Gold Nanocluster-Based Fluorescent Sensor Array for Antibiotic Sensing and Identification
Abstract
1. Introduction
2. Experiment
2.1. Reagents and Materials
2.2. Instrumentation
2.3. Synthesis of MUA-AuNCs
2.4. Preparation of MUA-AuNCs-Re3+ Fluorescent Sensor Array
2.5. Experimental Procedure of MUA-AuNCs-Re3+ Fluorescent Sensor Array for the Discrimination and Quantification of Antibiotics
2.5.1. Use Sensor Array to Distinguish Different Antibiotics
2.5.2. Response of the Sensor Array to Different Interfering Substances
2.5.3. Quantitative Detection of Different Antibiotics by the Sensor Array
2.5.4. Discrimination of Antibiotic Binary Mixtures by the Sensor Array
2.5.5. Blind Sample Detection
2.6. Data Processing and Analysis
3. Results and Discussion
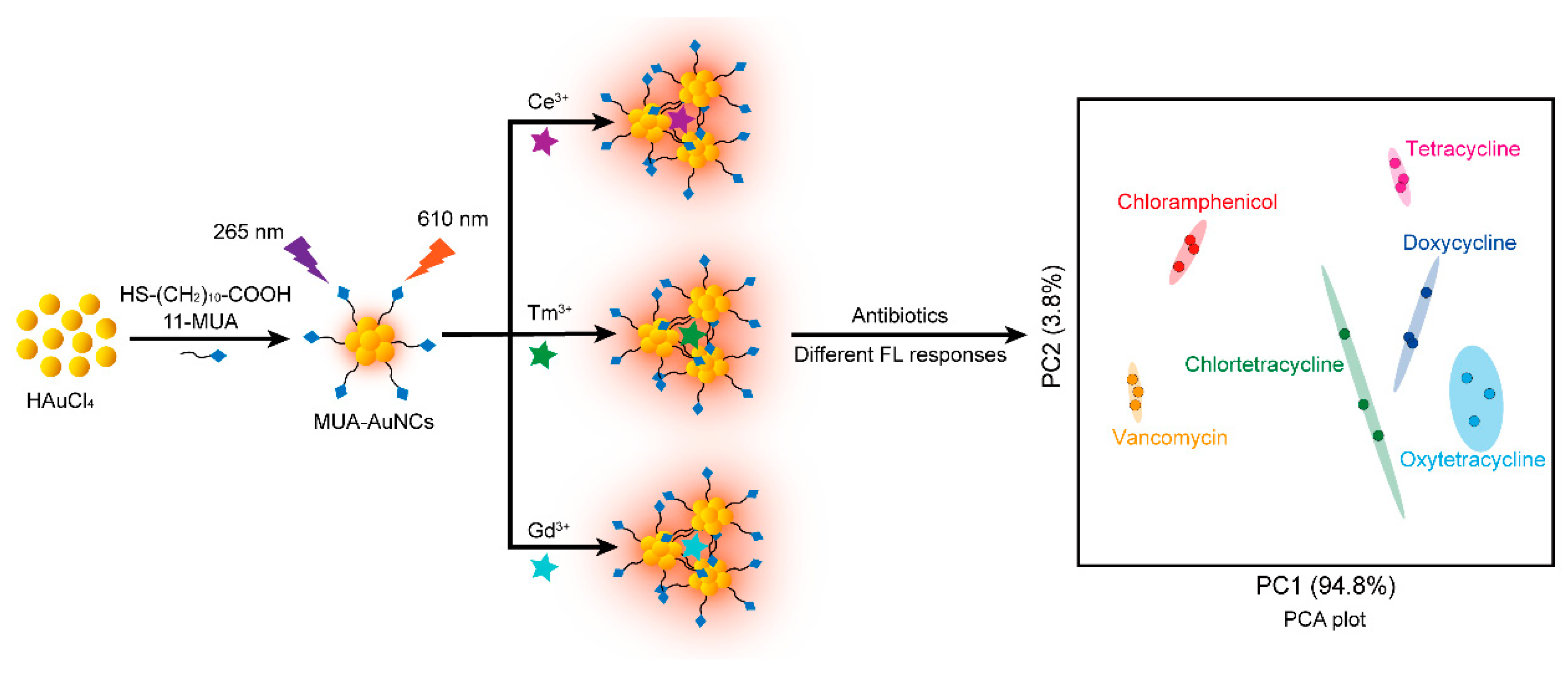
3.1. Design and Construction of MUA-AuNCs-Re3+ Fluorescent Sensor Arrays for the Detection of Antibiotics
3.2. Optimization of the Experimental Conditions
3.3. The Possible Mechanism of Sensor Array
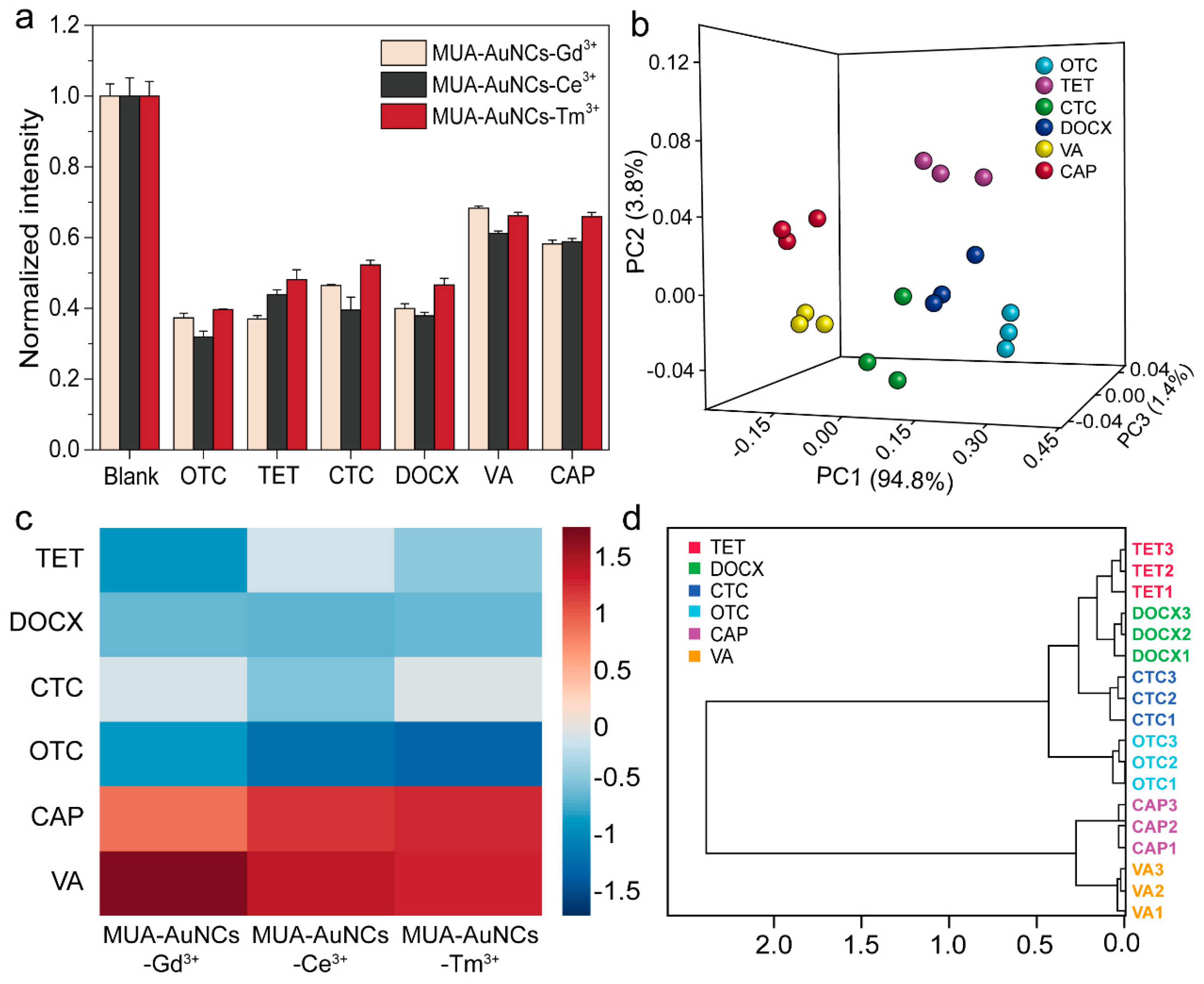
3.4. Discrimination of Antibiotics by Sensor Array
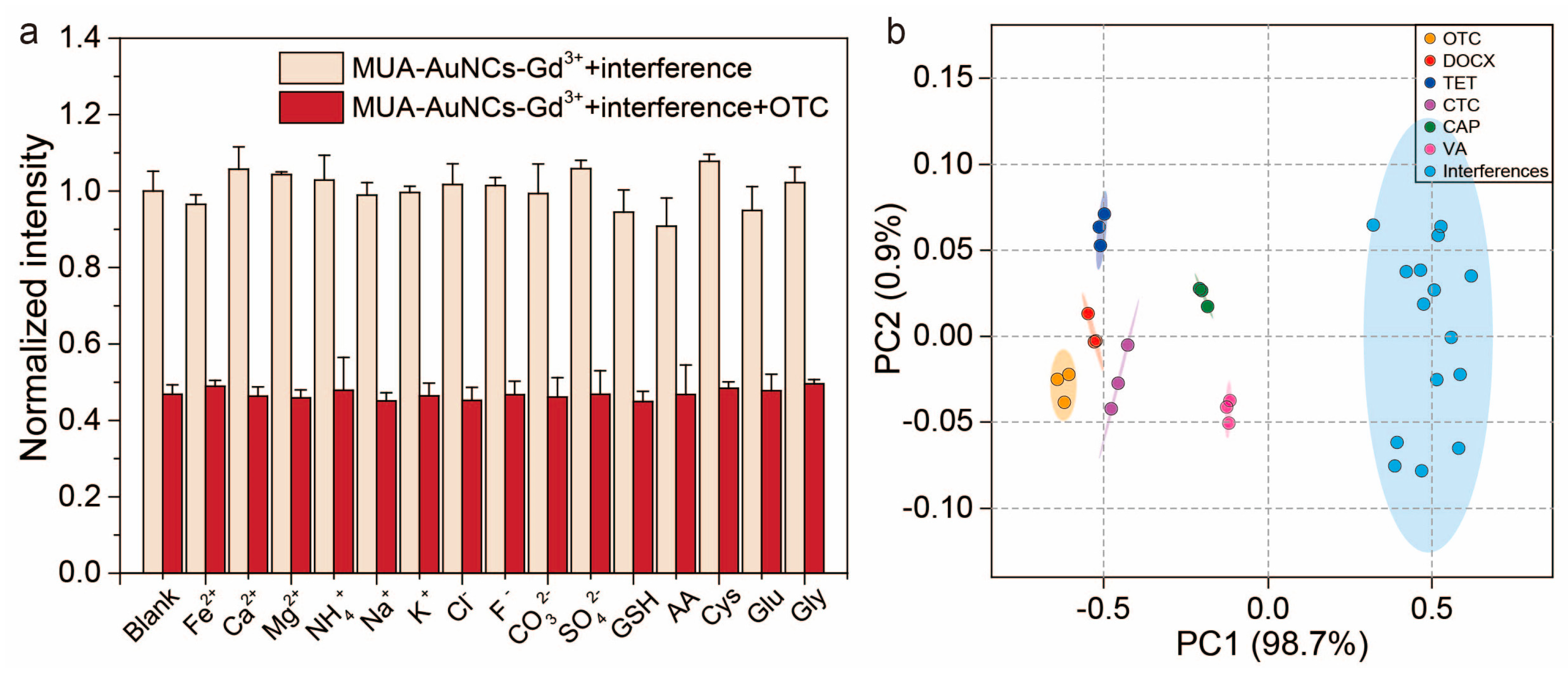
3.5. Response of Sensor Array to Interfering Substances
3.6. Quantitative Detection of Different Antibiotics by Sensor Array
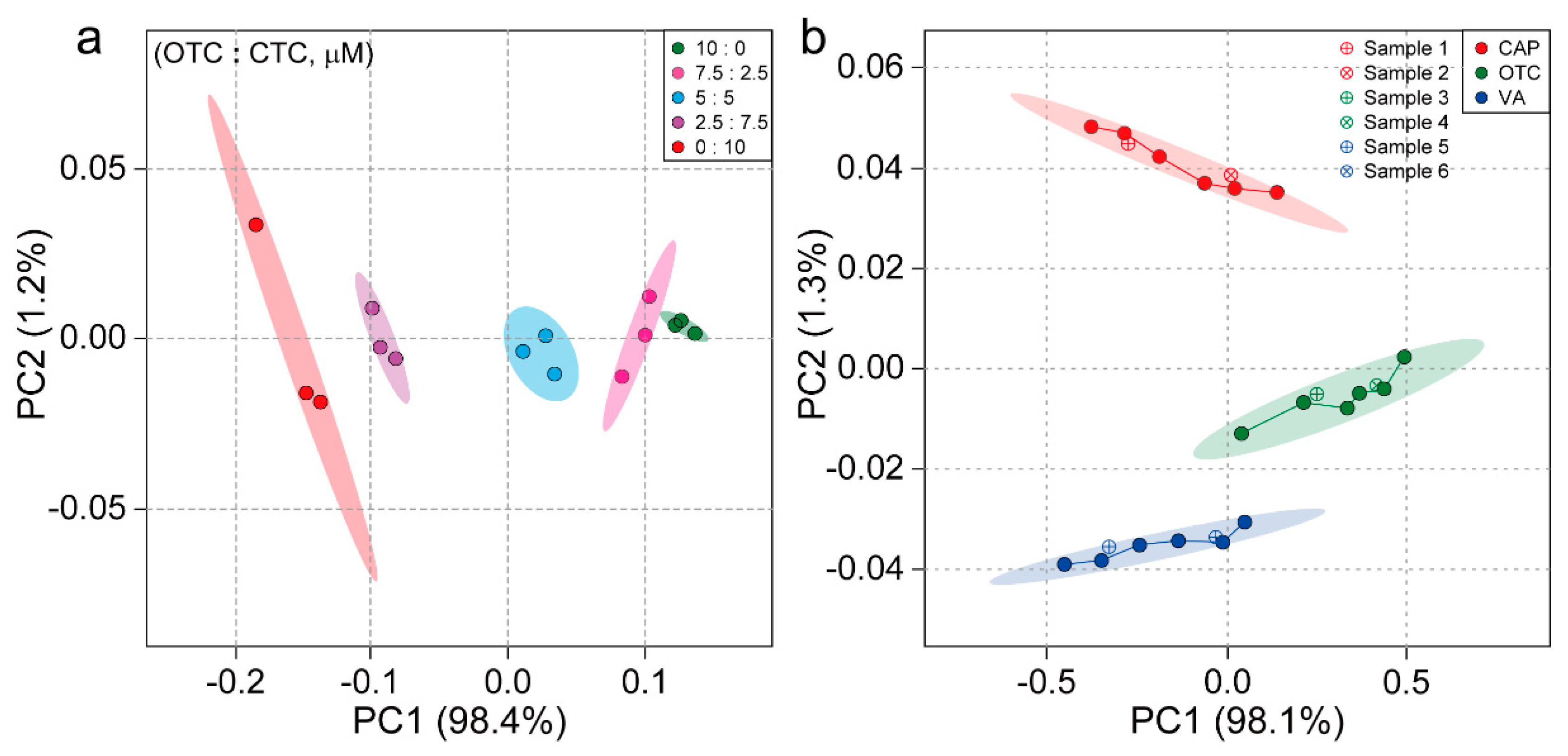
3.7. Discrimination of Antibiotic Binary Mixtures and Blind Samples by Sensor Arrays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Michalova, E.; Novotna, P.; Schlegelova, J. Tetracyclines in veterinary medicine and bacterial resistance to them. Vet. Med. 2004, 49, 79–100. [Google Scholar] [CrossRef]
