Microplasma-Enabled Sustainable Synthesis of Nitrogen-Doped Graphene Quantum Dots for Sensitive Detection of 4-Nitrophenol
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
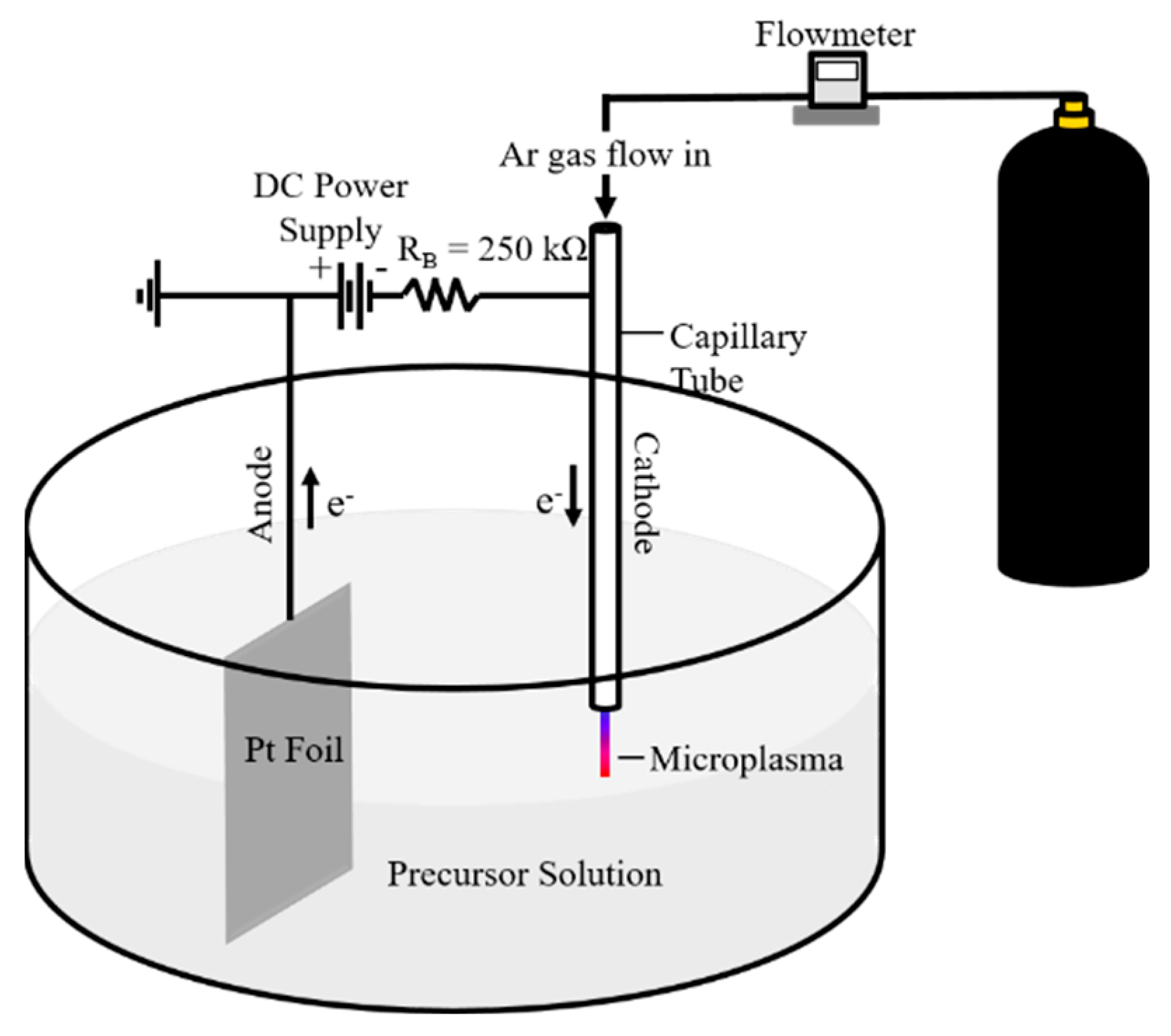
2.2. Synthesis of N-GQDs
2.3. Purification of N-GQDs
2.4. Characterization of N-GQDs
2.5. 4-NP Sensing
