Rapid Matching Antibodies Pair and Fast Detecting Melioidosis with Fluorescent Immunochromatographic Test Strips
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
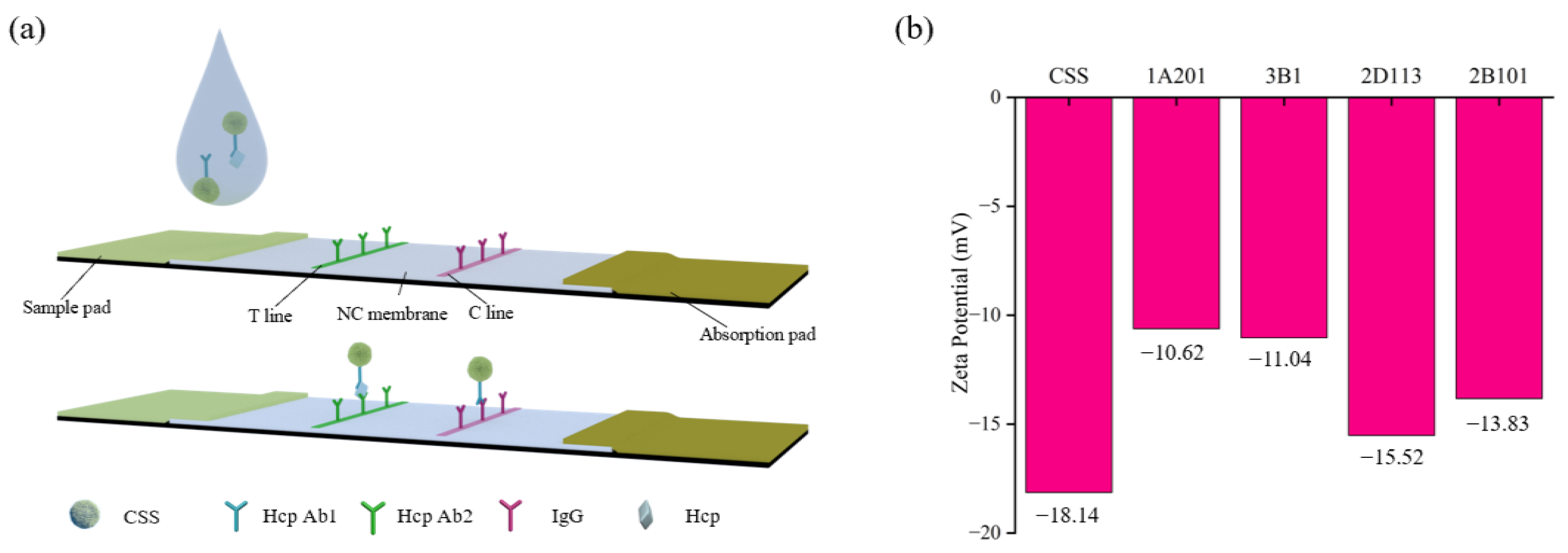
2.2. Fabrication of the CSS-Based LFA Test Strip
2.3. Expression and Purification of Hemolysin-Coregulated Protein 1
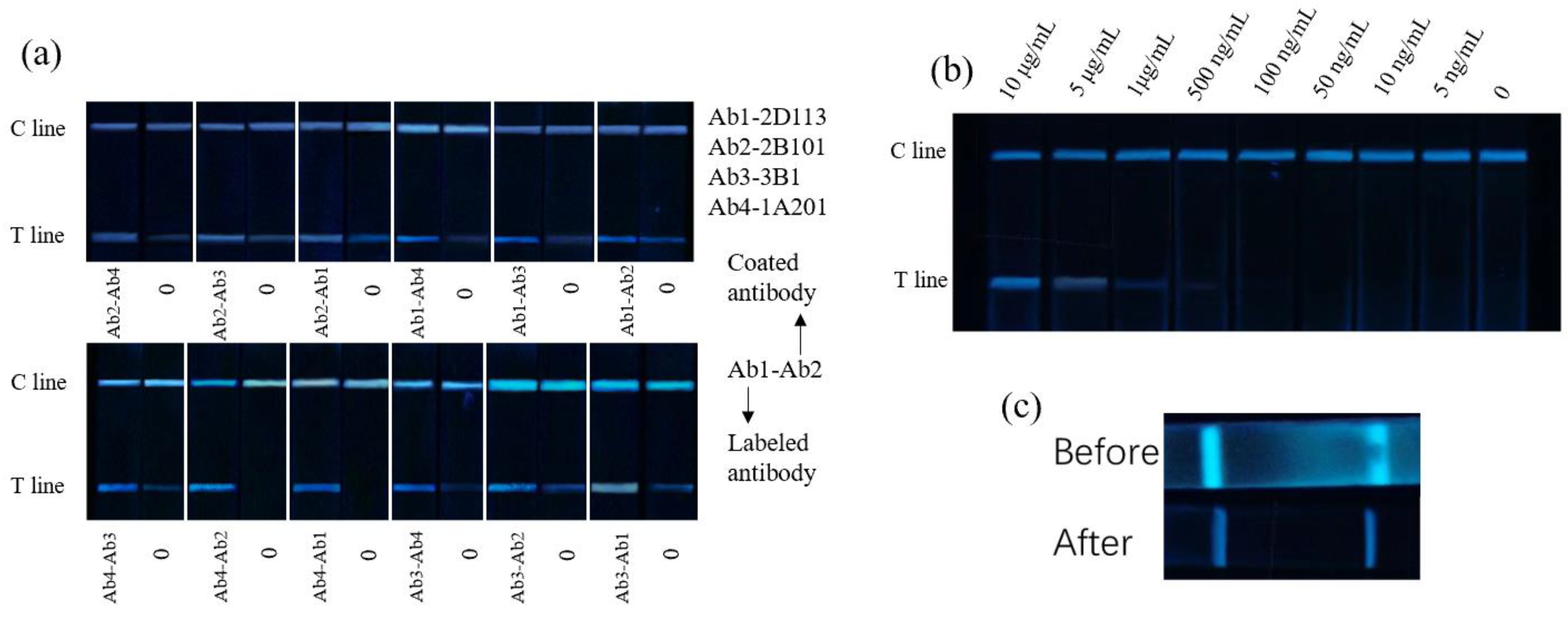
2.4. Development of Anti-Hcp-1 mAb
2.5. Synthesis of Dendritic SiO2 Spheres
2.6. In Situ Synthesis of CDs Dendritic SiO2 Spheres (CSS)
2.7. Preparation of CSS-Labeled Antibody Conjugate
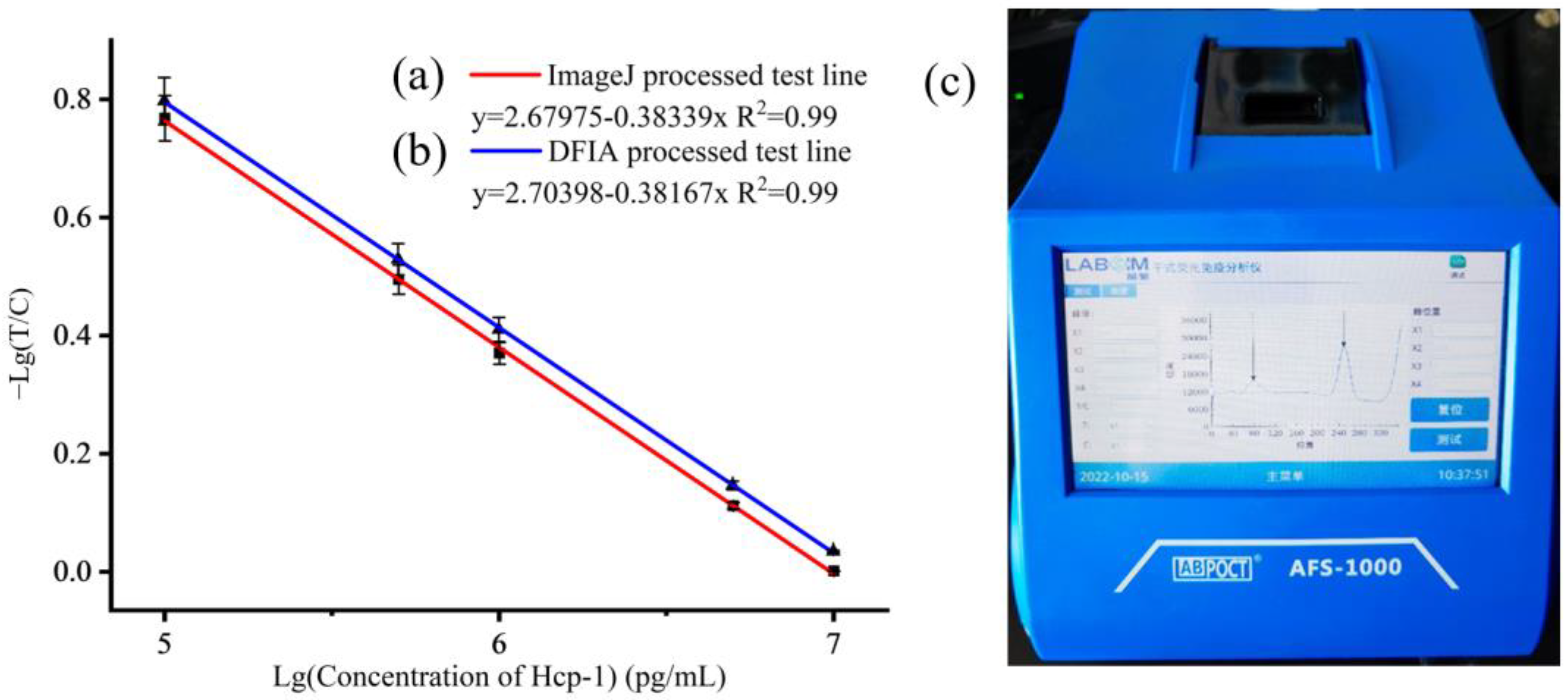
2.8. Detection of Hcp-1 with CSS-Based LFA Test Strips
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wiersinga, W.J.; Currie, B.J.; Peacock, S.J. Melioidosis. N. Engl. J. Med. 2012, 367, 1035–1044. [Google Scholar] [CrossRef]
- Limmathurotsakul, D.; Golding, N.; Dance, D.A.B.; Messina, J.P.; Pigott, D.M.; Moyes, C.L.; Rolim, D.B.; Bertherat, E.; Day, N.P.J.; Peacock, S.J.; et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat. Microbiol. 2016, 1, 15008. [Google Scholar] [CrossRef]
- Cheng, A.C.; Currie, B.J. Melioidosis: Epidemiology, pathophysiology, and management. Clin. Microbiol. Rev. 2005, 18, 383–416, Correction in Clin. Microbiol. Rev. 2007, 20, 533. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, Z.; Xia, Y.; Yuan, S.; Yan, J.; Rao, C.; Li, Q.; Yang, W.; Mao, X. Recombinant expression and immunological characterization of hemolysin coregulated protein 1,Burkholderia pseudomallei type Ⅵ secretion system protein. J. Third Mil. Med. Univ. 2020, 42, 2296–2301. [Google Scholar]
- Richardson, L.J.; Kaestli, M.; Mayo, M.; Bowers, J.R.; Tuanyok, A.; Schupp, J.; Engelthaler, D.; Wagner, D.M.; Keim, P.S.; Currie, B.J. Towards a rapid molecular diagnostic for melioidosis: Comparison of DNA extraction methods from clinical specimens. J. Microbiol. Methods 2012, 88, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Sharma, S.B.; Ahuja, S.; Saxena, S.N. Indirect (Passive) hemagglutination test for assay of antigen and antibody (a review). Acta Microbiol. Hung. 1991, 38, 81–90. [Google Scholar] [PubMed]
