Graphene-Based Electrodes for Monitoring of Estradiol
Abstract
1. Introduction
| Modifier/Electrode | Detection Technique | Linear Range (μM) | Limit of Detection (nM) | Ref. |
|---|---|---|---|---|
| Anti-E1/polyaniline (PANI)/graphene/SPE | EIS a | 0.37–0.76 | 0.0072 | [29] |
| Graphene quantum dots with poly-sulfosalicylic (PSSA/GO)/GCE | DPV b | 0.001–6.0 | 0.23 | [30] |
| Reduced graphene oxide/molecularly imprinted polymer/GCE | DPV | 0.16–15 | 27 | [31] |
| Reduced graphene oxide/di-hexadecyl phosphate/GCE | LSV c | 0.4–10 | 77 | [32] |
| Fe3O4 nanobeads/graphene-based molecularly imprinted polymer/GCE | DPV | 0.05–10 | 0.819 | [33] |
| Gold nanoparticle/graphene/molecularly imprinted polymer/GCE | DPV | 0.003–1 | 1 | [34] |
| Ultrasonicated exfoliated graphene in N-methyl-2-pyrrolidone/GCE | DPV | 0.01–15 | 4.9 | [35] |
| Reduced graphene oxide-platinum nanoparticles/MIP/GCE | DPV | 0.004–0.06 | 2 | [36] |
| Reduced graphene oxide on metallic Cu (II)-meso-tetra(thien-2-yl) porphyrin/GCE | DPV | 0.1–1.0 | 5.3 | [37] |
| Cysteamine/gold nanoparticle/fumed silica/graphene nanoribbon/GCE | DPV | 0.1–5.0 | 74 | [38] |
| Aptamer-reduced graphene oxide/GCE | EIS | 0.000012–0.00023 | 0.0005 | [39] |
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Experimental Instruments
2.3. Synthesis of Electrochemically Exfoliated Graphene (EEFGH) and Electrode Fabrication
3. Results and Discussion
3.1. Surface Characterization
3.2. Electrochemical Measurements
4. Electro-Oxidation Behavior of Estradiol
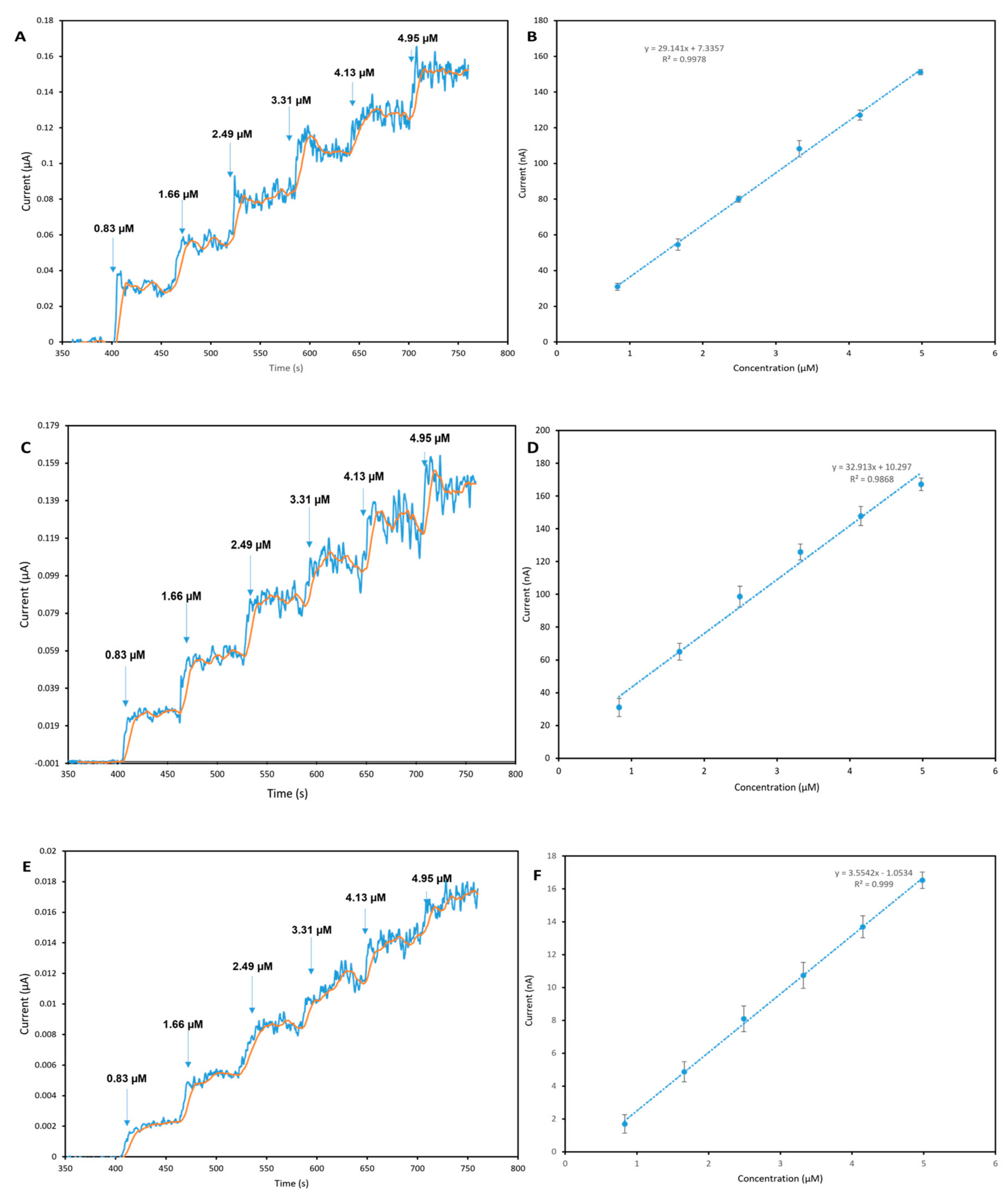
4.1. Amperometric Measurement and Calibration
4.2. Analytical Application
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Owen, R.; Jobling, S. The hidden costs of flexible fertility. Nature 2012, 485, 441. [Google Scholar] [CrossRef]
- Pu, H.; Huang, Z.; Sun, D.W.; Fu, H. Recent advances in the detection of 17β-estradiol in food matrices: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 2144–2157. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhou, Q.; Jiang, G. Nanomaterials for analysis and monitoring of emerging chemical pollutants. TrAC Trends Anal. Chem. 2014, 58, 10–22. [Google Scholar] [CrossRef]
- Adeel, M.; Song, X.; Wang, Y.; Francis, D.; Yang, Y. Environmental impact of estrogens on human, animal and plant life: A critical review. Environ. Int. 2017, 99, 107–119. [Google Scholar] [CrossRef]
- Li, C.; Wei, Y.; Zhang, S.; Tan, W. Advanced methods to analyse steroid estrogens in environmental samples. Environ. Chem. Lett. 2020, 18, 543–559. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, H.; Song, B.; Yuan, K.; Xiao, H.; Cao, Y.; Cao, Q. Metal–Organic Framework (MOF) Derivatives as Promising Chemiresistive Gas Sensing Materials: A Review. Int. J. Environ. Res. Public Health 2023, 20, 4388. [Google Scholar] [CrossRef] [PubMed]
- Musa, A.M.; Kiely, J.; Luxton, R.; Honeychurch, K.C. Recent progress in screen-printed electrochemical sensors and biosensors for the detection of estrogens. TrAC Trends Anal. Chem. 2021, 139, 116254. [Google Scholar] [CrossRef]
