Metal Oxide Semiconductor Gas Sensors for Lung Cancer Diagnosis
Abstract
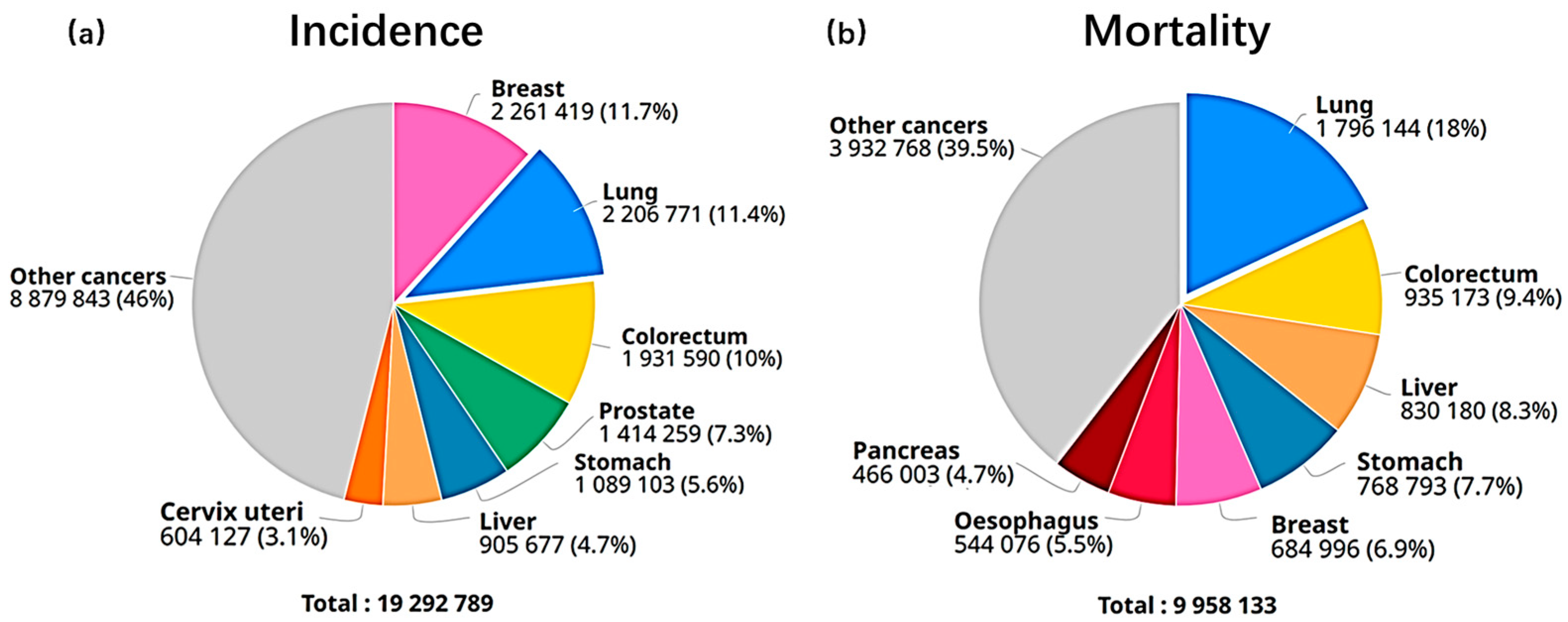
1. Introduction
2. MOS Gas Sensor for Lung Cancer Biomarker VOCs
2.1. Working Mechanism
2.2. Candidate Materials
2.3. Single MOS Gas Sensor and Performance Improvement Strategy
2.3.1. Structures and Gas-Sensing Performance
2.3.2. Noble Metal Modification and Gas Sensing Performance
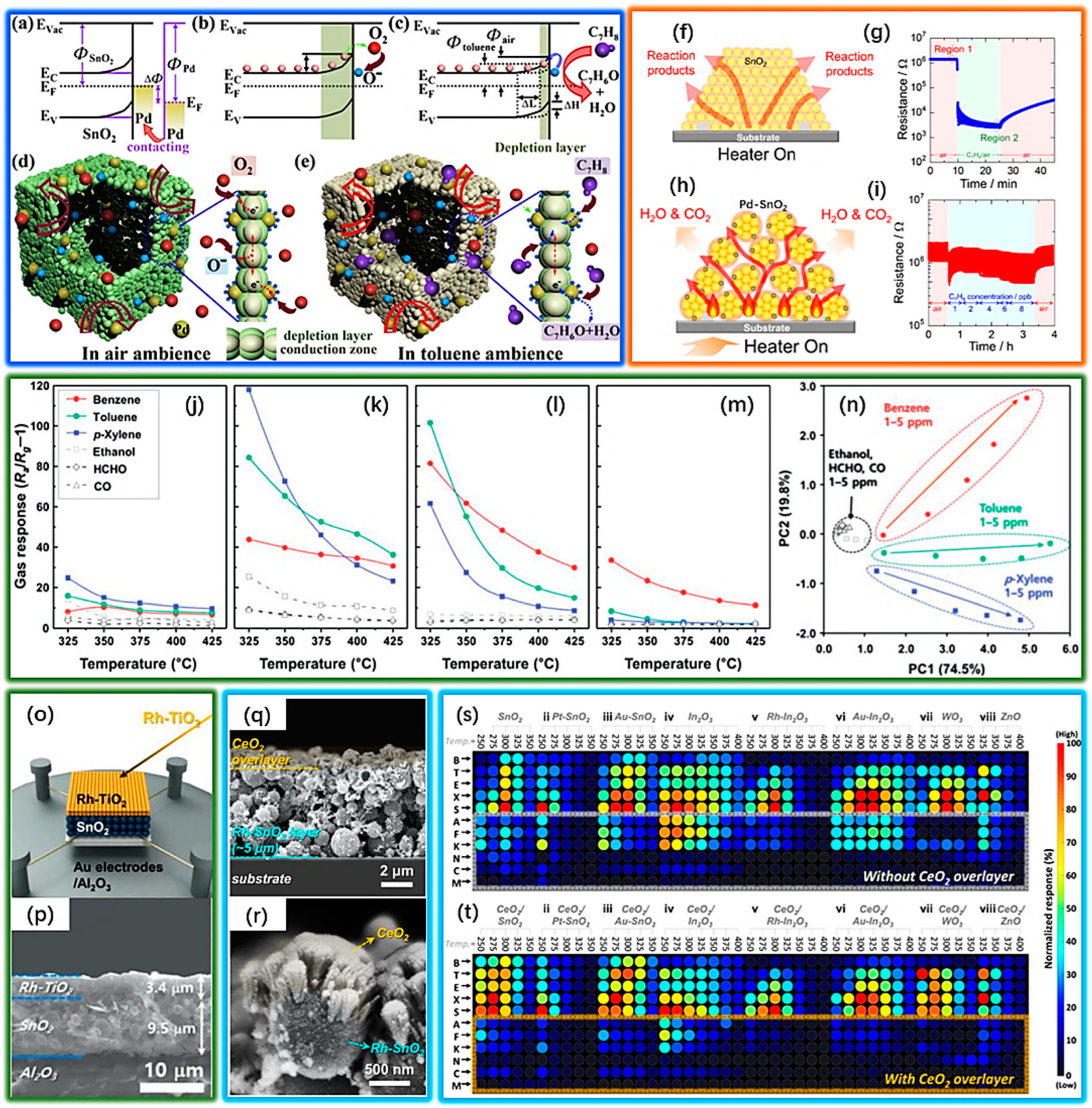
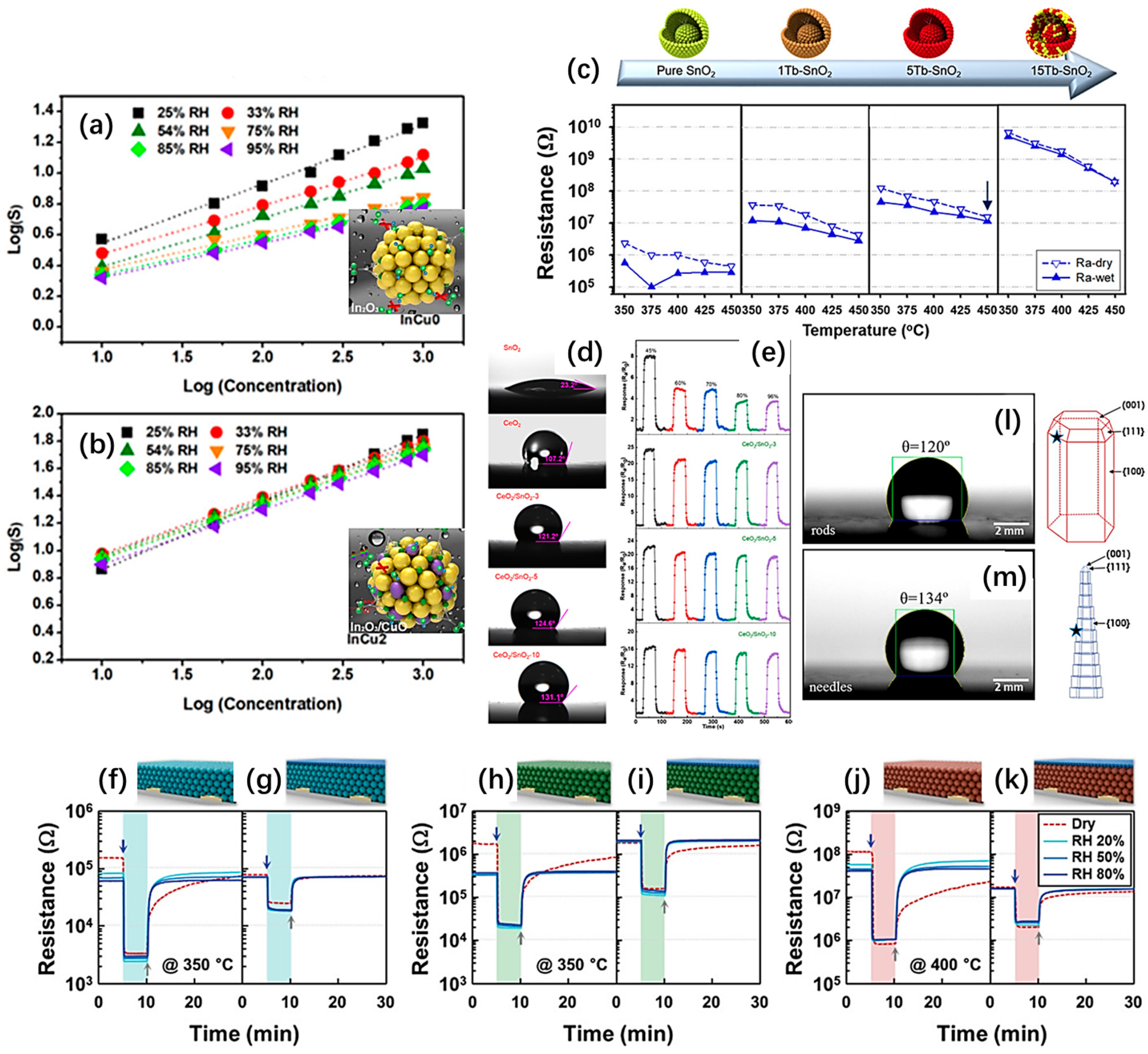
2.3.3. Improve the Humidity Resistance
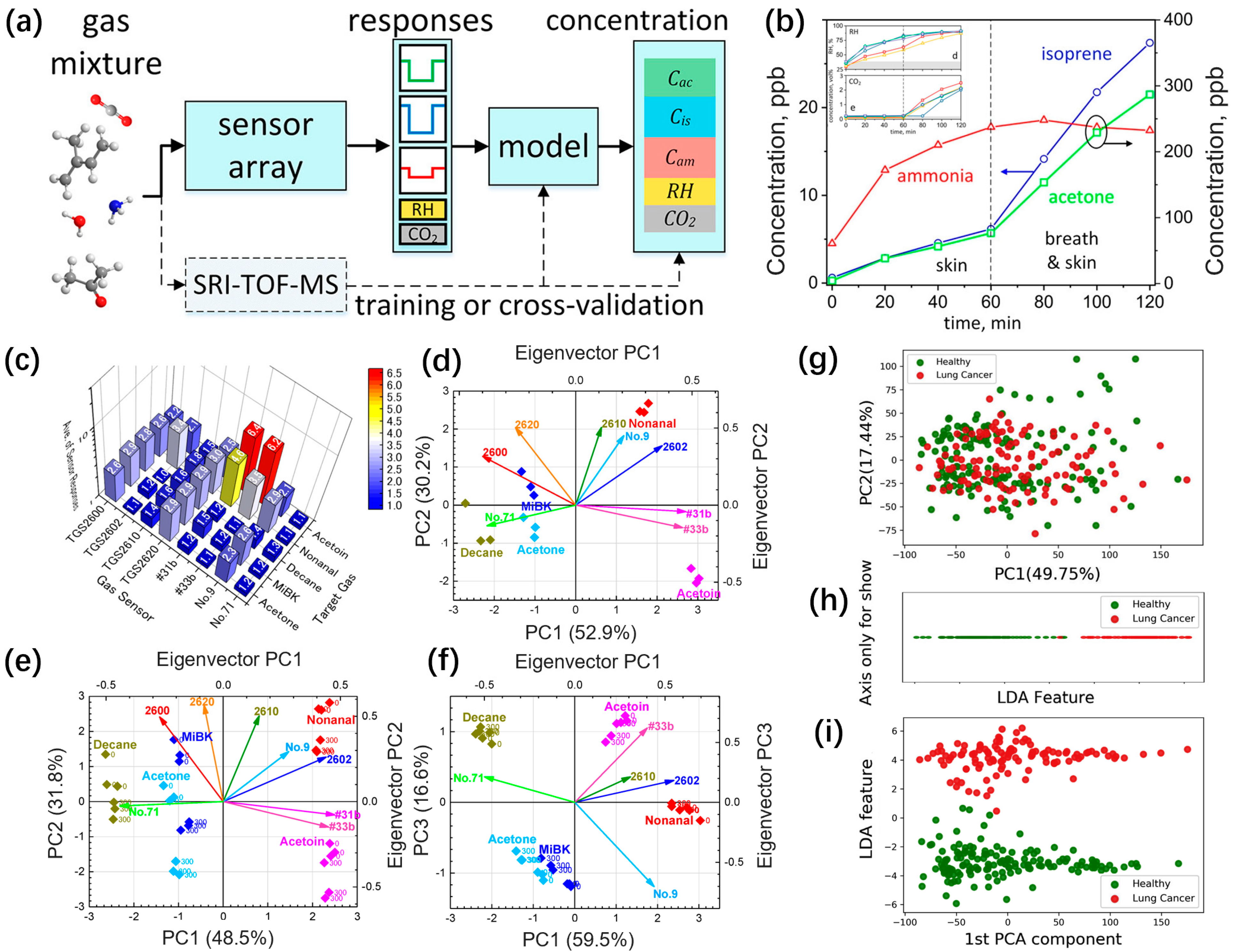
2.4. Sensor Array and Pattern Recognition
3. Summary and Outlook
- Clinical diagnosis. At present, the biomarkers of the exhaled breath of lung cancer patients have not been determined, which limits the application of MOS gas sensors in diagnosing lung cancer. We urgently need a single exhaled VOC, or a unified group of VOCs, as a standard marker for lung cancer to establish a highly reliable “breath prints” comparative database, which can significantly improve the accuracy of clinical diagnosis.
