS Chem. 2022, 4, 456–463. [Google Scholar] [CrossRef]
- Meng, L.C.; Ma, X.B.; Jiang, S.; Zhang, S.; Wu, Z.Y.; Xu, B.; Lei, Z.; Liu, L.J.; Tian, W.J. Twisted Intramolecular Charge Transfer-Aggregation-Induced Emission Fluorogen with Polymer Encapsulation-Enhanced Near-Infrared Emission for Bioimaging. CCS Chem. 2021, 3, 2084–2094. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Zhang, C.Y.; Zhu, Y.L.; Lu, Z.Y.; Liu, H.; Xu, B.; Zhang, X.Q.; Tian, W.J. Visualization of Macrophase Separation and Transformation in Immiscible Polymer Blends. Ccs Chem. 2022, 2022, 1–11. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Shen, X.C.; Feng, X.; Xia, H.; Mu, Y.; Liu, X.M. Covalent organic frameworks as pH responsive signaling scaffolds. Chem. Commun. 2016, 52, 11088–11091. [Google Scholar] [CrossRef] [PubMed]
- Long, C.; He, L.W.; Ma, F.Y.; Wei, L.; Wang, Y.X.; Silver, M.A.; Chen, L.H.; Lin, Z.; Gui, D.X.; Juan, D.W.; et al. Covalent Organic Framework Functionalized with 8-Hydroxyquinoline as a Dual-Mode Fluorescent and Colorimetric pH Sensor. ACS Appl. Mater. Inter. 2018, 10, 15364–15368. [Google Scholar]
- Ascherl, L.; Evans, E.W.; Gorman, J.; Orsborne, S.; Bessinger, D.; Bein, T.; Friend, R.H.; Auras, F. Perylene-Based Covalent Organic Frameworks for Acid Vapor Sensing. J. Am. Chem. Soc. 2019, 141, 15693–15699. [Google Scholar] [CrossRef]
- Bu, R.; Zhang, L.; Liu, X.Y.; Yang, S.L.; Li, G.; Gao, E.Q. Synthesis and Acid-Responsive Properties of a Highly Porous Vinylene-Linked Covalent Organic Framework. ACS Appl. Mater. Inter. 2021, 13, 26431–26440. [Google Scholar] [CrossRef]
- Hao, Q.; Li, Z.J.; Bai, B.; Zhang, X.; Zhong, Y.W.; Wan, L.J.; Wang, D. A Covalent Organic Framework Film for Three-State Near-Infrared Electrochromism and a Molecular Logic Gate. Angew. Chem. Int. Edit. 2021, 60, 12498–12503. [Google Scholar] [CrossRef]
- Sung, T.W.; Lo, Y.L. Ammonia vapor sensor based on CdSe/SiO2 core-shell nanoparticles embedded in sol-gel matrix. Sens. Actuat. B Chem. 2013, 188, 702–708. [Google Scholar] [CrossRef]
- Wang, Y.F.; Liu, X.; Wang, M.K.; Wang, X.X.; Ma, W.Y.; Li, J.Y. Facile synthesis of CDs@ZIF-8 nanocomposites as excellent peroxidase mimics for colorimetric detection of H2O2 and glutathione. Sens. Actuat. B Chem. 2021, 329, 129115. [Google Scholar] [CrossRef]
- Balaji, T.; Sasidharan, M.; Matsunaga, H. Optical sensor for the visual detection of mercury using mesoporous silica anchoring porphyrin moiety. Analyst 2005, 130, 1162–1167. [Google Scholar] [CrossRef]
- Richardson, T.H.; Dooling, C.M.; Jones, L.T.; Brook, R.A. Development and optimization of porphyrin gas sensing LB films. Adv. Colloid Interface Sci. 2005, 116, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.L.; Feng, D.W.; Wang, K.C.; Gu, Z.Y.; Wei, Z.W.; Chen, Y.P.; Zhou, H.C. An Exceptionally Stable, Porphyrinic Zr Metal-Organic Framework Exhibiting pH-Dependent Fluorescence. J. Am. Chem. Soc. 2013, 135, 13934–13938. [Google Scholar] [CrossRef] [PubMed]
- Grigoras, M.; Stafie, L. Synthesis and Characterization of Linear, Branched and Hyperbranched Triphenylamine-Based Polyazomethines. Des. Monomers Polym. 2009, 12, 177–196. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, W.; Gu, Z.; Pan, G.; Li, C.; Zhu, Y.; Liu, Z.; Liu, L.; Guo, Y.; Xu, B.; Tian, W. Dual-Response Photofunctional Covalent Organic Framework for Acid Detection in Various Solutions. Chemosensors 2023, 11, 214. https://doi.org/10.3390/chemosensors11040214
Ma W, Gu Z, Pan G, Li C, Zhu Y, Liu Z, Liu L, Guo Y, Xu B, Tian W. Dual-Response Photofunctional Covalent Organic Framework for Acid Detection in Various Solutions. Chemosensors. 2023; 11(4):214. https://doi.org/10.3390/chemosensors11040214
Chicago/Turabian StyleMa, Wenyue, Zijian Gu, Guocui Pan, Chunjuan Li, Yu Zhu, Zhaoyang Liu, Leijing Liu, Yupeng Guo, Bin Xu, and Wenjing Tian. 2023. "Dual-Response Photofunctional Covalent Organic Framework for Acid Detection in Various Solutions" Chemosensors 11, no. 4: 214. https://doi.org/10.3390/chemosensors11040214
APA StyleMa, W., Gu, Z., Pan, G., Li, C., Zhu, Y., Liu, Z., Liu, L., Guo, Y., Xu, B., & Tian, W. (2023). Dual-Response Photofunctional Covalent Organic Framework for Acid Detection in Various Solutions. Chemosensors, 11(4), 214. https://doi.org/10.3390/chemosensors11040214