- Musa, A.M.; Kiely, J.; Luxton, R.; Honeychurch, K.C. An Electrochemical Screen-Printed Sensor Based on Gold-Nanoparticle-Decorated Reduced Graphene Oxide–Carbon Nanotubes Composites for the Determination of 17-β Estradiol. Biosensors 2023, 13, 491. [Google Scholar] [CrossRef]
- Butler, D.; Guilbault, G.G. Disposable amperometric immunosensor for the detection of 17-β estradiol using screen-printed electrodes. Sens. Actuators B Chem. 2006, 113, 692–699. [Google Scholar] [CrossRef]
- Alam, A.U.; Clyne, D.; Jin, H.; Hu, N.X.; Deen, M.J. Fully Integrated, Simple, and Low-Cost Electrochemical Sensor Array for in Situ Water Quality Monitoring. ACS Sens. 2020, 5, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Enache, T.A.; Oliveira-Brett, A.M. Phenol and para-substituted phenols electrochemical oxidation pathways. J. Electroanal. Chem. 2011, 655, 9–16. [Google Scholar] [CrossRef]
- Hashemnia, S.; Khayatzadeh, S.; Hashemnia, M. Electrochemical Detection of Phenolic Compounds Using Composite Film of Multiwall Carbon Nanotube/Surfactant/Tyrosinase on a Carbon Paste Electrode. J. Solid State Electrochem. 2012, 16, 473–479. [Google Scholar] [CrossRef]
- Brocenschi, R.F.; Rocha-Filho, R.C.; Duran, B.; Swain, G.M. The analysis of estrogenic compounds by flow injection analysis with amperometric detection using a boron-doped diamond electrode. Talanta 2014, 126, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.H.; Pineda, S.; Woo, Y.C.; Xie, M.; Murdock, A.T.; Ang, E.Y.M.; Jiao, Y.; Park, M.J.; Il Lim, S.; Lawn, M.; et al. Anti-fouling graphene-based membranes for effective water desalination. Nat. Commun. 2018, 9, 683. [Google Scholar] [CrossRef]
- Rowley-Neale, S.J.; Brownson, D.A.C.; Smith, G.; Banks, C.E. Graphene Oxide Bulk-Modified Screen-Printed Electrodes Provide Beneficial Electroanalytical Sensing Capabilities. Biosensors 2020, 10, 27. [Google Scholar] [CrossRef]
- Ferrand, A.; Siaj, M.; Claverie, J.P. Graphene, the Swiss Army Knife of Nanomaterials Science. ACS Appl. Nano Mater. 2020, 3, 7305–7313. [Google Scholar] [CrossRef]
- Randviir, E.P.; Brownson, D.A.C.; Metters, J.P.; Kadara, R.O.; Banks, C.E. The fabrication, characterisation and electrochemical investigation of screen-printed graphene electrodes. Phys. Chem. Chem. Phys. 2014, 16, 4598–4611. [Google Scholar] [CrossRef]
- Zhao, Q.; Faraj, Y.; Liu, L.Y.; Wang, W.; Xie, R.; Liu, Z.; Ju, X.J.; Wei, J.; Chu, L.Y. Simultaneous determination of dopamine, uric acid and estriol in maternal urine samples based on the synergetic effect of reduced graphene oxide, silver nanowires and silver nanoparticles in their ternary 3D nanocomposite. Microchem. J. 2020, 158, 105185. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Fal’Ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for grapheme. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2016, 306, 666–669. [Google Scholar] [CrossRef]