- Materials. The prerequisite for the pattern recognition of the sensor array is that MOS responds to low-concentration VOCs gas; therefore, the LOD of MOS needs to be further reduced. The high-humidity environment of exhaled breath and MOS’s high-working temperature seriously affect its stability and repeatability; thus, it is necessary to develop better humidity-resistant and lower working-temperature MOS materials.
- Algorithms. Deep learning algorithms based on olfactory recognition are needed to identify gases accurately in complex environments. Although still in its early stages, this technology has demonstrated strong recognition ability in other fields. Collaborating with sensor arrays is essential to achieve precise gas identification.
- Devices. The collaborative design and manufacturing of gas sensors using MEMS and CMOS technology reduces their size. Multiple sensors are integrated into a sensor array, and data processing modules enable chip-level packaging and manufacturing.
- Mechanisms. Understanding the gas-sensing mechanism involves complex chemical reactions, which are still not fully understood. Further research can improve the sensor’s performance, address selectivity, and stability issues, and guide the development of gas-sensing materials.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The Ever-Increasing Importance of Cancer as a Leading Cause of Premature Death Worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Iii, C.A.P.; Burnett, R.T.; Thun, M.J.; Calle, E.E.; Krewski, D.; Ito, K.; Thurston, G.D. Lung Cancer, Cardiopulmonary Mortality, and Long-Term Exposure to Fine Particulate Air Pollution. JAMA 2002, 287, 1132–1141. [Google Scholar]
- Detterbeck, F.C.; Chansky, K.; Groome, P.; Bolejack, V.; Crowley, J.; Shemanski, L.; Kennedy, C.; Krasnik, M.; Peake, M.; Rami-Porta, R.; et al. The IASLC Lung Cancer Staging Project: Methodology and Validation Used in the Development of Proposals for Revision of the Stage Classification of NSCLC in the Forthcoming (Eighth) Edition of the TNM Classification of Lung Cancer. J. Thorac. Oncol. 2016, 11, 1433–1446. [Google Scholar] [CrossRef]
- Woodard, G.A.; Jones, K.D.; Jablons, D.M. Lung Cancer Staging and Prognosis. In Lung Cancer: Treatment and Research; Reckamp, K.L., Ed.; Cancer Treatment and Research; Springer International Publishing: Cham, Switzerland, 2016; pp. 47–75. [Google Scholar]
- Roointan, A.; Ahmad Mir, T.; Ibrahim Wani, S.; Mati-ur-Rehman; Hussain, K.K.; Ahmed, B.; Abrahim, S.; Savardashtaki, A.; Gandomani, G.; Gandomani, M.; et al. Early Detection of Lung Cancer Biomarkers through Biosensor Technology: A Review. J. Pharm. Biomed. Anal. 2019, 164, 93–103. [Google Scholar] [CrossRef]
- Nasim, F.; Sabath, B.F.; Eapen, G.A. Lung Cancer. Med. Clin. N. Am. 2019, 103, 463–473. [Google Scholar] [CrossRef]
- Chen, X.; Muhammad, K.G.; Madeeha, C.; Fu, W.; Xu, L.; Hu, Y.; Liu, J.; Ying, K.; Chen, L.; Yurievna, G.O. Calculated Indices of Volatile Organic Compounds (VOCs) in Exhalation for Lung Cancer Screening and Early Detection. Lung Cancer 2021, 154, 197–205. [Google Scholar] [CrossRef]
- Ratiu, I.A.; Ligor, T.; Bocos-Bintintan, V.; Mayhew, C.A.; Buszewski, B. Volatile Organic Compounds in Exhaled Breath as Fingerprints of Lung Cancer, Asthma and COPD. J. Clin. Med. 2021, 10, 32. [Google Scholar] [CrossRef]
- Honnorat, J.; Antoine, J.-C. Paraneoplastic Neurological Syndromes. Orphanet J. Rare Dis. 2007, 2, 22. [Google Scholar] [CrossRef]
- Klebe, S.; Henderson, D.W. Facts and Fiction: Premalignant Lesions of Lung Tissues. Pathology 2013, 45, 305–315. [Google Scholar] [CrossRef]
- D’Urso, V.; Doneddu, V.; Marchesi, I.; Collodoro, A.; Pirina, P.; Giordano, A.; Bagella, L. Sputum Analysis: Non-Invasive Early Lung Cancer Detection. J. Cell. Physiol. 2013, 228, 945–951. [Google Scholar] [CrossRef]
- Kvale, P.A.; Bode, L.F.R.; Kini, S. Diagnostic Accuracy in Lung Cancer: Comparison of Techniques Used in Association with Flexible Fiberoptic Bronchoscopy. Chest 1976, 69, 752–757. [Google Scholar] [CrossRef]
- Visser, M.P.J.; van Grimbergen, I.; Hölters, J.; Barendregt, W.B.; Vermeer, L.C.; Vreuls, W.; Janssen, J. Performance Insights of Endobronchial Ultrasonography (EBUS) and Mediastinoscopy for Mediastinal Lymph Node Staging in Lung Cancer. Lung Cancer 2021, 156, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Raj, R.; Raparia, K.; Lynch, D.A.; Brown, K.K. Surgical Lung Biopsy for Interstitial Lung Diseases. Chest 2017, 151, 1131–1140. [Google Scholar] [CrossRef]
- Park, S.; Jeong, W.; Moon, Y.S. X-Ray Image Segmentation Using Multi-Task Learning. KSII Trans. Internet Inf. Syst. TIIS 2020, 14, 1104–1120. [Google Scholar]
- The National Lung Screening Trial Research Team Results of Initial Low-Dose Computed Tomographic Screening for Lung Cancer. N. Engl. J. Med. 2013, 368, 1980–1991. [CrossRef] [PubMed]
- National Lung Screening Trial Research Team The National Lung Screening Trial: Overview and Study Design. Radiology 2011, 258, 243–253. [CrossRef]
- Choe, W.; Chae, J.D.; Lee, B.-H.; Kim, S.-H.; Park, S.Y.; Nimse, S.B.; Kim, J.; Warkad, S.D.; Song, K.-S.; Oh, A.-C.; et al. 9G TestTM Cancer/Lung: A Desirable Companion to LDCT for Lung Cancer Screening. Cancers 2020, 12, 3192. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.E.; Debowski, M.; Hukins, C.; Fielding, D.; Fong, K.M.; Bettington, C.S. Non-Small Cell Lung Cancer Brain Metastasis Screening in the Era of Positron Emission Tomography-CT Staging: Current Practice and Outcomes. J. Med. Imaging Radiat. Oncol. 2018, 62, 383–388. [Google Scholar] [CrossRef]
- Abdurixiti, M.; Nijiati, M.; Shen, R.; Ya, Q.; Abuduxiku, N.; Nijiati, M. Current Progress and Quality of Radiomic Studies for Predicting EGFR Mutation in Patients with Non-Small Cell Lung Cancer Using PET/CT Images: A Systematic Review. Br. J. Radiol. 2021, 94, 20201272. [Google Scholar] [CrossRef]
- Turner, C. Techniques and Issues in Breath and Clinical Sample Headspace Analysis for Disease Diagnosis. Bioanalysis 2016, 8, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Capuano, R.; Catini, A.; Paolesse, R.; Di Natale, C. Sensors for Lung Cancer Diagnosis. J. Clin. Med. 2019, 8, 235. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-E.; Lee, D.-S.; Ban, S.-W.; Oh, J.; Jung, M.Y.; Kim, S.-H.; Park, S.; Persaud, K.; Jheon, S. Analysis of Volatile Organic Compounds in Exhaled Breath for Lung Cancer Diagnosis Using a Sensor System. Sens. Actuators B Chem. 2018, 255, 800–807. [Google Scholar] [CrossRef]
- Saalberg, Y.; Wolff, M. VOC Breath Biomarkers in Lung Cancer. Clin. Chim. Acta 2016, 459, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.M.; Szidon, J.P.; Krotoszynski, B.K.; Gibbons, R.D.; O’Neill, H.J. Volatile Organic Compounds in Exhaled Air from Patients with Lung Cancer. Clin. Chem. 1985, 31, 1278–1282. [Google Scholar] [CrossRef]
- Konvalina, G.; Haick, H. Sensors for Breath Testing: From Nanomaterials to Comprehensive Disease Detection. Acc. Chem. Res. 2014, 47, 66–76. [Google Scholar] [CrossRef]
- Li, W.; Liu, H.-Y.; Jia, Z.-R.; Qiao, P.-P.; Pi, X.-T.; Chen, J.; Deng, L.-H. Advances in the Early Detection of Lung Cancer Using Analysis of Volatile Organic Compounds: From Imaging to Sensors. Asian Pac. J. Cancer Prev. 2014, 15, 4377–4384. [Google Scholar] [CrossRef]
- Pauling, L.; Robinson, A.B.; Teranishi, R.; Cary, P. Quantitative Analysis of Urine Vapor and Breath by Gas-Liquid Partition Chromatography. Proc. Natl. Acad. Sci. USA 1971, 68, 2374–2376. [Google Scholar] [CrossRef]
- Phillips, M.; Herrera, J.; Krishnan, S.; Zain, M.; Greenberg, J.; Cataneo, R.N. Variation in Volatile Organic Compounds in the Breath of Normal Humans. J. Chromatogr. B. Biomed. Sci. Appl. 1999, 729, 75–88. [Google Scholar] [CrossRef]