- Bahrami, F.; Morris, D.L.; Pourgholami, M.H. Tetracyclines: Drugs with huge therapeutic potential. Mini Rev. Med. Chem. 2012, 12, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.F.; Wei, T.T.; Jiang, M.D.; Xu, L.H.; Xu, Z.X. Voltammetric sensor for chloramphenicol determination based on a dual signal enhancement strategy with ordered mesoporous carbon@polydopamine and beta-cyclodextrin. Sens. Actuat. B Chem. 2018, 255, 2155–2162. [Google Scholar] [CrossRef]
- Mu, F.Y.; He, J.Q.; Fan, F.; Shi, G.Y. Dual-emission fluorescence biosensing of vancomycin based on AIEgen-peptide conjugates and aptamer-modified Au nanoclusters. Anal. Chim. Acta 2021, 1150, 238177. [Google Scholar] [CrossRef]
- Qiao, M.; Ying, G.G.; Singer, A.C.; Zhu, Y.G. Review of antibiotic resistance in China and its environment. Environ. Int. 2018, 110, 160–172. [Google Scholar] [CrossRef]
- Tuc, D.Q.; Alliot, F.; Moreau-Guigon, E.; Eurin, J.; Chevreuil, M.; Labadie, P. Measurement of trace levels of antibiotics in river water using on-line enrichment and triple-quadrupole LC-MS/MS. Talanta 2011, 85, 1238–1245. [Google Scholar]
- Verlicchi, P.; Al Aukidy, M.; Jelic, A.; Petrovic, M.; Barcelo, D. Comparison of measured and predicted concentrations of selected pharmaceuticals in wastewater and surface water: A case study of a catchment area in the Po Valley (Italy). Sci. Total Environ. 2014, 470, 844–854. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, L.X.; Lu, Z.H.; He, C.M.; Li, Q.W.; Na, G.S. Complex migration of antibiotic resistance in natural aquatic environments. Environ. Pollut. 2018, 232, 1–9. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Hu, H.W.; Gou, M.; Wang, J.T.; Chen, D.L.; He, J.Z. Temporal succession of soil antibiotic resistance genes following application of swine. cattle and poultry manures spiked with or without antibiotics. Environ. Pollut. 2017, 231, 1621–1632. [Google Scholar] [CrossRef]
- Wright, G.D. Antibiotic resistance in the environment: A link to the clinic? Curr. Opin. Microbiol. 2010, 13, 589–594. [Google Scholar] [CrossRef]
- Ben, Y.J.; Fu, C.X.; Hu, M.; Liu, L.; Wong, M.H.; Zheng, C.M. Human health risk assessment of antibiotic resistance associated with antibiotic residues in the environment: A review. Environ. Res. 2019, 169, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.; Lv, J.; Zhang, H.C.; Zhang, B.; Wei, W.L.; Qiao, Y. Photoresponsive colorimetric immunoassay based on chitosan modified AgI/TiO2 heterojunction for highly sensitive chloramphenicol detection. Biosens. Bioelectron. 2017, 87, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Kargin, I.D.; Sokolova, L.S.; Pirogov, A.V.; Shpigun, O.A. HPLC determination of tetracycline antibiotics in milk with post-column derivatization and fluorescence detection. Inorg. Mater. 2016, 52, 1365–1369. [Google Scholar] [CrossRef]
- Bol, D.S.; Amelin, V.G.; Nikeshina, T.B. Determination of antibiotics in drugs and biological fluids using capillary electrophoresis. J. Anal. Chem. 2016, 71, 215–233. [Google Scholar]