- Cinti, S.; Arduini, F. Graphene-based screen-printed electrochemical (bio)sensors and their applications: Efforts and criticisms, Biosens. Bioelectron 2017, 89, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Mattevi, C.; Eda, G.; Agnoli, S.; Miller, S.; Mkhoyan, K.A.; Celik, O.; Mastrogiovanni, D.; Granozzi, G.; Carfunkel, E.; Chhowalla, M. Evolution of electrical, chemical, and structural properties of transparent and conducting chemically derived graphene thin films. Adv. Funct. Mater. 2009, 19, 2577–2583. [Google Scholar] [CrossRef]
- Shivaraman, S.; Barton, R.A.; Yu, X.; Alden, J.; Herman, L.; Chandrashekhar, M.S.V.; Park, J.; McEuen, P.L.; Parpia, J.M.; Craighead, H.G.; et al. Free-standing epitaxial grapheme. Nano Lett. 2009, 9, 3100–3105. [Google Scholar] [CrossRef] [PubMed]
- Nuvoli, D.; Alzari, V.; Sanna, R.; Scognamillo, S.; Alongi, J.; Malucelli, G.; Mariani, A. Synthesis and characterization of graphene-based nanocomposites with potential use for biomedical applications. J. Nanopart. Res. 2013, 15, 1512. [Google Scholar] [CrossRef]
- Keeley, G.P.; O’Neill, A.; Holzinger, M.; Cosnier, S.; Coleman, J.N.; Duesberg, G.S. DMF-exfoliated graphene for electrochemical NADH detection. Phys. Chem. Chem. Phys. 2011, 13, 7747–7750. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.N. Liquid exfoliation of defect-free grapheme. Acc. Chem. Res. 2013, 46, 14–22. [Google Scholar] [CrossRef]
- Baradoke, A.; Pastoriza-Santos, I.; González-Romero, E. Screen-printed GPH electrode modified with Ru nanoplates and PoPD polymer film for NADH sensing: Design and characterization. Electrochim. Acta 2019, 300, 316–323. [Google Scholar] [CrossRef]
- Fan, X.; Xu, Y.; Sheng, T.; Zhao, D.; Yuan, H.; Liu, F.; Liu, X.; Zhu, X.; Zhang, L.; Lu, J. Amperometric Sensor for Dopamine Based on Surface-Graphenization Pencil Graphite Electrode Prepared by In-situ Electrochemical Delamination. Microchim. Acta 2019, 186, 1–8. [Google Scholar] [CrossRef]
- Barton, H.; Berbel-Filho, W.M.; Consuegra, S.; Francis, L.; Tizaoui, C.; Conlan, R.S.; Teixeira, S.R. Ultrasensitive environmental assessment of xeno-estrogens in water samples using label-free graphene immunosensors. Anal. Biochem. 2018, 548, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Arvand, M.; Hemmati, S. Analytical methodology for the electrocatalytic determination of estradiol and progesterone based on graphene quantum dots and poly(sulfosalicylic acid) co-modified electrode. Talanta 2017, 174, 243–255. [Google Scholar] [CrossRef]
- Braga, G.B.; Oliveira, A.E.F.; Pereira, A.C. Total Determination of Estrogenic Phenolic Compounds in River Water Using a Sensor Based on Reduced Graphene Oxide and Molecularly Imprinted Polymer. Electroanalysis 2018, 30, 2176–2184. [Google Scholar] [CrossRef]
- Janegitz, F.A.B.C.; Dos Santos, R.C.; Faria, V. Zucolotto, Electrochemical determination of estradiol using a thin film containing reduced graphene oxide and dihexadecylphosphate. Mater. Sci. Eng. C 2014, 37, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, X.; Li, P.; Huang, Y.; Wang, J.; Zhang, J. Highly sensitive Fe3O4 nanobeads/graphene-based molecularly imprinted electrochemical sensor for 17β-estradiol in water. Anal. Chim. Acta 2015, 884, 106–113. [Google Scholar] [CrossRef]