- de Lacy Costello, B.; Amann, A.; Al-Kateb, H.; Flynn, C.; Filipiak, W.; Khalid, T.; Osborne, D.; Ratcliffe, N.M. A Review of the Volatiles from the Healthy Human Body. J. Breath Res. 2014, 8, 014001. [Google Scholar] [CrossRef]
- Fenske, J.D.; Paulson, S.E. Human Breath Emissions of VOCs. J. Air Waste Manag. Assoc. 1999, 49, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Gleeson, K.; Hughes, J.M.B.; Greenberg, J.; Cataneo, R.N.; Baker, L.; McVay, W.P. Volatile Organic Compounds in Breath as Markers of Lung Cancer: A Cross-Sectional Study. Lancet 1999, 353, 1930–1933. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Cataneo, R.N.; Cummin, A.R.C.; Gagliardi, A.J.; Gleeson, K.; Greenberg, J.; Maxfield, R.A.; Rom, W.N. Detection of Lung Cancer with Volatile Markers in the Breatha. Chest 2003, 123, 2115–2123. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Altorki, N.; Austin, J.H.M.; Cameron, R.B.; Cataneo, R.N.; Greenberg, J.; Kloss, R.; Maxfield, R.A.; Munawar, M.I.; Pass, H.I.; et al. Prediction of Lung Cancer Using Volatile Biomarkers in Breath. Cancer Biomark. 2007, 3, 95–109. [Google Scholar] [CrossRef]
- Phillips, M.; Altorki, N.; Austin, J.H.M.; Cameron, R.B.; Cataneo, R.N.; Kloss, R.; Maxfield, R.A.; Munawar, M.I.; Pass, H.I.; Rashid, A.; et al. Detection of Lung Cancer Using Weighted Digital Analysis of Breath Biomarkers. Clin. Chim. Acta 2008, 393, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Hakim, M.; Broza, Y.Y.; Barash, O.; Peled, N.; Phillips, M.; Amann, A.; Haick, H. Volatile Organic Compounds of Lung Cancer and Possible Biochemical Pathways. Chem. Rev. 2012, 112, 5949–5966. [Google Scholar] [CrossRef]
- Preti, G.; Labows, J.N.; Kostelc, J.G.; Aldinger, S.; Daniele, R. Analysis of Lung Air from Patients with Bronchogenic Carcinoma and Controls Using Gas Chromatography-Mass Spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 1988, 432, 1–11. [Google Scholar] [CrossRef]
- Lai, X.; Cao, K.; Shen, G.; Xue, P.; Wang, D.; Hu, F.; Zhang, J.; Yang, Q.; Wang, X. Ordered Mesoporous NiFe2O4 with Ultrathin Framework for Low-Ppb Toluene Sensing. Sci. Bull. 2018, 63, 187–193. [Google Scholar] [CrossRef]
- Ligor, T.; Ligor, M.; Amann, A.; Ager, C.; Bachler, M.; Dzien, A.; Buszewski, B. The Analysis of Healthy Volunteers’ Exhaled Breath by the Use of Solid-Phase Microextraction and GC-MS. J. Breath Res. 2008, 2, 046006. [Google Scholar] [CrossRef]
- Koureas, M.; Kirgou, P.; Amoutzias, G.; Hadjichristodoulou, C.; Gourgoulianis, K.; Tsakalof, A. Target Analysis of Volatile Organic Compounds in Exhaled Breath for Lung Cancer Discrimination from Other Pulmonary Diseases and Healthy Persons. Metabolites 2020, 10, 317. [Google Scholar] [CrossRef]
- Schallschmidt, K.; Becker, R.; Jung, C.; Bremser, W.; Walles, T.; Neudecker, J.; Leschber, G.; Frese, S.; Nehls, I. Comparison of Volatile Organic Compounds from Lung Cancer Patients and Healthy Controls—Challenges and Limitations of an Observational Study. J. Breath Res. 2016, 10, 046007. [Google Scholar] [CrossRef]
- Adiguzel, Y.; Kulah, H. Breath Sensors for Lung Cancer Diagnosis. Biosens. Bioelectron. 2015, 65, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, P.J. Analysis of Volatile Organic Compounds in the Exhaled Breath for the Diagnosis of Lung Cancer. J. Thorac. Oncol. 2008, 3, 774–780. [Google Scholar] [CrossRef]
- Amann, A.; Costello, B.d.L.; Miekisch, W.; Schubert, J.; Buszewski, B.; Pleil, J.; Ratcliffe, N.; Risby, T. The Human Volatilome: Volatile Organic Compounds (VOCs) in Exhaled Breath, Skin Emanations, Urine, Feces and Saliva. J. Breath Res. 2014, 8, 034001. [Google Scholar] [CrossRef] [PubMed]
- Haick, H.; Broza, Y.Y.; Mochalski, P.; Ruzsanyi, V.; Amann, A. Assessment, Origin, and Implementation of Breath Volatile Cancer Markers. Chem. Soc. Rev. 2014, 43, 1423–1449. [Google Scholar] [CrossRef]
- Barash, O.; Tisch, U.; Haick, H. Volatile Organic Compounds and the Potential for a Lung Cancer Breath Test. Lung Cancer Manag. 2013, 2, 471–482. [Google Scholar] [CrossRef]
- Sutaria, S.R.; Gori, S.S.; Morris, J.D.; Xie, Z.; Fu, X.-A.; Nantz, M.H. Lipid Peroxidation Produces a Diverse Mixture of Saturated and Unsaturated Aldehydes in Exhaled Breath That Can Serve as Biomarkers of Lung Cancer—A Review. Metabolites 2022, 12, 561. [Google Scholar] [CrossRef]
- Bayley, J.-P.; Devilee, P. Warburg Tumours and the Mechanisms of Mitochondrial Tumour Suppressor Genes. Barking up the Right Tree? Curr. Opin. Genet. Dev. 2010, 20, 324–329. [Google Scholar] [CrossRef]
- Janfaza, S.; Khorsand, B.; Nikkhah, M.; Zahiri, J. Digging Deeper into Volatile Organic Compounds Associated with Cancer. Biol. Methods Protoc. 2019, 4, bpz014. [Google Scholar] [CrossRef]
- Lunt, S.Y.; Vander Heiden, M.G. Aerobic Glycolysis: Meeting the Metabolic Requirements of Cell Proliferation. Annu. Rev. Cell Dev. Biol. 2011, 27, 441–464. [Google Scholar] [CrossRef]
- Gashimova, E.M.; Temerdashev, A.Z.; Porkhanov, V.A.; Polyakov, I.S.; Perunov, D.V. Volatile Organic Compounds in Exhaled Breath as Biomarkers of Lung Cancer: Advances and Potential Problems. J. Anal. Chem. 2022, 77, 785–810. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; He, Z.; Chen, W.; Liu, H.; Chen, K.; Pi, X. Detection of Lung Cancer with Electronic Nose Using a Novel Ensemble Learning Framework. J. Breath Res. 2021, 15, 026014. [Google Scholar] [CrossRef]
- Chen, X.; Cao, M.; Li, Y.; Hu, W.; Wang, P.; Ying, K.; Pan, H. A Study of an Electronic Nose for Detection of Lung Cancer Based on a Virtual SAW Gas Sensors Array and Imaging Recognition Method. Meas. Sci. Technol. 2005, 16, 1535. [Google Scholar] [CrossRef]
- Jia, Z.; Patra, A.; Kutty, V.K.; Venkatesan, T. Critical Review of Volatile Organic Compound Analysis in Breath and In Vitro Cell Culture for Detection of Lung Cancer. Metabolites 2019, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.; Kohlbrenner, D.; Malesevic, S.; Huang, A.; Klein, S.D.; Puhan, M.A.; Kohler, M. Mapping the Landscape of Lung Cancer Breath Analysis: A Scoping Review (ELCABA). Lung Cancer 2023, 175, 131–140. [Google Scholar] [CrossRef]
- Miekisch, W.; Herbig, J.; Schubert, J.K. Data Interpretation in Breath Biomarker Research: Pitfalls and Directions. J. Breath Res. 2012, 6, 036007. [Google Scholar] [CrossRef] [PubMed]
- Smolinska, A.; Hauschild, A.-C.; Fijten, R.R.R.; Dallinga, J.W.; Baumbach, J.; Schooten, F.J. van Current Breathomics—A Review on Data Pre-Processing Techniques and Machine Learning in Metabolomics Breath Analysis. J. Breath Res. 2014, 8, 027105. [Google Scholar] [CrossRef]
- Kim, C.; Lee, K.K.; Kang, M.S.; Shin, D.-M.; Oh, J.-W.; Lee, C.-S.; Han, D.-W. Artificial Olfactory Sensor Technology That Mimics the Olfactory Mechanism: A Comprehensive Review. Biomater. Res. 2022, 26, 40. [Google Scholar] [CrossRef]
- Peled, N.; Hakim, M.; Bunn, P.A.; Miller, Y.E.; Kennedy, T.C.; Mattei, J.; Mitchell, J.D.; Hirsch, F.R.; Haick, H. Non-Invasive Breath Analysis of Pulmonary Nodules. J. Thorac. Oncol. 2012, 7, 1528–1533. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, J.; Fu, W.; Muhammad, K.G.; Li, Y.; Liu, W.; Xu, L.; Dong, H.; Wang, D.; Liu, J.; et al. Smartphone-Based Platforms for Clinical Detections in Lung-Cancer-Related Exhaled Breath Biomarkers: A Review. Biosensors 2022, 12, 223. [Google Scholar] [CrossRef]
- Salimi, M.; Milani Hosseini, S.M.R. Smartphone-Based Detection of Lung Cancer-Related Volatile Organic Compounds (VOCs) Using Rapid Synthesized ZnO Nanosheet. Sens. Actuators B Chem. 2021, 344, 130127. [Google Scholar] [CrossRef]