- Gilmore, G.; Barnes, J.; Ketheesan, N.; Norton, R. Use of antigens derived from Burkholderia pseudomallei, B. thailandensis, and B. cepacia in the indirect hemagglutination assay for melioidosis. Clin. Vaccine Immunol. 2007, 14, 1529–1531. [Google Scholar] [CrossRef] [PubMed]
- Tiyawisutsri, R.; Peacock, S.J.; Langa, S.; Limmathurotsakul, D.; Cheng, A.C.; Chierakul, W.; Chaowagul, W.; Day, N.P.J.; Wuthiekanun, V. Antibodies from patients with melioidosis recognize Burkholderia mallei but not Burkholderia thailandensis antigens in the indirect hemagglutination assay. J. Clin. Microbiol. 2005, 43, 4872–4874. [Google Scholar] [CrossRef] [PubMed]
- Tandhavanant, S.; Wongsuvan, G.; Wuthiekanun, V.; Teerawattanasook, N.; Day, N.P.J.; Limmathurotsakul, D.; Peacock, S.J.; Chantratita, N. Monoclonal Antibody-Based Immunofluorescence Microscopy for the Rapid Identification of Burkholderia Pseudomallei in Clinical Specimens. Am. J. Trop. Med. Hyg. 2013, 89, 165–168. [Google Scholar] [CrossRef]
- Posthuma-Trumpie, G.A.; Korf, J.; van Amerongen, A. Lateral flow (immuno) assay: Its strengths, weaknesses, opportunities and threats. A literature survey. Anal. Bioanal. Chem. 2009, 393, 569–582. [Google Scholar] [CrossRef]
- Alrashoudi, A.A.; Albalawi, H.I.; Aldoukhi, A.H.; Moretti, M.; Bilalis, P.; Abedalthagafi, M.; Hauser, C.A.E. Fabrication of a Lateral Flow Assay for Rapid In-Field Detection of COVID-19 Antibodies using Additive Manufacturing Printing Technologies. Int. J. Bioprint. 2021, 7, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, W.W.W.; Le, T.N.; Pham, D.M.; Ko, H.H.; Chang, H.C.; Lee, C.C.; Sharma, N.; Lee, C.K.; Chiang, W.H. Recent Advances in Novel Lateral Flow Technologies for Detection of COVID-19. Biosensors 2021, 11, 295. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Song, S.; Park, S.; Joo, C. Recent advances in high-sensitivity detection methods for paper-based lateral-flow assay. Biosens. Bioelectron. 2020, 152, 112015. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Wang, W.; Du, T.E. Dry-reagent nucleic acid biosensor based on blue dye doped latex beads and lateral flow strip. Talanta 2013, 114, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Tok, A.I.Y.; Lee, C.; Ganapathy, R.; Palaniappan, A.; Liedberg, B. Magnetic field assisted preconcentration of biomolecules for lateral flow assaying. Sens. Actuators B Chem. 2019, 285, 431–437. [Google Scholar] [CrossRef]
- Hui, W.L.; Zhang, S.N.; Zhang, C.; Wan, Y.S.; Zhu, J.L.; Zhao, G.; Wu, S.D.; Xi, D.J.; Zhang, Q.L.; Li, N.N.; et al. A novel lateral flow assay based on GoldMag nanoparticles and its clinical applications for genotyping of MTHFR C677T polymorphisms. Nanoscale 2016, 8, 3579–3587. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Yu, X.Z.; Wen, K.; Li, C.L.; Marti, G.M.; Jiang, H.Y.; Shi, W.M.; Shen, J.Z.; Wang, Z.H. Multiplex Lateral Flow Immunoassays Based on Amorphous Carbon Nanoparticles for Detecting Three Fusarium Mycotoxins in Maize. J. Agric. Food Chem. 2017, 65, 8063–8071. [Google Scholar] [CrossRef]
- Qin, K.M.; Ding, M.Y.; Zhang, C.S.; Zhang, X.J.; Mao, Y.X.; Dang, M.; Li, Z.Z.; Wang, Y.Y.; Zhang, S.H.; Sun, Y.H.; et al. Development of a sensitive monoclonal antibody-based immunochromatographic strip for neomycin detection in milk. Food Agric. Immunol. 2022, 33, 315–327. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, Q.Q.; Xie, J.; Wang, H.; Tang, Y. Development of a high sensitivity quantum dot-based fluorescent quenching lateral flow assay for the detection of zearalenone. Anal. Bioanal. Chem. 2019, 411, 2169–2175. [Google Scholar] [CrossRef]
- Xu, L.D.; Wang, S.W.; Zhu, J.; Zhou, T.T.; Ding, S.N. Dendritic Silica Nanospheres Loaded with Red-Emissive Enhanced Carbon Dots for Zika Virus Immunoassay. Chemistryselect 2021, 6, 9787–9793. [Google Scholar] [CrossRef]