- Li, T.H.; Wang, D.; Lan, H.Z.; Gan, N. Determination of 17β-estradiol based on electropolymerized-molecularly imprinted polymer on gold nanoparticles-graphene modified electrode. Adv. Mater. Res. 2014, 881, 93–97. [Google Scholar] [CrossRef]
- Hu, L.; Cheng, Q.; Chen, D.; Ma, M.; Wu, K. Liquid-phase exfoliated graphene as highly sensitive sensor for simultaneous determination of endocrine disruptors: Diethylstilbestrol and estradiol. J. Hazard. Mater. 2015, 283, 157–163. [Google Scholar] [CrossRef]
- Wen, T.; Xue, C.; Li, Y.; Wang, Y.; Wang, R.; Hong, J.; Zhou, X.; Jiang, H. Reduced graphene oxide-platinum nanoparticles composites based imprinting sensor for sensitively electrochemical analysis of 17β-estradiol. J. Electroanal. Chem. 2012, 682, 121–127. [Google Scholar] [CrossRef]
- Moraes, F.C.; Rossi, B.; Donatoni, M.C.; De Oliveira, K.T.; Pereira, E.C. Sensitive determination of 17β-estradiol in river water using a graphene based electrochemical sensor. Anal. Chim. Acta 2015, 881, 37–43. [Google Scholar] [CrossRef]
- Özcan, A.; Topçuoğulları, D. Voltammetric determination of 17-Β-estradiol by cysteamine self-assembled gold nanoparticle modified fumed silica decorated graphene nanoribbon nanocomposite. Sens. Actuators B Chem. 2017, 250, 85–90. [Google Scholar] [CrossRef]
- Rather, J.A.; Khudaish, E.A.; Kannan, P. Graphene-amplified femtosensitive aptasensing of estradiol, an endocrine disruptor. Analyst 2018, 143, 1835–1845. [Google Scholar] [CrossRef] [PubMed]
- Karuwan, C.; Wisitsoraat, A.; Phokharatkul, D.; Sriprachuabwong, C.; Lomas, T.; Nacapricha, D.; Tuantranont, A. A disposable screen printed graphene-carbon paste electrode and its application in electrochemical sensing. RSC Adv. 2013, 3, 25792–25799. [Google Scholar] [CrossRef]
- Pasakon, P.; Mensing, J.P.; Phokaratkul, D.; Karuwan, C.; Lomas, T.; Wisitsoraat, A.; Tuantranont, A. A high-performance, disposable screen-printed carbon electrode modified with multi-walled carbon nanotubes/graphene for ultratrace level electrochemical sensors. J. Appl. Electrochem. 2019, 49, 217–227. [Google Scholar] [CrossRef]
- Santos, A.M.; Wong, A.; Prado, T.M.; Fava, E.L.; Fatibello-Filho, O.; Sotomayor, M.D.P.T.; Moraes, F.C. Voltammetric determination of ethinylestradiol using screen-printed electrode modified with functionalized graphene, graphene quantum dots and magnetic nanoparticles coated with molecularly imprinted polymers. Talanta 2021, 224, 121804. [Google Scholar] [CrossRef]
- Ferrari, A.G.M.; Foster, C.W.; Kelly, P.J.; Brownson, D.A.C.; Banks, C.E. Determination of the electrochemical area of screen-printed electrochemical sensing platforms. Biosensors 2018, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, E.M. Electroanalysis and Raman Spectroscopy of Graphene-Modified Electrodes Influenced by the Synthesis and Transfer of the Two-Dimensional Nanomaterial. Ph.D. Thesis, University of Regensburg, Regensburg, Germany, June 2020. Available online: https://epub.uni-regensburg.de/43493/ (accessed on 5 May 2021).