- Di Natale, C.; Macagnano, A.; Martinelli, E.; Paolesse, R.; D’Arcangelo, G.; Roscioni, C.; Finazzi-Agrò, A.; D’Amico, A. Lung Cancer Identification by the Analysis of Breath by Means of an Array of Non-Selective Gas Sensors. Biosens. Bioelectron. 2003, 18, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Li, D.; Du, W.; Yan, M.; Wang, Y.; Huo, D.; Hou, C. Rapid Recognition of Volatile Organic Compounds with Colorimetric Sensor Arrays for Lung Cancer Screening. Anal. Bioanal. Chem. 2018, 410, 3671–3681. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Rotaru, A.; Robeyns, K.; Garcia, Y. A Colorimetric Sensor for the Highly Selective, Ultra-Sensitive, and Rapid Detection of Volatile Organic Compounds and Hazardous Gases. Ind. Eng. Chem. Res. 2021, 60, 8788–8798. [Google Scholar] [CrossRef]
- Ji, H.; Zeng, W.; Li, Y. Gas Sensing Mechanisms of Metal Oxide Semiconductors: A Focus Review. Nanoscale 2019, 11, 22664–22684. [Google Scholar] [CrossRef] [PubMed]
- Drmosh, Q.A.; Olanrewaju Alade, I.; Qamar, M.; Akbar, S. Zinc Oxide-Based Acetone Gas Sensors for Breath Analysis: A Review. Chem.–Asian J. 2021, 16, 1519–1538. [Google Scholar] [CrossRef] [PubMed]
- Ahmadipour, M.; Pang, A.L.; Ardani, M.R.; Pung, S.-Y.; Ooi, P.C.; Hamzah, A.A.; Mohd Razip Wee, M.F.; Aniq Shazni Mohammad Haniff, M.; Dee, C.F.; Mahmoudi, E.; et al. Detection of Breath Acetone by Semiconductor Metal Oxide Nanostructures-Based Gas Sensors: A Review. Mater. Sci. Semicond. Process. 2022, 149, 106897. [Google Scholar] [CrossRef]
- Raju, P.; Li, Q. Review—Semiconductor Materials and Devices for Gas Sensors. J. Electrochem. Soc. 2022, 169, 057518. [Google Scholar] [CrossRef]
- Cheng, L.; Meng, Q.-H.; Lilienthal, A.J.; Qi, P.-F. Development of Compact Electronic Noses: A Review. Meas. Sci. Technol. 2021, 32, 062002. [Google Scholar] [CrossRef]
- Yuan, H.; Li, N.; Fan, W.; Cai, H.; Zhao, D. Metal-Organic Framework Based Gas Sensors. Adv. Sci. 2022, 9, 2104374. [Google Scholar] [CrossRef]
- Yang, X.; Deng, Y.; Yang, H.; Liao, Y.; Cheng, X.; Zou, Y.; Wu, L.; Deng, Y. Functionalization of Mesoporous Semiconductor Metal Oxides for Gas Sensing: Recent Advances and Emerging Challenges. Adv. Sci. 2023, 10, 2204810. [Google Scholar] [CrossRef]
- Vajhadin, F.; Mazloum-Ardakani, M.; Amini, A. Metal Oxide-Based Gas Sensors for the Detection of Exhaled Breath Markers. Med. Devices Sens. 2021, 4, e10161. [Google Scholar] [CrossRef] [PubMed]
- Seiyama, T.; Kato, A.; Fujiishi, K.; Nagatani, M. A New Detector for Gaseous Components Using Semiconductive Thin Films. Anal. Chem. 1962, 34, 1502–1503. [Google Scholar] [CrossRef]
- Engelhard, E.; Gudden, B. Zur Frage Der Gültigkeit Des Ohmschen Gesetzes Bei Cu2O. Bemerkung Zur Arbeit “Variable Widerstände Und Ihre Hydrodynamische Analogie” von R. Auerbach. Z. Phys. 1931, 70, 701–705. [Google Scholar] [CrossRef]
- Persaud, K.; Dodd, G. Analysis of Discrimination Mechanisms in the Mammalian Olfactory System Using a Model Nose. Nature 1982, 299, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Yoon, Y.S.; Kim, D.-J. Two-Dimensional Transition Metal Dichalcogenides and Metal Oxide Hybrids for Gas Sensing. ACS Sens. 2018, 3, 2045–2060. [Google Scholar] [CrossRef]
- Das, S.; Mojumder, S.; Saha, D.; Pal, M. Influence of Major Parameters on the Sensing Mechanism of Semiconductor Metal Oxide Based Chemiresistive Gas Sensors: A Review Focused on Personalized Healthcare. Sens. Actuators B Chem. 2022, 352, 131066. [Google Scholar] [CrossRef]
- Yamazoe, N. New Approaches for Improving Semiconductor Gas Sensors. Sens. Actuators B Chem. 1991, 5, 7–19. [Google Scholar] [CrossRef]
- Ji, H.; Zeng, W.; Li, Y. Assembly of 2D Nanosheets into Flower-like MoO3: New Insight into the Petal Thickness Affect on Gas-Sensing Properties. Mater. Res. Bull. 2019, 118, 110476. [Google Scholar] [CrossRef]
- Wang, H.; Ma, J.; Zhang, J.; Feng, Y.; Vijjapu, M.T.; Yuvaraja, S.; Surya, S.G.; Salama, K.N.; Dong, C.; Wang, Y.; et al. Gas Sensing Materials Roadmap. J. Phys. Condens. Matter 2021, 33, 303001. [Google Scholar] [CrossRef]
- Li, Z.; Yu, J.; Dong, D.; Yao, G.; Wei, G.; He, A.; Wu, H.; Zhu, H.; Huang, Z.; Tang, Z. E-Nose Based on a High-Integrated and Low-Power Metal Oxide Gas Sensor Array. Sens. Actuators B Chem. 2023, 380, 133289. [Google Scholar] [CrossRef]
- Rath, R.J.; Farajikhah, S.; Oveissi, F.; Dehghani, F.; Naficy, S. Chemiresistive Sensor Arrays for Gas/Volatile Organic Compounds Monitoring: A Review. Adv. Eng. Mater. 2023, 25, 2200830. [Google Scholar] [CrossRef]
- Wang, M.; Hou, T.; Shen, Z.; Zhao, X.; Ji, H. MOF-Derived Fe2 Why 3: Phase Control and Effects of Phase Composition on Gas Sensing Performance. Sens. Actuators B Chem. 2019, 292, 171–179. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Neri, G.; Pinna, N. Nanostructured Materials for Room-Temperature Gas Sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef] [PubMed]
- Sahm, T.; Gurlo, A.; Bârsan, N.; Weimar, U. Basics of Oxygen and SnO2 Interaction; Work Function Change and Conductivity Measurements. Sens. Actuators B Chem. 2006, 118, 78–83. [Google Scholar] [CrossRef]
- Manno, D.; Micocci, G.; Rella, R.; Serra, A.; Taurino, A.; Tepore, A. Titanium Oxide Thin Films for NH3 Monitoring: Structural and Physical Characterizations. J. Appl. Phys. 1997, 82, 54–59. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Conduction Model of Metal Oxide Gas Sensors. J. Electroceramics 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Gurlo, A. Interplay between O2 and SnO2: Oxygen Ionosorption and Spectroscopic Evidence for Adsorbed Oxygen. ChemPhysChem 2006, 7, 2041–2052. [Google Scholar] [CrossRef]
- Morsy, M.; Abdel-Salam, A.I.; Mostafa, M.; Elzwawy, A. Promoting the Humidity Sensing Capabilities of Titania Nanorods/RGO Nanocomposite via de-Bundling and Maximizing Porosity and Surface Area through Lyophilization. Micro Nano Eng. 2022, 17, 100163. [Google Scholar] [CrossRef]
- Bai, J.; Zhao, C.; Gong, H.; Wang, Q.; Huang, B.; Sun, G.; Wang, Y.; Zhou, J.; Xie, E.; Wang, F. Debye-Length Controlled Gas Sensing Performances in NiO@ZnO p-n Junctional Core–Shell Nanotubes. J. Phys. Appl. Phys. 2019, 52, 285103. [Google Scholar] [CrossRef]
- Nakate, U.T.; Ahmad, R.; Patil, P.; Wang, Y.; Bhat, K.S.; Mahmoudi, T.; Yu, Y.T.; Suh, E.; Hahn, Y.-B. Improved Selectivity and Low Concentration Hydrogen Gas Sensor Application of Pd Sensitized Heterojunction N-ZnO/p-NiO Nanostructures. J. Alloys Compd. 2019, 797, 456–464. [Google Scholar] [CrossRef]
- Moseley, P.T.; Norris, J.O.; Williams, D.E. Techniques and Mechanisms in Gas Sensing; Adam Hilger Bristol; CRC Press: Boca Raton, FL, USA, 1991; Volume 234. [Google Scholar]
- Jain, G.H. MOS Gas Sensors: What Determines Our Choice? In Proceedings of the 2011 Fifth International Conference on Sensing Technology, Palmerston North, New Zealand, 28 November–1 December 2011; pp. 66–72. [Google Scholar]
- Hübner, M.; Simion, C.E.; Tomescu-Stănoiu, A.; Pokhrel, S.; Bârsan, N.; Weimar, U. Influence of Humidity on CO Sensing with P-Type CuO Thick Film Gas Sensors. Sens. Actuators B Chem. 2011, 153, 347–353. [Google Scholar] [CrossRef]
- Mohammed, R.S.; Fakhri, M.A. Titanium Dioxide–Based Sensors: A Review. AIP Conf. Proc. 2022, 2660, 020133. [Google Scholar]
- Yan, Z.; Zhang, Y.; Kang, W.; Deng, N.; Pan, Y.; Sun, W.; Ni, J.; Kang, X. TiO2 Gas Sensors Combining Experimental and DFT Calculations: A Review. Nanomaterials 2022, 12, 3611. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Cui, X.; Lai, T.; Ren, J.; Yang, Z.; Xiao, M.; Wang, B.; Xiao, X.; Wang, Y. Gas Sensors Based on TiO2 Nanostructured Materials for the Detection of Hazardous Gases: A Review. Nano Mater. Sci. 2021, 3, 390–403. [Google Scholar] [CrossRef]