- Shen, L.; Chen, J.; Li, N.; He, P.L.; Li, Z. Rapid colorimetric sensing of tetracycline antibiotics with in situ growth of gold nanoparticles. Anal. Chim. Acta 2014, 839, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Chafer-Pericas, C.; Maquicira, A.; Puchades, R.; Company, B.; Miralles, J.; Moreno, A. Multiresidue determination of antibiotics in aquaculture fish samples by HPLC-MS/MS. Aquac. Res. 2010, 41, e217–e225. [Google Scholar] [CrossRef]
- Jia, Q.; Liu, Y.; Duan, Y.; Zhou, J. Interfere-free detection of hydroxyl radical and arthritis diagnosis by rare-earth nanoprobe utilizing SWIR emission as reference. Anal. Chem. 2019, 97, 11433–11439. [Google Scholar] [CrossRef]
- Jia, Q.; Ma, L.; Zhai, X.; Fu, W.; Liu, Y.; Liao, X.; Zhou, J. Orthogonal NIR-II imaging enables spatially distinguishing tissues based on lanthanide-doped nanoprobes. Anal. Chem. 2020, 92, 14762–14768. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, Q.; Zhai, X.; Mao, F.; Jiang, A.; Zhou, J. Rationally Designed Pure-inorganic Upconversion Nanoprobes for Ultra-highly Selective Hydrogen Sulfide Imaging and Elimination In Vivo. Chem. Sci. 2019, 10, 1193–1200. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, T.L.; Zhao, M.Y.; Wang, P.X.; Hao, Z.; Lin, X.D.; Wang, S.; Liu, Y.Q. Chemical-tongue sensor array for determination of multiple metal ions based on trichromatic lanthanide-based nanomaterials. Sens. Actuators B Chem. 2021, 343, 130107. [Google Scholar] [CrossRef]
- Huang, C.Y.; Luo, Y.X.; Li, J.C.; Liu, C.; Zhou, T.S.; Deng, J.J. pH-regulated H4TCPE@Eu/AMP ICP sensor array and its fingerprinting on test papers: Toward point-of-use systematic analysis of environmental antibiotics. Anal. Chem. 2021, 93, 9183–9192. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Jiang, K.; Qian, S.H.; Wang, Y.H.; Lin, H.W. Applying carbon dots-metal ions ensembles as a multichannel fluorescent sensor array: Detection and discrimination of phosphate anions. Anal. Chem. 2017, 89, 5542–5548. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.C. Quantum sized. thiolate-protected gold nanoclusters. Nanoscale 2010, 2, 343–362. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Zeng, C.J.; Zhou, M.; Jin, R.C. Surface engineering of Au36(SR)24 nanoclusters for photoluminescence enhancement. Part. Part. Syst. Charact. 2017, 34, 1600388. [Google Scholar] [CrossRef]
- Yuan, X.; Luo, Z.T.; Yu, Y.; Yao, Q.F.; Xie, J.P. Luminescent noble metal nanoclusters as an emerging optical probe for sensor development. Chem. Asian J. 2013, 8, 858–871. [Google Scholar] [CrossRef]
- Tan, X.P.; Zhang, Z.; Cao, T.W.; Zeng, W.J.; Huang, T.; Zhao, G.F. Control Assembly of Pillar[6]arene-Modified Ag Nanoparticles on Covalent Organic Framework Surface for Enhanced Sensing Performance toward Paraquat. ACS Sustain. Chem. Eng. 2020, 7, 20051–20059. [Google Scholar] [CrossRef]
- Wang, H.H.; Lin, C.A.; Lee, C.H.; Lin, Y.C.; Tseng, Y.M.; Hsieh, C.L.; Chen, C.H.; Tsai, C.H.; Hsieh, C.T.; Shen, J.L.; et al. Fluorescent gold nanoclusters as a biocompatible marker for in vitro and in vivo tracking of endothelial cells. ACS Nano 2011, 5, 4337–4344. [Google Scholar] [CrossRef]
- Cantelli, A.; Battistelli, G.; Guidetti, G.; Manzi, J.; Di Giosia, M.; Montalti, M. Luminescent gold nanoclusters as biocompatible probes for optical imaging and theranostics. Dyes Pigments 2016, 135, 64–79. [Google Scholar] [CrossRef]