- Marques, A.C.; Cardoso, A.R.; Martins, R.; Sales, M.G.F.; Fortunato, E. Laser-Induced Graphene-Based Platforms for Dual Biorecognition of Molecules. ACS Appl. Nano Mater. 2020, 3, 2795–2803. [Google Scholar] [CrossRef]
- Griffiths, K.; Dale, C.; Hedley, J.; Kowal, M.D.; Kaner, R.B.; Keegan, N. Laser-scribed graphene presents an opportunity to print a new generation of disposable electrochemical sensors. Nanoscale 2014, 6, 13613–13622. [Google Scholar] [CrossRef]
- Luo, J.; Dai, Z.; Feng, M.; Gu, M.; Xie, Y. Graphitic Carbon Nitride/Ferroferric Oxide/Reduced Graphene Oxide Nanocomposite as Highly Active Visible Light Photocatalyst. Nano Res. 2023, 16, 371–376. [Google Scholar] [CrossRef]
- Karuwan, C.; Sriprachuabwong, C.; Wisitsoraat, A.; Phokharatkul, D.; Sritongkham, P.; Tuantranont, A. Inkjet-printed gra-phene-poly(3,4-ethylenedioxythiophene):poly(styrene- sulfonate) modified on screen printed carbon electrode for electro-chemical sensing of salbutamol. Sens. Actuators B Chem. 2012, 161, 549–555. [Google Scholar] [CrossRef]
- Metters, J.P.; Kadara, R.O.; Banks, C.E. New Directions in Screen Printed Electroanalytical Sensors: An Overview of Recent Developments. Analyst 2011, 136, 1067–1076. [Google Scholar] [CrossRef]
- Settu, K.; Lai, Y.C.; Liao, C.T. Carbon Nanotube Modified Laser-Induced Graphene Electrode for Hydrogen Peroxide Sensing. Mater. Lett. 2021, 300, 130106. [Google Scholar] [CrossRef]
- Olowu, R.A.; Arotiba, O.; Mailu, S.N.; Waryo, T.T.; Baker, P.; Iwuoha, E. Electrochemical Aptasensor for Endocrine Disrupting 17β-Estradiol Based on a Poly(3,4-ethylenedioxylthiopene)-Gold Nanocomposite Platform. Sensors 2010, 10, 9872–9890. [Google Scholar] [CrossRef]