- Hazra, A.; Das, S.; Kanungo, J.; Sarkar, C.K.; Basu, S. Studies on a Resistive Gas Sensor Based on Sol–Gel Grown Nanocrystalline p-TiO2 Thin Film for Fast Hydrogen Detection. Sens. Actuators B Chem. 2013, 183, 87–95. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Dong, J.; Qin, N.; Xu, J. Selective BTEX Sensor Based on a SnO2/V2O5 Composite. Sens. Actuators B Chem. 2013, 186, 126–131. [Google Scholar] [CrossRef]
- Peng, G.; Tisch, U.; Adams, O.; Hakim, M.; Shehada, N.; Broza, Y.Y.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Haick, H. Diagnosing Lung Cancer in Exhaled Breath Using Gold Nanoparticles. Nat. Nanotechnol. 2009, 4, 669–673. [Google Scholar] [CrossRef]
- Ferrus, L.; Guenard, H.; Vardon, G.; Varene, P. Respiratory Water Loss. Respir. Physiol. 1980, 39, 367–381. [Google Scholar] [CrossRef]
- Bag, A.; Lee, N. Recent Advancements in Development of Wearable Gas Sensors. Adv. Mater. Technol. 2021, 6, 2000883. [Google Scholar] [CrossRef]
- Jeong, S.-Y.; Kim, J.-S.; Lee, J.-H. Rational Design of Semiconductor-Based Chemiresistors and Their Libraries for Next-Generation Artificial Olfaction. Adv. Mater. 2020, 32, 2002075. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, T. Recent Progress of Nanostructured Sensing Materials from 0D to 3D: Overview of Structure–Property-Application Relationship for Gas Sensors. Small Methods 2021, 5, 2100515. [Google Scholar] [CrossRef]
- Mahajan, S.; Jagtap, S. Metal-Oxide Semiconductors for Carbon Monoxide (CO) Gas Sensing: A Review. Appl. Mater. Today 2020, 18, 100483. [Google Scholar] [CrossRef]
- Hu, Y.; Hwang, J.; Lee, Y.; Conlin, P.; Schlom, D.G.; Datta, S.; Cho, K. First Principles Calculations of Intrinsic Mobilities in Tin-Based Oxide Semiconductors SnO, SnO2, and Ta2SnO6. J. Appl. Phys. 2019, 126, 185701. [Google Scholar] [CrossRef]
- Batzill, M. Surface Science Studies of Gas Sensing Materials: SnO2. Sensors 2006, 6, 1345–1366. [Google Scholar] [CrossRef]
- Ruhland, B.; Becker, T.; Müller, G. Gas-Kinetic Interactions of Nitrous Oxides with SnO2 Surfaces. Sens. Actuators B Chem. 1998, 50, 85–94. [Google Scholar] [CrossRef]
- Cirera, A.; Diéguez, A.; Diaz, R.; Cornet, A.; Morante, J.R. New Method to Obtain Stable Small-Sized SnO2 Powders for Gas Sensors. Sens. Actuators B Chem. 1999, 58, 360–364. [Google Scholar] [CrossRef]
- Li, Y.; Chen, N.; Deng, D.; Xing, X.; Xiao, X.; Wang, Y. Formaldehyde Detection: SnO2 Microspheres for Formaldehyde Gas Sensor with High Sensitivity, Fast Response/Recovery and Good Selectivity. Sens. Actuators B Chem. 2017, 238, 264–273. [Google Scholar] [CrossRef]
- Hwang, I.-S.; Choi, J.-K.; Woo, H.-S.; Kim, S.-J.; Jung, S.-Y.; Seong, T.-Y.; Kim, I.-D.; Lee, J.-H. Facile Control of C2H5OH Sensing Characteristics by Decorating Discrete Ag Nanoclusters on SnO2 Nanowire Networks. ACS Appl. Mater. Interfaces 2011, 3, 3140–3145. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhu, L.F.; Yang, Y.H.; Xu, N.S.; Yang, G.W. Fabrication of a SnO2 Nanowire Gas Sensor and Sensor Performance for Hydrogen. J. Phys. Chem. C 2008, 112, 6643–6647. [Google Scholar] [CrossRef]
- Bulemo, P.M.; Cho, H.-J.; Kim, N.-H.; Kim, I.-D. Mesoporous SnO2 Nanotubes via Electrospinning–Etching Route: Highly Sensitive and Selective Detection of H2S Molecule. ACS Appl. Mater. Interfaces 2017, 9, 26304–26313. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, W.; Liu, X.; Zhang, L.; Zheng, L.; Yang, C.; Pinna, N.; Zhang, J. Platinum Single Atoms on Tin Oxide Ultrathin Films for Extremely Sensitive Gas Detection. Mater. Horiz. 2020, 7, 1519–1527. [Google Scholar] [CrossRef]
- Kim, R.; Jang, J.-S.; Kim, D.-H.; Kang, J.-Y.; Cho, H.-J.; Jeong, Y.J.; Kim, I.-D. A General Synthesis of Crumpled Metal Oxide Nanosheets as Superior Chemiresistive Sensing Layers. Adv. Funct. Mater. 2019, 29, 1903128. [Google Scholar] [CrossRef]
- Kumar, R.; Liu, X.; Zhang, J.; Kumar, M. Room-Temperature Gas Sensors Under Photoactivation: From Metal Oxides to 2D Materials. Nano-Micro Lett. 2020, 12, 164. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.J.; Yoon, J.-W.; Lee, J.-H.; Kang, Y.C. One-Pot Synthesis of Pd-Loaded SnO2 Yolk–Shell Nanostructures for Ultraselective Methyl Benzene Sensors. Chem.—Eur. J. 2014, 20, 2737–2741. [Google Scholar] [CrossRef]
- Qiao, L.; Bing, Y.; Wang, Y.; Yu, S.; Liang, Z.; Zeng, Y. Enhanced Toluene Sensing Performances of Pd- Loaded SnO2 Cubic Nanocages with Porous Nanoparticle-Assembled Shells. Sens. Actuators B Chem. 2017, 241, 1121–1129. [Google Scholar] [CrossRef]
- Bing, Y.; Liu, C.; Qiao, L.; Zeng, Y.; Yu, S.; Liang, Z.; Liu, J.; Luo, J.; Zheng, W. Multistep Synthesis of Non-Spherical SnO2 @SnO2 Yolk-Shell Cuboctahedra with Nanoparticle-Assembled Porous Structure for Toluene Detection. Sens. Actuators B Chem. 2016, 231, 365–375. [Google Scholar] [CrossRef]
- Wang, T.; Xu, H.; Wang, Y.; Zeng, Y.; Liu, B. Porous SnO2 Triple-Shelled Hollow Nanoboxes for High Sensitive Toluene Detection. Mater. Lett. 2020, 264, 127320. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, S.; Zhou, P.; Cui, B.; Liu, W.; Wei, D.; Shen, Y. Room-Temperature NO2 Sensing Properties and Mechanism of CuO Nanorods with Au Functionalization. Sens. Actuators B Chem. 2021, 328, 129070. [Google Scholar] [CrossRef]
- Liu, C.; Kuang, Q.; Xie, Z.; Zheng, L. The Effect of Noble Metal (Au, Pd and Pt) Nanoparticles on the Gas Sensing Performance of SnO2-Based Sensors: A Case Study on the {221} High-Index Faceted SnO2 Octahedra. CrystEngComm 2015, 17, 6308–6313. [Google Scholar] [CrossRef]
- Suematsu, K.; Harano, W.; Oyama, T.; Shin, Y.; Watanabe, K.; Shimanoe, K. Pulse-Driven Semiconductor Gas Sensors Toward Ppt Level Toluene Detection. Anal. Chem. 2018, 90, 11219–11223. [Google Scholar] [CrossRef] [PubMed]
- Suematsu, K.; Harano, W.; Yamasaki, S.; Watanabe, K.; Shimanoe, K. One-Trillionth Level Toluene Detection Using a Dual-Designed Semiconductor Gas Sensor: Material and Sensor-Driven Designs. ACS Appl. Electron. Mater. 2020, 2, 4122–4126. [Google Scholar] [CrossRef]
- Li, G.; Cheng, Z.; Xiang, Q.; Yan, L.; Wang, X.; Xu, J. Bimetal PdAu Decorated SnO2 Nanosheets Based Gas Sensor with Temperature-Dependent Dual Selectivity for Detecting Formaldehyde and Acetone. Sens. Actuators B Chem. 2019, 283, 590–601. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, J.-H.; Park, Y.; Kim, J.-Y.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Toluene- and Benzene-Selective Gas Sensors Based on Pt- and Pd-Functionalized ZnO Nanowires in Self-Heating Mode. Sens. Actuators B Chem. 2019, 294, 78–88. [Google Scholar] [CrossRef]
- Hanh, N.H.; Duy, L.V.; Hung, C.M.; Xuan, C.T.; Duy, N.V.; Hoa, N.D. High-Performance Acetone Gas Sensor Based on Pt–Zn2SnO4 Hollow Octahedra for Diabetic Diagnosis. J. Alloys Compd. 2021, 886, 161284. [Google Scholar] [CrossRef]
- Moon, Y.K.; Jeong, S.-Y.; Jo, Y.-M.; Jo, Y.K.; Kang, Y.C.; Lee, J.-H. Highly Selective Detection of Benzene and Discrimination of Volatile Aromatic Compounds Using Oxide Chemiresistors with Tunable Rh-TiO2 Catalytic Overlayers. Adv. Sci. 2021, 8, 2004078. [Google Scholar] [CrossRef]
- Jeong, S.-Y.; Moon, Y.K.; Wang, J.; Lee, J.-H. Exclusive Detection of Volatile Aromatic Hydrocarbons Using Bilayer Oxide Chemiresistors with Catalytic Overlayers. Nat. Commun. 2023, 14, 233. [Google Scholar] [CrossRef]
- Kwak, C.-H.; Kim, T.-H.; Jeong, S.-Y.; Yoon, J.-W.; Kim, J.-S.; Lee, J.-H. Humidity-Independent Oxide Semiconductor Chemiresistors Using Terbium-Doped SnO2 Yolk–Shell Spheres for Real-Time Breath Analysis. ACS Appl. Mater. Interfaces 2018, 10, 18886–18894. [Google Scholar] [CrossRef]
- Egashira, M.; Nakashima, M.; Kawasumi, S.; Selyama, T. Temperature Programmed Desorption Study of Water Adsorbed on Metal Oxides. 2. Tin Oxide Surfaces. J. Phys. Chem. 1981, 85, 4125–4130. [Google Scholar] [CrossRef]