- Xu, L.D.; Zhu, J.; Ding, S.N. Highly-fluorescent carbon dots grown onto dendritic silica nanospheres for anthrax protective antigen detection. Anal. Methods 2022, 14, 1836–1840. [Google Scholar] [CrossRef]
- Hu, Q.S.; Wei, Q.Z.; Zhang, P.P.; Li, S.; Xue, L.; Yang, R.F.; Wang, C.B.; Zhou, L. An up-converting phosphor technology-based lateral flow assay for point-of-collection detection of morphine and methamphetamine in saliva. Analyst 2018, 143, 4646–4654. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.E.; Ryu, E.; Kim, J.; Shin, C.J.; Kwon, O.S. Fluorophore-encapsulated nanobeads for on-site, rapid, and sensitive lateral flow assay. Sens. Actuators B Chem. 2023, 381, 133364. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.Y.; Xie, H.Y.; Zhang, Z.L.; Wu, L.L.; Hu, J.; Tang, M.; Wu, M.; Pang, D.W. Fluorescent/magnetic micro/nano-spheres based on quantum dots and/or magnetic nanoparticles: Preparation, properties, and their applications in cancer studies. Nanoscale 2016, 8, 12406–12429. [Google Scholar] [CrossRef] [PubMed]
- Raysyan, A.; Galvidis, I.A.; Schneider, R.J.; Eremin, S.A.; Burkin, M.A. Development of a latex particles-based lateral flow immunoassay for group determination of macrolide antibiotics in breast milk. J. Pharm. Biomed. Anal. 2020, 189, 113450. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Luo, P.J.; Liu, Z.W.; He, Z.X.; Pang, Y.M.; Lei, H.T.; Xu, Z.L.; Wang, H.; Li, X.M. Rainbow latex microspheres lateral flow immunoassay with smartphone-based device for simultaneous detection of three mycotoxins in cereals. Anal. Chim. Acta 2022, 1221, 340138. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Xu, H.; Wei, M.; Gu, H.C.; Xu, Q.F.; Zhu, W. Study of superparamagnetic nanoparticles as labels in the quantitative lateral flow immunoassay. Mater. Sci. Eng. C-Biomim. Supramol. Syst. 2009, 29, 714–718. [Google Scholar] [CrossRef]