- Chang, Z.; Zhu, B.; Liu, J.J.; Zhu, X.; Xu, M.; Travas-Sejdic, J. Electrochemical aptasensor for 17β-estradiol using disposable Laser scribed graphene electrodes. Biosens. Bioelectron. 2021, 185, 113247. [Google Scholar] [CrossRef]
- Yoon, H.; Nah, J.; Kim, H.; Ko, S.; Sharifuzzaman, M.; Barman, S.C.; Xuan, X.; Kim, J.; Park, J.Y. A chemically modified laser-induced porous graphene based flexible and ultrasensitive electrochemical biosensor for sweat glucose detection. Sens. Actuators B Chem. 2020, 311, 127866. [Google Scholar] [CrossRef]
- Smajdor, J.; Piech, R.; Ławrywianiec, M.; Paczosa-Bator, B. Glassy Carbon Electrode Modified with Carbon Black for Sensitive Estradiol Determination by Means of Voltammetry and Flow Injection Analysis with Amperometric Detection. Anal. Biochem. 2018, 544, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Berovic, M. Legisa, Citric acid production, Biotechnol. Annu. Rev. 2007, 13, 303–343. [Google Scholar] [CrossRef]
- Ambrosi, A.; Pumera, M. Electrochemically Exfoliated Graphene and Graphene Oxide for Energy Storage and Electrochemistry Applications. Chem. A Eur. J. 2016, 22, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.L.G.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-induced porous graphene films from commercial polymers. Nat. Commun. 2014, 5, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, P.; Nia, A.S.; Feng, X. Emerging 2D Materials Produced via Electrochemistry. Adv. Mater. 2020, 32, 1907857. [Google Scholar] [CrossRef]
- Torrinha, Á.; Carneiro, P.; Dias, D.; Delerue-Matos, C.; Morais, S. The Simpler the Better: Highly Sensitive 17α-ethinylestradiol Sensor Based on an Unmodified Carbon Paper Transducer. Talanta 2022, 245, 123457. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Li, Y. A Sensitive Determination of Estrogens with a Pt Nano-Clusters/Multi-Walled Carbon Nanotubes Modified Glassy Carbon Electrode. Biosens. Bioelectron. 2006, 22, 253–259. [Google Scholar] [CrossRef]