- Großmann, K.; Wicker, S.; Weimar, U.; Barsan, N. Impact of Pt Additives on the Surface Reactions between SnO2, Water Vapour, CO and H2; an Operando Investigation. Phys. Chem. Chem. Phys. 2013, 15, 19151–19158. [Google Scholar] [CrossRef]
- Singh, S.; Deb, J.; Sarkar, U.; Sharma, S. MoS2/MoO3 Nanocomposite for Selective NH3 Detection in a Humid Environment. ACS Sustain. Chem. Eng. 2021, 9, 7328–7340. [Google Scholar] [CrossRef]
- Ma, N.; Suematsu, K.; Yuasa, M.; Kida, T.; Shimanoe, K. Effect of Water Vapor on Pd-Loaded SnO2 Nanoparticles Gas Sensor. ACS Appl. Mater. Interfaces 2015, 7, 5863–5869. [Google Scholar] [CrossRef]
- Choi, K.-I.; Kim, H.-J.; Kang, Y.C.; Lee, J.-H. Ultraselective and Ultrasensitive Detection of H2S in Highly Humid Atmosphere Using CuO-Loaded SnO2 Hollow Spheres for Real-Time Diagnosis of Halitosis. Sens. Actuators B Chem. 2014, 194, 371–376. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Z.; Suematsu, K.; Li, P.; Yu, Z.; Zhang, W.; Hu, J.; Shimanoe, K. Rapid and Stable Detection of Carbon Monoxide in Changing Humidity Atmospheres Using Clustered In2O3/CuO Nanospheres. ACS Sens. 2020, 5, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Park, J.K.; Lee, J.; Kwon, Y.J.; Choi, H.; Yang, S.-M.; Lee, J.-H.; Jeong, Y.K. Synergistic Approach to Simultaneously Improve Response and Humidity-Independence of Metal-Oxide Gas Sensors. J. Hazard. Mater. 2022, 424, 127524. [Google Scholar] [CrossRef]
- Zhu, X.; Chang, X.; Tang, S.; Chen, X.; Gao, W.; Niu, S.; Li, J.; Jiang, Y.; Sun, S. Humidity-Tolerant Chemiresistive Gas Sensors Based on Hydrophobic CeO2/SnO2 Heterostructure Films. ACS Appl. Mater. Interfaces 2022, 14, 25680–25692. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.-Y.; Moon, Y.K.; Kim, J.K.; Park, S.-W.; Jo, Y.K.; Kang, Y.C.; Lee, J.-H. A General Solution to Mitigate Water Poisoning of Oxide Chemiresistors: Bilayer Sensors with Tb4O7 Overlayer. Adv. Funct. Mater. 2021, 31, 2007895. [Google Scholar] [CrossRef]
- Si, Y.; Dong, Z.; Jiang, L. Bioinspired Designs of Superhydrophobic and Superhydrophilic Materials. ACS Cent. Sci. 2018, 4, 1102–1112. [Google Scholar] [CrossRef]
- Vallejos, S.; Gràcia, I.; Pizúrová, N.; Figueras, E.; Čechal, J.; Hubálek, J.; Cané, C. Gas Sensitive ZnO Structures with Reduced Humidity-Interference. Sens. Actuators B Chem. 2019, 301, 127054. [Google Scholar] [CrossRef]
- Peng, L.; Jiang, D.; Wang, Z.; Hua, L.; Li, H. Dopant-Assisted Negative Photoionization Ion Mobility Spectrometry Coupled with on-Line Cooling Inlet for Real-Time Monitoring H2S Concentration in Sewer Gas. Talanta 2016, 153, 295–300. [Google Scholar] [CrossRef]
- Han, B.; Wang, H.; Yang, W.; Wang, J.; Wei, X. Hierarchical Pt-Decorated In2O3 Microspheres with Highly Enhanced Isoprene Sensing Properties. Ceram. Int. 2021, 47, 9477–9485. [Google Scholar] [CrossRef]
- Wang, Y.; Hua, L.; Li, Q.; Jiang, J.; Hou, K.; Wu, C.; Li, H. Direct Detection of Small N-Alkanes at Sub-Ppbv Level by Photoelectron-Induced O2+ Cation Chemical Ionization Mass Spectrometry at KPa Pressure. Anal. Chem. 2018, 90, 5398–5404. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Fahad, H.M.; Amani, M.; Song, X.; Scott, M.; Javey, A. Elimination of Response to Relative Humidity Changes in Chemical-Sensitive Field-Effect Transistors. ACS Sens. 2019, 4, 1857–1863. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Duan, Z.; Zhang, B.; Zhao, Y.; Yuan, Z.; Zhang, Y.; Wu, Y.; Jiang, Y.; Tai, H. Local Gaussian Process Regression with Small Sample Data for Temperature and Humidity Compensation of Polyaniline-Cerium Dioxide NH3 Sensor. Sens. Actuators B Chem. 2023, 378, 133113. [Google Scholar] [CrossRef]
- Konvalina, G.; Haick, H. Effect of Humidity on Nanoparticle-Based Chemiresistors: A Comparison between Synthetic and Real-World Samples. ACS Appl. Mater. Interfaces 2012, 4, 317–325. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, C.; Liu, B.; Jiang, Y.; Li, Z.; Tai, H.; Li, X. Self-Adaptive Temperature and Humidity Compensation Based on Improved Deep BP Neural Network for NO2 Detection in Complex Environment. Sens. Actuators B Chem. 2022, 362, 131812. [Google Scholar] [CrossRef]
- Yan, M.; Wu, Y.; Hua, Z.; Lu, N.; Sun, W.; Zhang, J.; Fan, S. Humidity Compensation Based on Power-Law Response for MOS Sensors to VOCs. Sens. Actuators B Chem. 2021, 334, 129601. [Google Scholar] [CrossRef]
- Venkatraman, M.; Kadian, A.; Choudhary, S.; Subramanian, A.; Singh, A.; Sikarwar, S. Ultra-Fast Benzene Gas (C6H6) Detection Characteristics of Cobalt-Doped Aluminum Oxide Sensors. ChemistrySelect 2023, 8, e202204531. [Google Scholar] [CrossRef]
- Kim, S.-J.; Choi, S.-J.; Jang, J.-S.; Kim, N.-H.; Hakim, M.; Tuller, H.L.; Kim, I.-D. Mesoporous WO3 Nanofibers with Protein-Templated Nanoscale Catalysts for Detection of Trace Biomarkers in Exhaled Breath. ACS Nano 2016, 10, 5891–5899. [Google Scholar] [CrossRef]
- Dasari, S.G.; Nagaraju, P.; Yelsani, V.; Tirumala, S.; MV, R.R. Nanostructured Indium Oxide Thin Films as a Room Temperature Toluene Sensor. ACS Omega 2021, 6, 17442–17454. [Google Scholar] [CrossRef]
- Wang, X.; Chen, F.; Yang, M.; Guo, L.; Xie, N.; Kou, X.; Song, Y.; Wang, Q.; Sun, Y.; Lu, G. Dispersed WO3 Nanoparticles with Porous Nanostructure for Ultrafast Toluene Sensing. Sens. Actuators B Chem. 2019, 289, 195–206. [Google Scholar] [CrossRef]
- Gao, H.; Zhao, L.; Wang, L.; Sun, P.; Lu, H.; Liu, F.; Chuai, X.; Lu, G. Ultrasensitive and Low Detection Limit of Toluene Gas Sensor Based on SnO2-Decorated NiO Nanostructure. Sens. Actuators B Chem. 2018, 255, 3505–3515. [Google Scholar] [CrossRef]
- Xing, Y.; Zhang, L.-X.; Li, C.-T.; Yin, Y.-Y.; Bie, L.-J. Pt Decoration and Oxygen Defects Synergistically Boosted Xylene Sensing Performance of Polycrystalline SnO2 Nanosheet Assembled Microflowers. Sens. Actuators B Chem. 2022, 354, 131220. [Google Scholar] [CrossRef]
- Jeong, H.-M.; Kim, J.-H.; Jeong, S.-Y.; Kwak, C.-H.; Lee, J.-H. Co3O4–SnO2 Hollow Heteronanostructures: Facile Control of Gas Selectivity by Compositional Tuning of Sensing Materials via Galvanic Replacement. ACS Appl. Mater. Interfaces 2016, 8, 7877–7883. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhou, Y.; Guo, J.; Zhao, L.; Wang, T.; Yan, X.; Wang, C.; Liu, F.; Sun, P.; Lu, G. Highly Sensitive and Selective Xylene Sensor Based on P-p Heterojunctions Composites Derived from off-Stoichiometric Cobalt Tungstate. Sens. Actuators B Chem. 2022, 351, 130973. [Google Scholar] [CrossRef]
- Liu, B.; Li, Y.; Gao, L.; Zhou, F.; Duan, G. Ultrafine Pt NPs-Decorated SnO2/α-Fe2O3 Hollow Nanospheres with Highly Enhanced Sensing Performances for Styrene. J. Hazard. Mater. 2018, 358, 355–365. [Google Scholar] [CrossRef]
- Han, B.; Wang, J.; Yang, W.; Chen, X.; Wang, H.; Chen, J.; Zhang, C.; Sun, J.; Wei, X. Hydrothermal Synthesis of Flower-like In2O3 as a Chemiresistive Isoprene Sensor for Breath Analysis. Sens. Actuators B Chem. 2020, 309, 127788. [Google Scholar] [CrossRef]
- Park, Y.; Yoo, R.; Park, S.; Lee, J.H.; Jung, H.; Lee, H.-S.; Lee, W. Highly Sensitive and Selective Isoprene Sensing Performance of ZnO Quantum Dots for a Breath Analyzer. Sens. Actuators B Chem. 2019, 290, 258–266. [Google Scholar] [CrossRef]
- Zheng, Q.; Lee, J.H.; Kim, S.-J.; Lee, H.-S.; Lee, W. Excellent Isoprene-Sensing Performance of In2O3 Nanoparticles for Breath Analyzer Applications. Sens. Actuators B Chem. 2021, 327, 128892. [Google Scholar] [CrossRef]
- Wu, X.; Wang, H.; Wang, J.; Chen, J.; Shi, L.; Han, B.; Tian, X. Hydrothermal Synthesis of Flower-like Cr2O3-Doped In2O3 Nanorods Clusters for Ultra-Low Isoprene Detection. Colloids Surf. Physicochem. Eng. Asp. 2021, 620, 126606. [Google Scholar] [CrossRef]
- Yao, Y.; Li, Z.; Han, Y.; Xie, L.; Zhao, X.; Zhu, Z. Fabrication and Characterization of a MnO2/Ti3C2Tx Based Gas Sensor for Highly Sensitive and Selective Detection of Lung Cancer Marker Hexanal. Chem. Eng. J. 2023, 451, 139029. [Google Scholar] [CrossRef]