- Tao, Y.; Li, M.Q.; Ren, J.S.; Qu, X.G. Metal nanoclusters: Novel probes for diagnostic and therapeutic applications. Chem. Soc. Rev. 2015, 44, 8636–8663. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Wang, B.; Wang, K.; Yan, P. Identification of proteins and bacteria based on a metal ion-gold nanocluster sensor array. Anal. Methods 2018, 10, 3939–3944. [Google Scholar] [CrossRef]
- Yang, H.W.; Lu, F.N.; Sun, Y.; Yuan, Z.Q.; Lu, C. Fluorescent gold nanocluster-based sensor array for nitrophenol isomer discrimination via an integration of host-guest interaction and inner filter effect. Anal. Chem. 2018, 90, 12846–12853. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Mei, Q.S.; Zhang, K.; Zhang, Z.P. Protein-gold nanoclusters for identification of amino acids by metal ions modulated ratiometric fluorescence. Analyst 2012, 137, 1618–1623. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.Z.; Lin, L.P.; Rong, M.C.; Chen, X. A cross-reactive sensor array for the fluorescence qualitative analysis of heavy metal ions. Talanta 2014, 129, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.N.; Li, C.M.; Gao, B.H.; Kargbo, O.; Wan, N.; Chen, X.; Zhou, C. Probing phosphate ion via the europium(III)-modulated fluorescence of gold nanoclusters. Microchim. Acta 2014, 181, 1957–1963. [Google Scholar] [CrossRef]
- Wang, J.X.; Lin, X.F.; Shu, T.; Su, L.; Liang, F.; Zhang, X.J. Self-assembly of metal nanoclusters for aggregation-induced emission. Int. J. Mol. Sci. 2019, 20, 1891. [Google Scholar] [CrossRef]
- Shu, T.; Wang, J.X.; Su, L.; Zhang, X.J. Luminescent organometallic nanomaterials with aggregation-induced emission. Crit. Rev. Anal. Chem. 2018, 48, 330–336. [Google Scholar] [CrossRef]