- Xie, Z.H.; Feng, S.S.; Pei, F.B.; Xia, M.Z.; Hao, Q.L.; Liu, B.; Tong, Z.Y.; Wang, J.; Lei, W.; Mu, X.H. Magnetic/fluorescent dual-modal lateral flow immunoassay based on multifunctional nanobeads for rapid and accurate SARS-CoV-2 nucleocapsid protein detection. Anal. Chim. Acta 2022, 1233, 340486. [Google Scholar] [CrossRef]
- Yan, L.Z.; Dou, L.N.; Bu, T.; Huang, Q.; Wang, R.; Yang, Q.F.; Huang, L.J.; Wang, J.L.; Zhang, D.H. Highly sensitive furazolidone monitoring in milk by a signal amplified lateral flow assay based on magnetite nanoparticles labeled dual-probe. Food Chem. 2018, 261, 131–138. [Google Scholar] [CrossRef]
- He, H.; Liu, B.L.; Wen, S.H.; Liao, J.Y.; Lin, G.G.; Zhou, J.J.; Jin, D.Y. Quantitative Lateral Flow Strip Sensor using Highly Doped Upconversion Nanoparticles. Anal. Chem. 2018, 90, 12356–12360. [Google Scholar] [CrossRef]
- Kim, H.S.; Ko, H.; Kang, M.J.; Pyun, J.C. Highly sensitive rapid test with chemiluminescent signal bands. Biochip J. 2010, 4, 155–160. [Google Scholar] [CrossRef]
- Wang, Y.; Fill, C.; Nugen, S.R. Development of chemiluminescent lateral flow assay for the detection of nucleic acids. Biosensors 2012, 2, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.X.; Xu, L.Y.; Yang, L.; Cui, Y. Minimizing Cross-Reactivity for the Chemiluminescent Lateral Flow Immunoassay of Cardiac Troponin I Based on PEGylation of Gold Nanoparticles. Anal. Chem. 2023, 95, 6646–6654. [Google Scholar] [CrossRef]
- Baker, S.N.; Baker, G.A. Luminescent Carbon Nanodots: Emergent Nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.D.; Zhang, Q.; Ding, S.N.; Xu, J.J.; Chen, H.Y. Ultrasensitive Detection of Severe Fever with Thrombocytopenia Syndrome Virus Based on Immunofluorescent Carbon Dots/SiO2 Nanosphere-Based Lateral Flow Assay. Acs Omega 2019, 4, 21431–21438. [Google Scholar] [CrossRef]
- Shen, J.; Shang, S.M.; Chen, X.Y.; Wang, D.; Cai, Y. Facile synthesis of fluorescence carbon dots from sweet potato for Fe3+ sensing and cell imaging. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 856–864. [Google Scholar] [CrossRef]