- McCreery, R.L.; McDermott, M.T. Comment on electrochemical kinetics at ordered graphite electrodes. Anal. Chem. 2012, 84, 2602–2605. [Google Scholar] [CrossRef]
- Batista, I.V.; Lanza, M.R.V.; Dias, I.L.T.; Tanaka, S.M.C.N.; Tanaka, A.A.; Sotomayor, M.D.P.T. Electrochemical Sensor Highly Selective for Estradiol Valerate Determination Based on a Modified Carbon Paste with Iron Tetrapyridinoporphyrazine. Analyst 2008, 133, 1692–1699. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yang, J.; Hu, X. Electrochemical determination of estradiol using a poly (L-serine) film-modified electrode. J. Appl. Electrochem. 2008, 38, 833–836. [Google Scholar] [CrossRef]
- Konopka, S.J.; McDuffie, B. Diffusion Coefficients of Ferri- and Ferrocyanide Ions in Aqueous Media, Using Twin-Electrode Thin-Layer Electrochemistry. Anal. Chem. 1970, 42, 1741–1746. [Google Scholar] [CrossRef]
- Morrin, A.; Killard, A.J.; Smyth, M.R. Electrochemical characterization of commercial and home-made screen-printed carbon electrodes. Anal. Lett. 2003, 36, 2021–2039. [Google Scholar] [CrossRef]

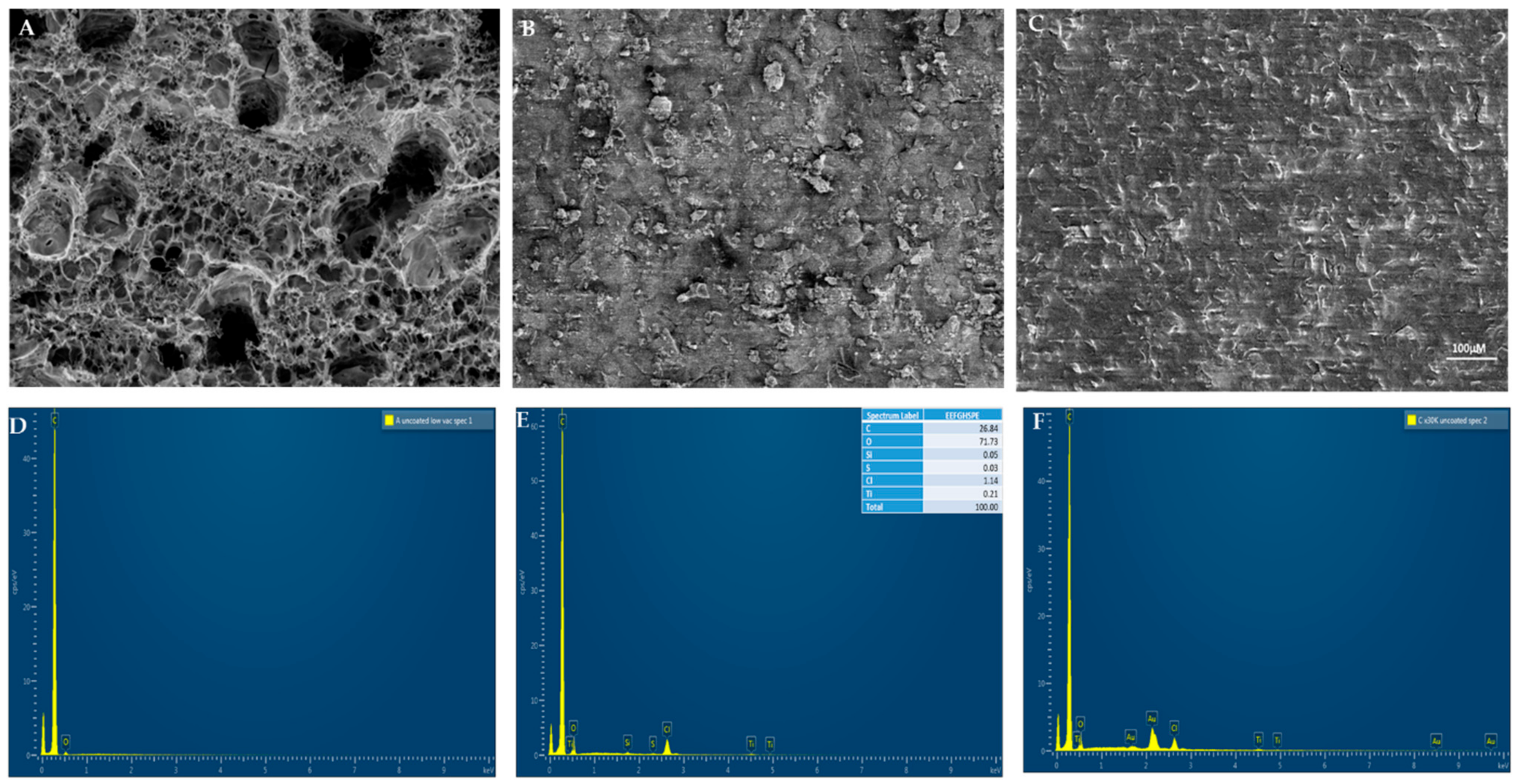
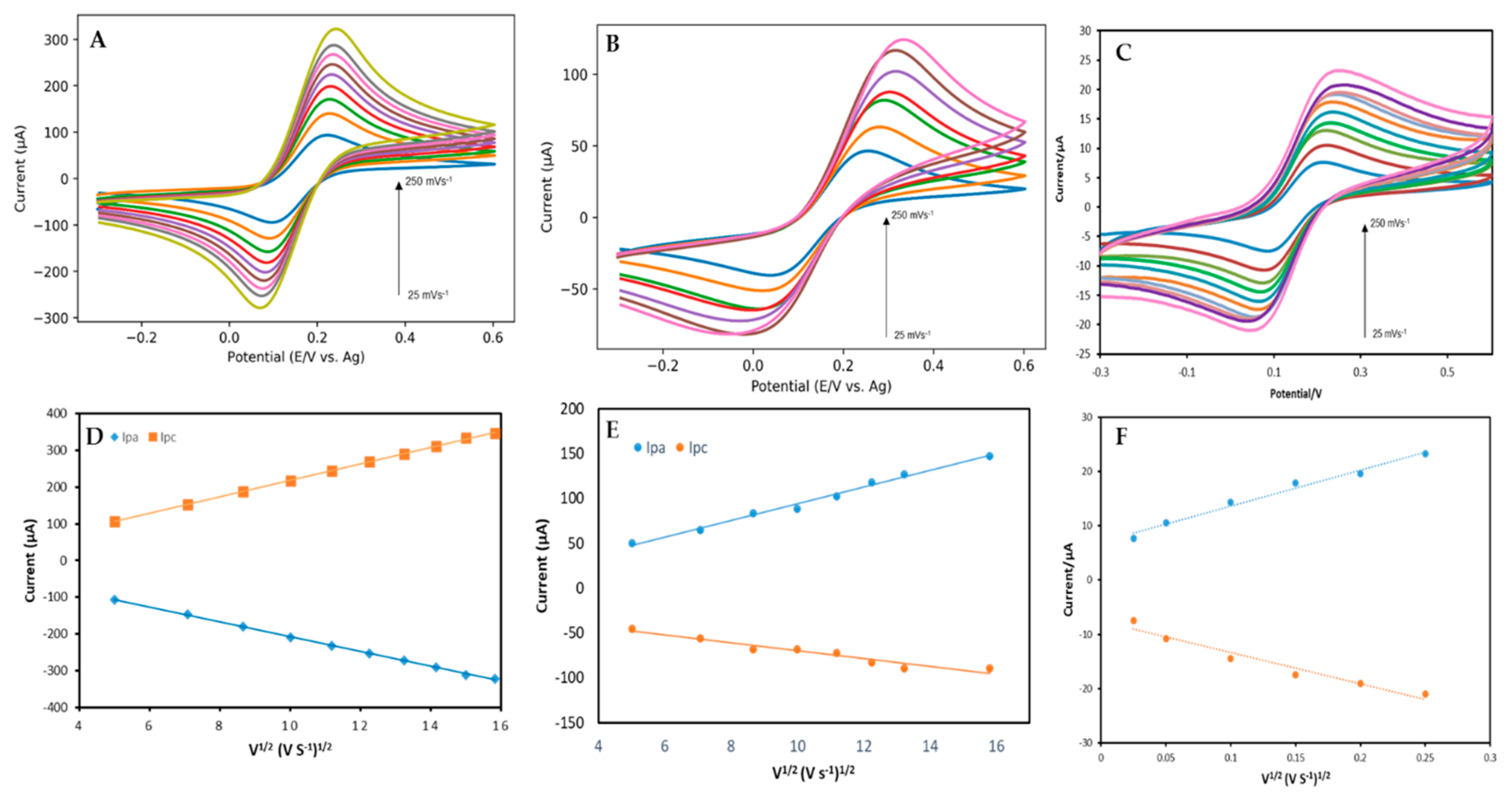


| GHSPE | 3D-GFSPE | EEFGH | |
|---|---|---|---|
| ΔEp/mV | 391 | 89 | 163 |
| Ipa/µA | 89 | 197 | 14.2 |
| a ECSA/cm2 | 0.2 | 0.079 | 0.0125 |
| GHSPE | 3D-GFSPE | EEFGH | |
|---|---|---|---|
| Working potential (V) | +0.65 | +0.65 | +0.65 |
| Linear range (µM) | 0.83–4.98 | 0.83–4.98 | 0.83–4.98 |
| Reproducibility (%RSD) | 5.7 | 4.45 | 6.3 |
| Detection limit (µM) | 0.0041 | 0.097 | 0.018 |
| Sensitivity (µAµM−1cm−2) | 0.151 | 0.429 | 0.273 |