- Zhang, Y.; Lv, M.; Gao, P.F.; Zhang, G.M.; Shi, L.H.; Yuan, M.J.; Shuang, S.M. The synthesis of high bright silver nanoclusters with aggregation-induced emission for detection of tetracycline. Sens. Actuators B Chem. 2021, 326, 129009. [Google Scholar] [CrossRef]
- Chen, B.N.; Yang, Z.S.; Qu, X.L.; Zheng, S.R.; Yin, D.Q.; Fu, H.Y. Screening and discrimination of perfluoroalkyl substances in aqueous solution using a luminescent metal-organic framework sensor array. ACS Appl. Mater. Interfaces 2021, 13, 47706–47716. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, M.; Yuan, H.; Zhang, J.; Wang, J.; Liu, Y.; Peng, J.; Zhao, L. Gold Nanocluster-Based Fluorescent Sensor Array for Antibiotic Sensing and Identification. Chemosensors 2023, 11, 330. https://doi.org/10.3390/chemosensors11060330
Mo M, Yuan H, Zhang J, Wang J, Liu Y, Peng J, Zhao L. Gold Nanocluster-Based Fluorescent Sensor Array for Antibiotic Sensing and Identification. Chemosensors. 2023; 11(6):330. https://doi.org/10.3390/chemosensors11060330
Chicago/Turabian StyleMo, Mengjiao, Haoyi Yuan, Jingyu Zhang, Jian Wang, Ying Liu, Juanjuan Peng, and Lingzhi Zhao. 2023. "Gold Nanocluster-Based Fluorescent Sensor Array for Antibiotic Sensing and Identification" Chemosensors 11, no. 6: 330. https://doi.org/10.3390/chemosensors11060330
APA StyleMo, M., Yuan, H., Zhang, J., Wang, J., Liu, Y., Peng, J., & Zhao, L. (2023). Gold Nanocluster-Based Fluorescent Sensor Array for Antibiotic Sensing and Identification. Chemosensors, 11(6), 330. https://doi.org/10.3390/chemosensors11060330