- Shi, N.N.; Sun, K.Y.; Zhang, Z.D.; Zhao, J.; Geng, L.N.; Lei, Y.H. Amino-modified carbon dots as a functional platform for drug delivery: Load-release mechanism and cytotoxicity. J. Ind. Eng. Chem. 2021, 101, 372–378. [Google Scholar] [CrossRef]
- Sun, Y.Q.; Li, J.A.; He, D.W.; Wang, X.R.; Shi, Y.; Pan, L.J. Recent progress on performances and mechanisms of carbon dots for gas sensing. Luminescence 2022. ahead of print. [Google Scholar] [CrossRef]
- Li, H.T.; He, X.D.; Kang, Z.H.; Huang, H.; Liu, Y.; Liu, J.L.; Lian, S.Y.; Tsang, C.H.A.; Yang, X.B.; Lee, S.T. Water-Soluble Fluorescent Carbon Quantum Dots and Photocatalyst Design. Angew. Chem.-Int. Ed. 2010, 49, 4430–4434. [Google Scholar] [CrossRef]
- He, C.; Xu, P.; Zhang, X.H.; Long, W.J. The synthetic strategies, photoluminescence mechanisms and promising applications of carbon dots: Current state and future perspective. Carbon 2022, 186, 91–127. [Google Scholar] [CrossRef]
- Houghton, R.L.; Reed, D.E.; Hubbard, M.A.; Dillon, M.J.; Chen, H.J.; Currie, B.J.; Mayo, M.; Sarovich, D.S.; Theobald, V.; Limmathurotsakul, D.; et al. Development of a Prototype Lateral Flow Immunoassay (LFI) for the Rapid Diagnosis of Melioidosis. PLoS Negl. Trop. Dis. 2014, 8, e2727. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S. A short history, principles, and types of ELISA, and our laboratory experience with peptide/protein analyses using ELISA. Peptides 2015, 72, 4–15. [Google Scholar] [CrossRef]
- Zhou, T.T.; Yue, Y.; Zheng, F.; Liang, X.D.; Cao, Q.X.; Wang, Y.W.; Zhu, J. Monoclonal antibody against L-lectin module of SraP blocks adhesion and protects mice against Staphylococcus aureus challenge. J. Microbiol. Immunol. Infect. 2021, 54, 420–428. [Google Scholar] [CrossRef]
- Radhakrishnan, A.; Behera, B.; Mishra, B.; Mohapatra, P.R.; Kumar, R.; Singh, A.K. Clinico-microbiological description and evaluation of rapid lateral flow immunoassay and PCR for detection of Burkholderia pseudomallei from patients hospitalized with sepsis and pneumonia: A twenty-one months study from Odisha, India. Acta Trop. 2021, 221, 105994. [Google Scholar] [CrossRef]