| Electrode | Technique | Linear Range (μM) | LOD (μM) | Sample | Ref. |
|---|---|---|---|---|---|
| Boron-doped diamond electrode | Amperometry | 0.1–3.0 | 0.1 | River water | [13] |
| Carbon fiber paper | Differential pulse voltammetry | 0.001–0.0001 | 0.00014 | River Water | [59] |
| Glassy carbon electrode with platinum/multi-walled carbon nanotube MWCNTs | Square wave voltammetry | 0.5–1 | 0.018 | Serum | [60] |
| Carbon paste modified with iron tetrapyridinoporphyrazine | Amperometry | 45–450 | 0.013 | injection | [62] |
| Glassy carbon with poly(L-serine) | Square wave voltammetry | 0.10–30.0 | 0.02 | Serum | [63] |
| 3D graphene foam screen-printed electrode | Amperometry | 0.83–0.15 | 0.097 | Tap water | This work |
| Graphene ink screen-printed electrode | Amperometry | 0.83–4.98 | 0.0041 | Tap water | This work |
| Electrochemically exfoliated graphene (EEFGH) screen-printed electrode | Amperometry | 0.83–4.98 | 0.018 | Tap water | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musa, A.M.; Kiely, J.; Luxton, R.; Honeychurch, K.C. Graphene-Based Electrodes for Monitoring of Estradiol. Chemosensors 2023, 11, 337. https://doi.org/10.3390/chemosensors11060337
Musa AM, Kiely J, Luxton R, Honeychurch KC. Graphene-Based Electrodes for Monitoring of Estradiol. Chemosensors. 2023; 11(6):337. https://doi.org/10.3390/chemosensors11060337
Chicago/Turabian StyleMusa, Auwal M., Janice Kiely, Richard Luxton, and Kevin C. Honeychurch. 2023. "Graphene-Based Electrodes for Monitoring of Estradiol" Chemosensors 11, no. 6: 337. https://doi.org/10.3390/chemosensors11060337
APA StyleMusa, A. M., Kiely, J., Luxton, R., & Honeychurch, K. C. (2023). Graphene-Based Electrodes for Monitoring of Estradiol. Chemosensors, 11(6), 337. https://doi.org/10.3390/chemosensors11060337