- Malara, A.; Bonaccorsi, L.; Donato, A.; Frontera, P.; Piscopo, A.; Poiana, M.; Leonardi, S.G.; Neri, G. Sensing Properties of Indium, Tin and Zinc Oxides for Hexanal Detection. In Proceedings of the Sensors; Andò, B., Baldini, F., Di Natale, C., Ferrari, V., Marletta, V., Marrazza, G., Militello, V., Miolo, G., Rossi, M., Scalise, L., et al., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 39–44. [Google Scholar]
- Kulkarni, S.; Kummara, S.; Gorthala, G.; Ghosh, R. CuO Nanoflake-Based Sensors for Detecting Linalool, Hexanal, and Methyl Salicylate. ACS Agric. Sci. Technol. 2022, 2, 1285–1291. [Google Scholar] [CrossRef]
- Bonaccorsi, L.; Donato, A.; Fotia, A.; Frontera, P.; Gnisci, A. Competitive Detection of Volatile Compounds from Food Degradation by a Zinc Oxide Sensor. Appl. Sci. 2022, 12, 2261. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, J.; Li, H.; Liao, H. Role of Ruthenium Incorporation on Room-Temperature Nonanal Sensing Properties of Ru-Loaded Urchin-like W18O49 Hierarchical Nanostructure. Sens. Actuators B Chem. 2022, 353, 131096. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, K.; Xu, K.; Zhang, C. Investigation on Microstructure and Nonanal Sensing Properties of Hierarchical Sb2WO6 Microspheres. Ceram. Int. 2022, 48, 30249–30259. [Google Scholar] [CrossRef]
- Masuda, Y.; Kato, K.; Kida, M.; Otsuka, J. Selective Nonanal Molecular Recognition with SnO2 Nanosheets for Lung Cancer Sensor. Int. J. Appl. Ceram. Technol. 2019, 16, 1807–1811. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Zhao, L.; Liu, F.; Sun, X.; Hu, X.; Lu, G. Preparation of Ce-Doped SnO2 Cuboids with Enhanced 2-Butanone Sensing Performance. Sens. Actuators B Chem. 2021, 341, 130039. [Google Scholar] [CrossRef]
- Oliveira, T.N.T.; Zito, C.A.; Perfecto, T.M.; Azevedo, G.M.; Volanti, D.P. ZnO Twin-Rods Decorated with Pt Nanoparticles for Butanone Detection. New J. Chem. 2020, 44, 15574–15583. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, M.; Shen, Z.; Wei, Q. A Nanostructured Cr2O3/WO3 p–n Junction Sensor for Highly Sensitive Detection of Butanone. J. Mater. Sci. Mater. Electron. 2017, 28, 12056–12062. [Google Scholar] [CrossRef]
- Jiang, Z.; Guo, Z.; Sun, B.; Jia, Y.; Li, M.; Liu, J. Highly Sensitive and Selective Butanone Sensors Based on Cerium-Doped SnO2 Thin Films. Sens. Actuators B Chem. 2010, 145, 667–673. [Google Scholar] [CrossRef]
- Zhu, H.; Qin, W.; Li, J.; Yuan, Z.; Meng, F.; Shen, Y. Investigation on Butanone Sensing Properties of ZnO Sensor Under Different Calcination Temperature. IEEE Sens. J. 2022, 22, 25–32. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, D.; Liu, C.; Fang, S.; Ge, K.; Wu, Y.; Wu, M.; Liu, Q. Sea Urchin-like Mesoporous WO3 (SUS-WO3) for Sensitive 3-Hydroxy-2-Butanone Biomarker Detection. Mater. Sci. Semicond. Process. 2022, 137, 106160. [Google Scholar] [CrossRef]
- Zhu, H.-M.; Qin, W.-B.; Yuan, Z.-Y.; Han, C.; Li, J.; Shen, Y.-B.; Meng, F.-L. Ppb-Level Butanone Sensor Based on Porous Spherical NiO and the Influence of Silver Modification. Chin. J. Anal. Chem. 2022, 50, 100034. [Google Scholar] [CrossRef]
- Yang, M.; Lu, J.; Wang, X.; Zhang, H.; Chen, F.; Sun, J.; Yang, J.; Sun, Y.; Lu, G. Acetone Sensors with High Stability to Humidity Changes Based on Ru-Doped NiO Flower-like Microspheres. Sens. Actuators B Chem. 2020, 313, 127965. [Google Scholar] [CrossRef]
- Sharma, B.; Sharma, A.; Myung, J. Highly Selective Detection of Acetone by TiO2-SnO2 Heterostructures for Environmental Biomarkers of Diabetes. Sens. Actuators B Chem. 2021, 349, 130733. [Google Scholar] [CrossRef]
- Bai, J.; Shi, Y.; Liang, W.; Wang, C.; Liu, Y.; Wang, H.; Liu, F.; Sun, P.; Zhang, Y.; Lu, G. PtCu Nanocrystals with Crystalline Control: Twin Defect-Driven Enhancement of Acetone Sensing. Sens. Actuators B Chem. 2022, 354, 131210. [Google Scholar] [CrossRef]
- Chen, L.; Song, Y.; Liu, W.; Dong, H.; Wang, D.; Liu, J.; Liu, Q.; Chen, X. MOF-Based Nanoscale Pt Catalyst Decorated SnO2 Porous Nanofibers for Acetone Gas Detection. J. Alloys Compd. 2022, 893, 162322. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, J.; Meng, F. High Response N-Propanol Sensor Based on Co-Modified ZnO Nanorods. J. Alloys Compd. 2022, 910, 164971. [Google Scholar] [CrossRef]
- Yin, Y.; Shen, Y.; Zhou, P.; Lu, R.; Li, A.; Zhao, S.; Liu, W.; Wei, D.; Wei, K. Fabrication, Characterization and n-Propanol Sensing Properties of Perovskite-Type ZnSnO3 Nanospheres Based Gas Sensor. Appl. Surf. Sci. 2020, 509, 145335. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, S.; Zhai, X.; Shao, L.; Bai, X.; Liu, Y.; Wang, T.; Li, Y.; Zhang, L.; Fan, F.; et al. Construction of Zn/Ni Bimetallic Organic Framework Derived ZnO/NiO Heterostructure with Superior N-Propanol Sensing Performance. ACS Appl. Mater. Interfaces 2021, 13, 9206–9215. [Google Scholar] [CrossRef]
- Du, L.; Wang, D.; Gu, K.; Zhang, M. Construction of PdO-Decorated Double-Shell ZnSnO3 Hollow Microspheres for n-Propanol Detection at Low Temperature. Inorg. Chem. Front. 2021, 8, 787–795. [Google Scholar] [CrossRef]
- Kortidis, I.; Lushozi, S.; Leshabane, N.; Nkosi, S.S.; Ndwandwe, O.M.; Tshilongo, J.; Ntsasa, N.; Motaung, D.E. Selective Detection of Propanol Vapour at Low Operating Temperature Utilizing ZnO Nanostructures. Ceram. Int. 2019, 45, 16417–16423. [Google Scholar] [CrossRef]
- Wang, N.; Zhou, Y.; Chen, K.; Wang, T.; Sun, P.; Wang, C.; Chuai, X.; Zhang, S.; Liu, X.; Lu, G. Double Shell Cu2O Hollow Microspheres as Sensing Material for High Performance n-Propanol Sensor. Sens. Actuators B Chem. 2021, 333, 129540. [Google Scholar] [CrossRef]
- Mokoena, T.P.; Swart, H.C.; Hillie, K.T.; Tshabalala, Z.P.; Jozela, M.; Tshilongo, J.; Motaung, D.E. Enhanced Propanol Gas Sensing Performance of P-Type NiO Gas Sensor Induced by Exceptionally Large Surface Area and Crystallinity. Appl. Surf. Sci. 2022, 571, 151121. [Google Scholar] [CrossRef]
- Luo, Y.; Ly, A.; Lahem, D.; Martin, J.D.M.; Romain, A.-C.; Zhang, C.; Debliquy, M. Role of Cobalt in Co-ZnO Nanoflower Gas Sensors for the Detection of Low Concentration of VOCs. Sens. Actuators B Chem. 2022, 360, 131674. [Google Scholar] [CrossRef]
- Luo, Y.; Ly, A.; Lahem, D.; Zhang, C.; Debliquy, M. A Novel Low-Concentration Isopropanol Gas Sensor Based on Fe-Doped ZnO Nanoneedles and Its Gas Sensing Mechanism. J. Mater. Sci. 2021, 56, 3230–3245. [Google Scholar] [CrossRef]
- Hsieh, Y.-C.; Yao, D.-J. Intelligent Gas-Sensing Systems and Their Applications. J. Micromech. Microeng. 2018, 28, 093001. [Google Scholar] [CrossRef]
- Broza, Y.Y.; Haick, H. Nanomaterial-Based Sensors for Detection of Disease by Volatile Organic Compounds. Nanomedicine 2013, 8, 785–806. [Google Scholar] [CrossRef]
- Güntner, A.T.; Pineau, N.J.; Mochalski, P.; Wiesenhofer, H.; Agapiou, A.; Mayhew, C.A.; Pratsinis, S.E. Sniffing Entrapped Humans with Sensor Arrays. Anal. Chem. 2018, 90, 4940–4945. [Google Scholar] [CrossRef]
- Tarca, A.L.; Carey, V.J.; Chen, X.; Romero, R.; Drăghici, S. Machine Learning and Its Applications to Biology. PLoS Comput. Biol. 2007, 3, e116. [Google Scholar] [CrossRef]
- Saha, P.; Ghorai, S.; Tudu, B.; Bandyopadhyay, R.; Bhattacharyya, N. Optimization of Sensor Array in Electronic Nose by Combinational Feature Selection Method. In Sensing Technology: Current Status and Future Trends II; Mason, A., Mukhopadhyay, S.C., Jayasundera, K.P., Bhattacharyya, N., Eds.; Smart Sensors, Measurement and Instrumentation; Springer International Publishing: Cham, Switzerland, 2014; pp. 189–205. [Google Scholar]
- Gardner, J.W.; Boilot, P.; Hines, E.L. Enhancing Electronic Nose Performance by Sensor Selection Using a New Integer-Based Genetic Algorithm Approach. Sens. Actuators B Chem. 2005, 106, 114–121. [Google Scholar] [CrossRef]