- Shaw, T.; Tellapragada, C.; Vandana, K.E.; AuCoin, D.P.; Mukhopadhyayl, C. Performance evaluation of Active Melioidosis Detect-Lateral Flow Assay (AMD-LFA) for diagnosis of melioidosis in endemic settings with limited resources. PLoS ONE 2018, 13, e0194595. [Google Scholar] [CrossRef] [PubMed]
- Wongsuvan, G.; Hantrakun, V.; Teparrukkul, P.; Imwong, M.; West, T.E.; Wuthiekanun, V.; Day, N.P.J.; AuCoin, D.; Limmathurotsakul, D. Sensitivity and specificity of a lateral flow immunoassay (LFI) in serum samples for diagnosis of melioidosis. Trans. R. Soc. Trop. Med. Hyg. 2018, 112, 568–570. [Google Scholar] [CrossRef] [PubMed]
- Currie, B.J.; Woerle, C.; Mayo, M.; Meumann, E.M.; Baird, R.W. What is the Role of Lateral Flow Immunoassay for the Diagnosis of Melioidosis? Open Forum Infect. Dis. 2022, 9, ofac149. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-Z.; Zhou, T.-T.; Zhu, J.; Ding, S.-N. Rapid Matching Antibodies Pair and Fast Detecting Melioidosis with Fluorescent Immunochromatographic Test Strips. Chemosensors 2023, 11, 351. https://doi.org/10.3390/chemosensors11060351
Lin Y-Z, Zhou T-T, Zhu J, Ding S-N. Rapid Matching Antibodies Pair and Fast Detecting Melioidosis with Fluorescent Immunochromatographic Test Strips. Chemosensors. 2023; 11(6):351. https://doi.org/10.3390/chemosensors11060351
Chicago/Turabian StyleLin, Yi-Zhi, Ting-Ting Zhou, Jin Zhu, and Shou-Nian Ding. 2023. "Rapid Matching Antibodies Pair and Fast Detecting Melioidosis with Fluorescent Immunochromatographic Test Strips" Chemosensors 11, no. 6: 351. https://doi.org/10.3390/chemosensors11060351
APA StyleLin, Y.-Z., Zhou, T.-T., Zhu, J., & Ding, S.-N. (2023). Rapid Matching Antibodies Pair and Fast Detecting Melioidosis with Fluorescent Immunochromatographic Test Strips. Chemosensors, 11(6), 351. https://doi.org/10.3390/chemosensors11060351