- Yan, J.; Guo, X.; Duan, S.; Jia, P.; Wang, L.; Peng, C.; Zhang, S. Electronic Nose Feature Extraction Methods: A Review. Sensors 2015, 15, 27804–27831. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Akamatsu, T.; Tsuruta, A.; Shin, W. Selective Detection of Target Volatile Organic Compounds in Contaminated Humid Air Using a Sensor Array with Principal Component Analysis. Sensors 2017, 17, 1662. [Google Scholar]
- Li, W.; Jia, Z.; Xie, D.; Chen, K.; Cui, J.; Liu, H. Recognizing Lung Cancer Using a Homemade E-Nose: A Comprehensive Study. Comput. Biol. Med. 2020, 120, 103706. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Alwadie, A.S.; Irfan, M.; Nawaz, R.; Raza, M.; Javed, E.; Awais, M. Wireless E-Nose Sensors to Detect Volatile Organic Gases through Multivariate Analysis. Micromachines 2020, 11, 597. [Google Scholar] [CrossRef] [PubMed]
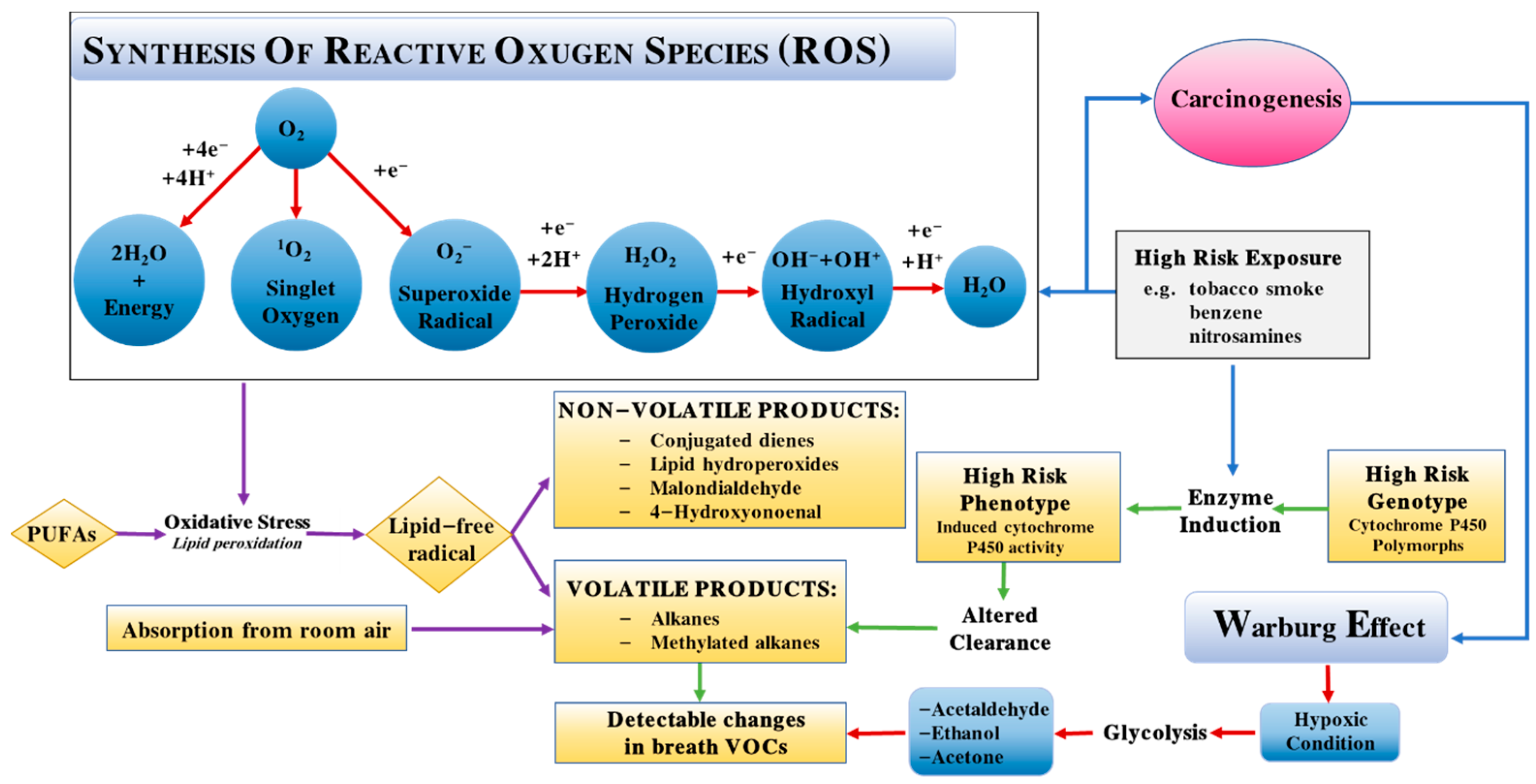

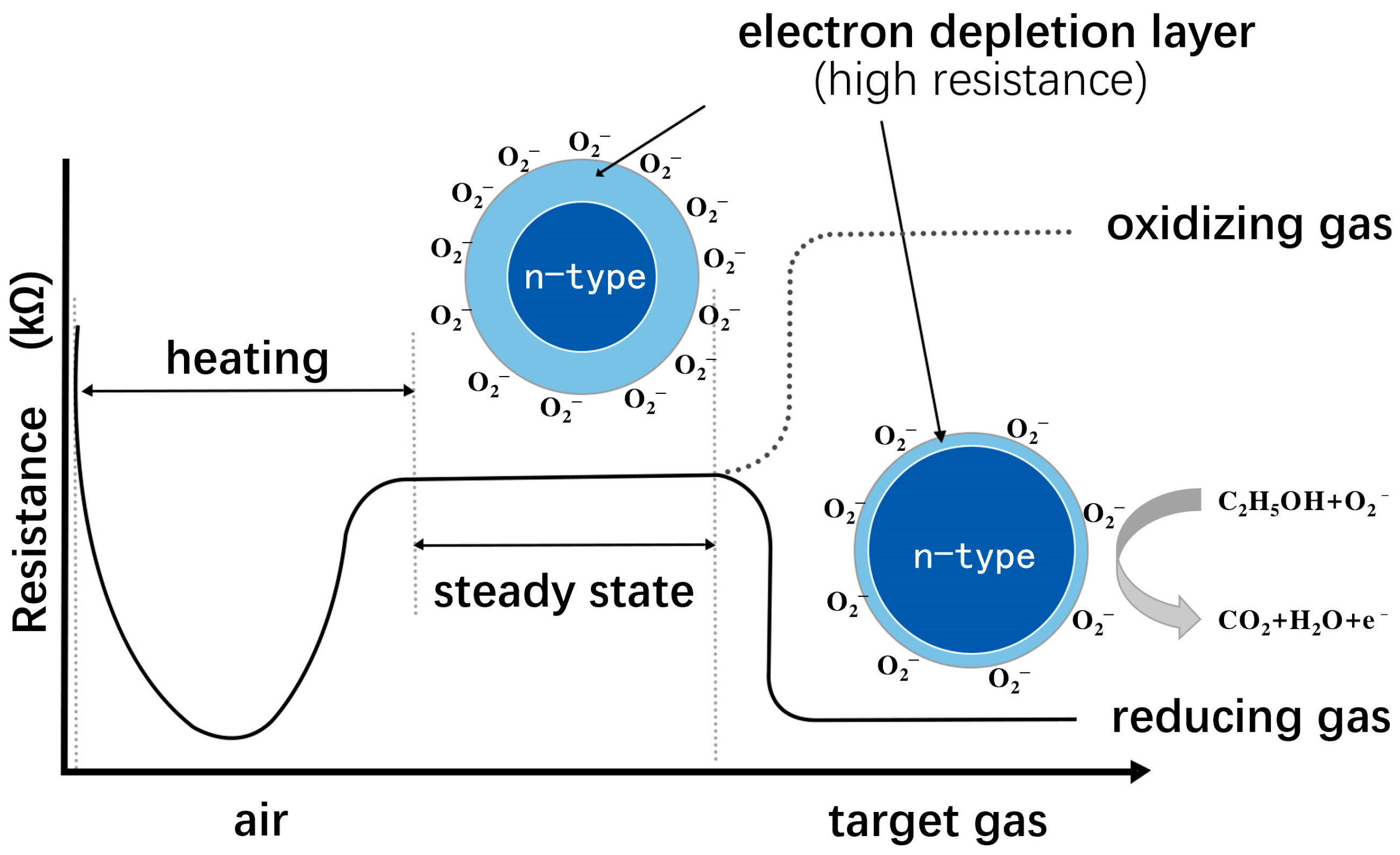
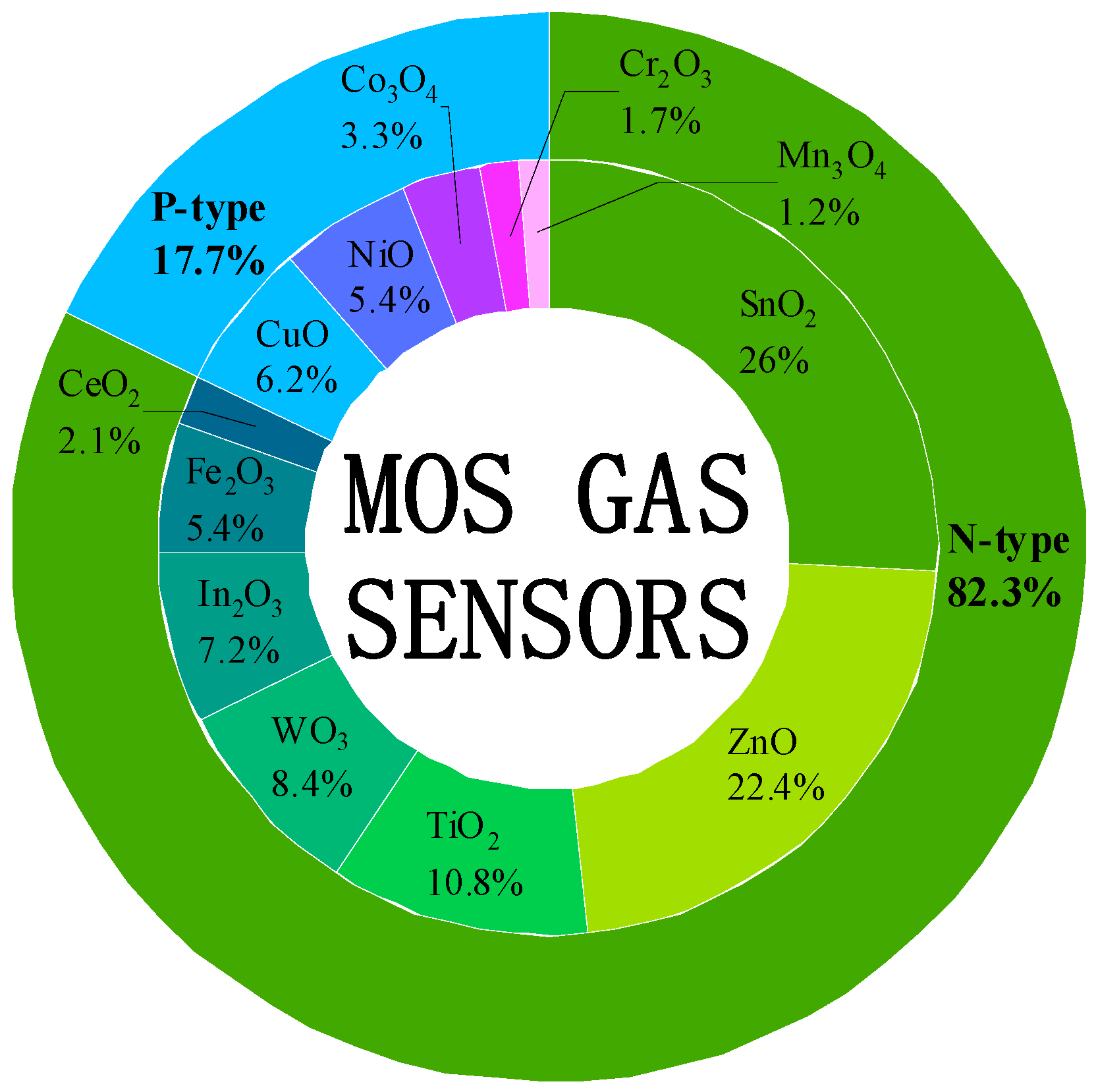

| Target Gas | Material and Structure | Concentration | Temperature (°C) | Response | Res/Rec Time (s) | LOD | Interference Gas | Ref. |
|---|---|---|---|---|---|---|---|---|
| Benzene | Co-Al2O3 | 5/10/50 ppm | 100/100/100 | 1.66/2.53/21.86 | 1.95/2.18, 2.23/2.59, 2.87/3.15 | - | - | [151] |
| Benzene | 2Rh-TiO2/SnO2, dual- layer sensor | 5 ppm | 325 | 35 | - | - | - | [129] |
| Toluene | WO3 mesoporous nanofibers | 1 ppm | 350 | 11 | 8.56/9.2 | 100 ppb | H2, H2S, CO, ethanol, NH3, CH4 | [152] |
| Toluene | ln2O3 | 50 ppm | 27 | 9 | 26/28 | - | methanol, ethanol, acetone, n-butanol, and benzene | [153] |
| Toluene | WO3 porous nanostructure | 100 ppm | 225 | 132 | 2/6 | - | methanol, acetone, glycol, formaldehyde, ethanol, C2H2, NH3, NO2, and CO | [154] |
| Toluene | Pd-SnO2 CNPs | 1 ppb | 250 | 3 | - | 200 ppt | O2, H2, N2 | [124] |
| Toluene | Pd-SnO2 CNPs | 7.9 ppb | 250 | 15 | - | 7 ppt | Air | [125] |
| Toluene | 1Rh-TiO2/SnO2, dual- layer | 5 ppm | 325 | 103 | - | - | - | [129] |
| Toluene | SnO2@SnO2 yolk-shell cuboctahedra | 20 ppm | 250 | 28.6 | 1.8/4.1 | - | benzene, methanol, acetone, and ethanol | [120] |
| Toluene | Three-, two-, one-layer hollow cubic SnO2 | 20 ppm | 250 | 38.7, 33.4, 22.1 | 0.8/6.1, 2.3/5.8, 2.0/6.5 | - | - | [121] |
| Toluene | Pd-loaded SnO2 cubic cages | 20 ppm | 230 | 41.4 | 0.4/16.5 | 100 ppb | - | [119] |
| Toluene | SnO2/NiO nanoparticle | 100 ppm | 250 | 66.2 | - | 10 ppb | - | [155] |
| Xylene | 0.5Rh-TiO2/SnO2, dual-layer | 5 ppm | 325 | 120 | - | - | - | [129] |
| Xylene | Pt/SnO2 nanosheet flowers | 200 ppm | 200 | 154 | 29/47 | - | - | [156] |
| Xylene | Co3O4-SnO2 hollow nanostructures | 5 ppm | 275 | 18.6 | 243/- | - | ethanol, toluene | [157] |
| Xylene | CoWO4-Co3O4 heterojunctions composites | 100 ppm | 200 | 51.6 | - | 300 ppb | ethanol, methanol, formaldehyde, benzene, toluene, acetone, NH3, NO2, H2O | [158] |
| Styrene | Pt-SnO2/α-Fe2O3 hollow nanospheres | 1 ppm | 206 | 10.56 | 3/15 | 50 ppb | - | [159] |
| Isoprene | In2O3 nanoflowers | 500 ppb | 190 | 3.1 | 53/299 | 5 ppb | NH3, ethanol, H2, CO | [160] |
| Isoprene | ZnO quantum dots | 1 ppm | 350 | 42 | 42/8 | 10 ppb | - | [161] |
| Isoprene | In2O3/nanoparticles | 1 ppm | 350 | 231 | 3/35–200 | 1 ppb | acetone, H2, CO2, CO, CH4, | [162] |
| Isoprene | Pt-decorated In2O3/microspheres | 5 ppm | 200 | 103.5 | 124/204 | 5 ppb | H2O, CO, H2, ethanol, ammonia | [144] |
| Isoprene | 1 wt%Cr2O3/In2O3 nanorods clusters | 500 ppb | 240 | 1.9 | 135/830 | 5 ppb | benzene, acetone, octane, pentane, ethanol, NH3, NO2 | [163] |
| Hexanal | MnO2/Ti3C2Tx | 20 ppm | 100 | 52 | 134/381 | - | - | [164] |
| Hexanal | In2O3 nanoparticle | 50 ppm | 300 | 18 | - | - | - | [165] |
| Hexanal | CuO nanoflake | 200 ppm | 250 | 3.7 | - | 1.85 ppm | linalool, methyl salicylate | [166] |
| Hexanal | ZnO nanoparticle | 5 ppm | 250 | 2.12 | - | - | 1-pentanol, 1-octen-3-ol | [167] |
| Nonanal | Ru-W18049 urchin-like | 30 ppm | RT | 16.1 | 25/154 | - | SO2, H2S, CO, NH3, ethanol, acetone, | [168] |
| Nonanal | Sb2WO6 hierarchical microspheres | 30 ppm | RT | 62 | 32/145 | 1.6 ppm | C8H16O, C9H14O, C6H12O, C10H18O | [169] |
| Nonanal | SnO2 nanosheets | 0.1/0.3 ppm | 250 | 1.383/2 | - | - | CO, NO2, acetone, H2, ethanol, NH3, H2S, formaldehyde, acetaldehyde, butanal | [170] |
| Butanone | Ce-SnO2 cuboids | 20 ppm | 175 | 23.9 | 20/- | 500 ppb | ethanol, toluene, acetone | [171] |
| Butanone | Pt-ZnO twin-rods | 100 ppm | 450 | 35.3 | 8/- | - | - | [172] |
| Butanone | Cr2O3/WO3 nanosheets | 100 ppm | 180 | 40.51 | 9/15 | - | - | [173] |
| Butanone | 1 at% Ce-SnO2 thin films | 100 ppm | 210 | 181 | - | - | - | [174] |
| Butanone | ZnO small size | 100 ppm | 350 | 151 | 4.5/5 | 200 ppb | chlorobenzene, vinyl benzene, xylene, toluene, benzene, acetaldehyde, formaldehyde | [175] |
| Butanone | WO3 urchin-like mesoporous | 50 ppm | 240 | 188.5 | 7/13 | 100 ppb | - | [176] |
| Butanone | Ag-modified NiO porous spherical | 100 ppb | 320 | 3.2 | 5.5/8 | 50 ppb | Formaldehyde, methanol, acetone, acetaldehyde | [177] |
| Acetone | Ru-NiO flower-like microspheres | 100 ppm | 200 | 12 | 71/23 | - | ethanol, methanol, formaldehyde, benzene | [178] |
| Acetone | TiO2/SnO2 | 100 ppm | 300 | 301.5 | - | 20 ppb | ethanol, acetone, NO2 | [179] |
| Acetone | PtCu-SnO2 | 5 ppm | 240 | 27.8 | - | 5 ppb | ethanol, toulene, pentane | [180] |
| Acetone | Pt-ZnO-SnO2 porous nanofibers | 100 ppm | 170 | 104.26 | - | - | C7H8, benzene, C3H6O | [181] |
| 1-Propanol | Co-ZnO nanorods | 100 ppm | 250 | 491 | 2/19 | 10 ppb | formaldehyde, methyl alcohol, ethanol, triethylamine, 2-Propanol, benzene, ammonia, glacial acetic acid, formic acid | [182] |
| 1-Propanol | ZnSnO3 nanospheres | 10 ppm | 200 | 10.3 | 10/90 | 500 ppb | acetone, xylene, ammonia, hydrogen, methane | [183] |
| 1-Propanol | ZnO/NiO one-dimensional chain MOF | 500 ppm | 275 | 280.2 | 31.5/18.2 | 200 ppb | methanol, ethanol, isopropanol, hexanol, acetone | [184] |
| 1-Propanol | PdO-ZnSnO3 hollow microspheres | 100 ppm | 140 | 30.8 | 1/25 | - | formaldehyde, ethanol, acetone, xylene, methanol, ammonia | [185] |
| 1-Propanol | ZnO nanoparticles | 40 ppm | 125 | 6.6 | 190/200 | - | H2O, ethanol, acetone, benzene, toluene | [186] |
| 1-Propanol | Cu2O double-shell hollow microspheres | 100 ppm | 187 | 11 | 50/40 | 10 ppm | acetone, carbon monoxide, ethyne, formaldehyde, isopropanol, ethanol, methanol | [187] |
| 1-Propanol | NiO porous nanoparticles | 20 ppb | 75 | 1.59 | - | 20 ppb | ethanol, propanol, toluene, methane, NO2 | [188] |
| 2-Propanol | 10 at% Co-ZnO nanoflower | 5 ppm | 225 | 22.5 | 330/475 | / | N2, O2, CO2, acetaldehyde, isoprene, ethanol, acetone, methanol | [189] |
| 2-Propanol | Fe-doped ZnO | 250 ppb | 275 | 4.7 | 51/762 | 250 ppb | H2O, ethanol, acetone, methanol | [190] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Zhu, X.; Liu, J.; Li, S.; Liu, X. Metal Oxide Semiconductor Gas Sensors for Lung Cancer Diagnosis. Chemosensors 2023, 11, 251. https://doi.org/10.3390/chemosensors11040251
Li G, Zhu X, Liu J, Li S, Liu X. Metal Oxide Semiconductor Gas Sensors for Lung Cancer Diagnosis. Chemosensors. 2023; 11(4):251. https://doi.org/10.3390/chemosensors11040251
Chicago/Turabian StyleLi, Guangyao, Xitong Zhu, Junlong Liu, Shuyang Li, and Xiaolong Liu. 2023. "Metal Oxide Semiconductor Gas Sensors for Lung Cancer Diagnosis" Chemosensors 11, no. 4: 251. https://doi.org/10.3390/chemosensors11040251
APA StyleLi, G., Zhu, X., Liu, J., Li, S., & Liu, X. (2023). Metal Oxide Semiconductor Gas Sensors for Lung Cancer Diagnosis. Chemosensors, 11(4), 251. https://doi.org/10.3390/chemosensors11040251