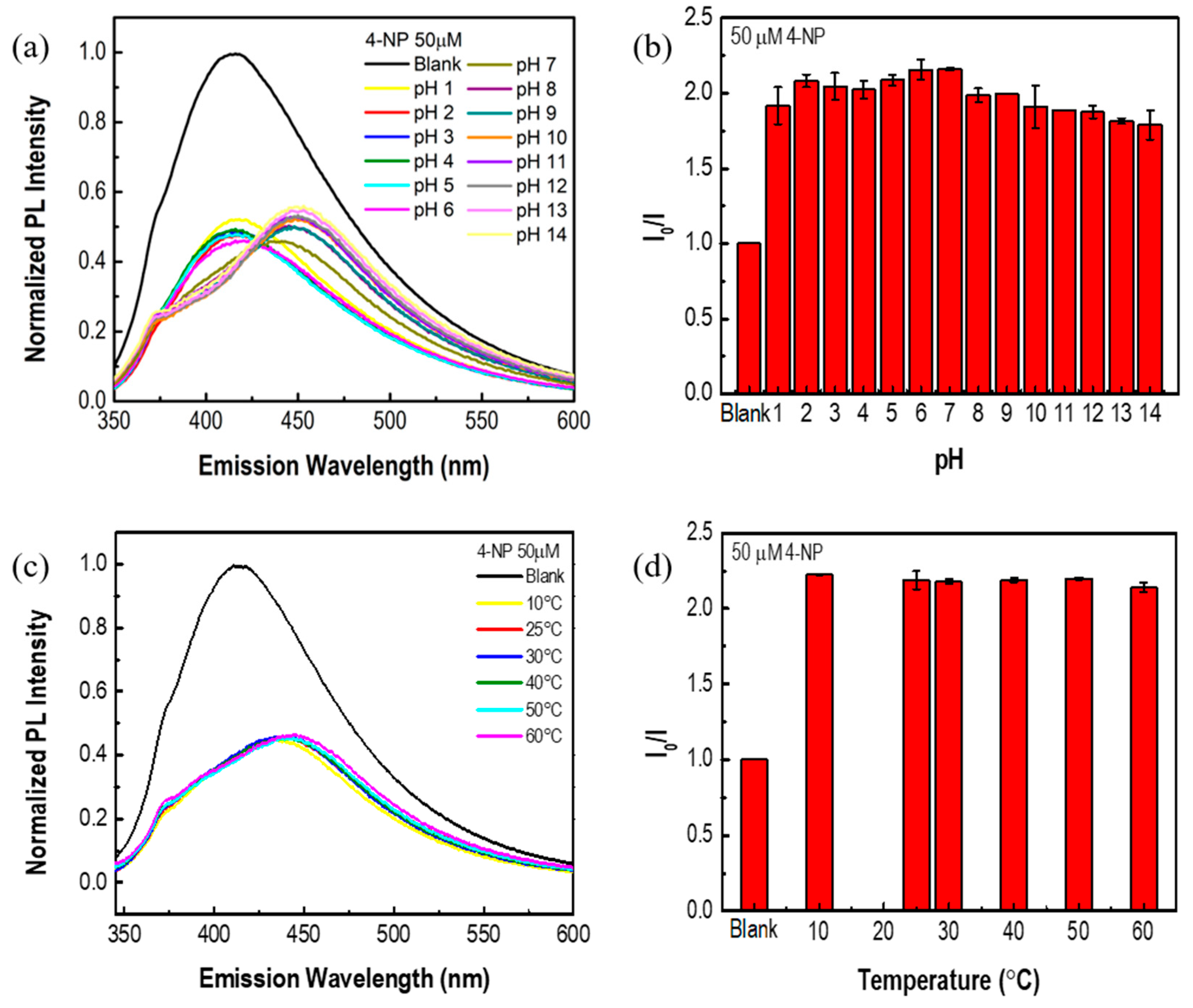
2.6. pH Stability
2.7. Temperature Stability
3. Results and Discussion
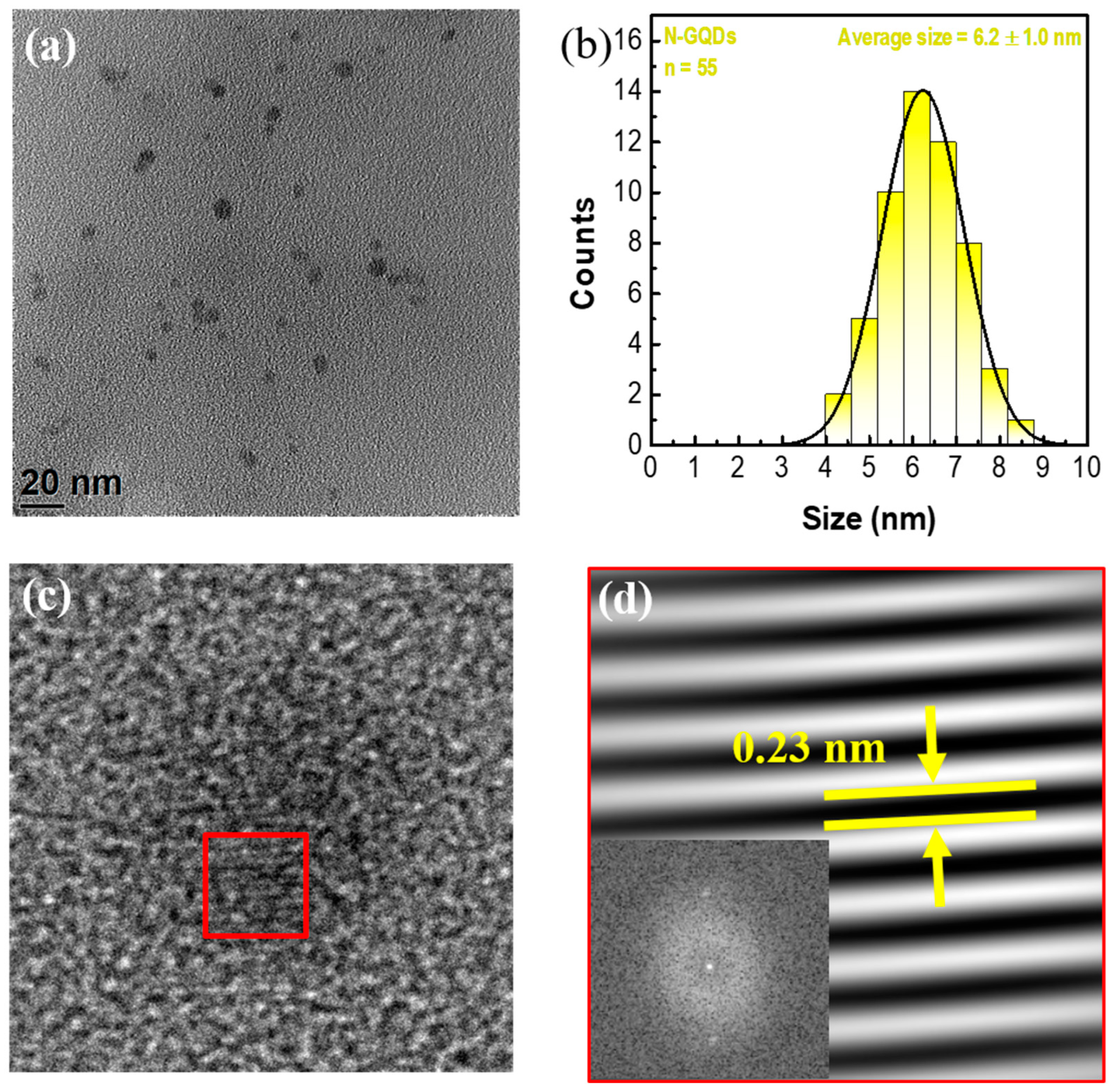
3.1. Synthesis of N-GQDs
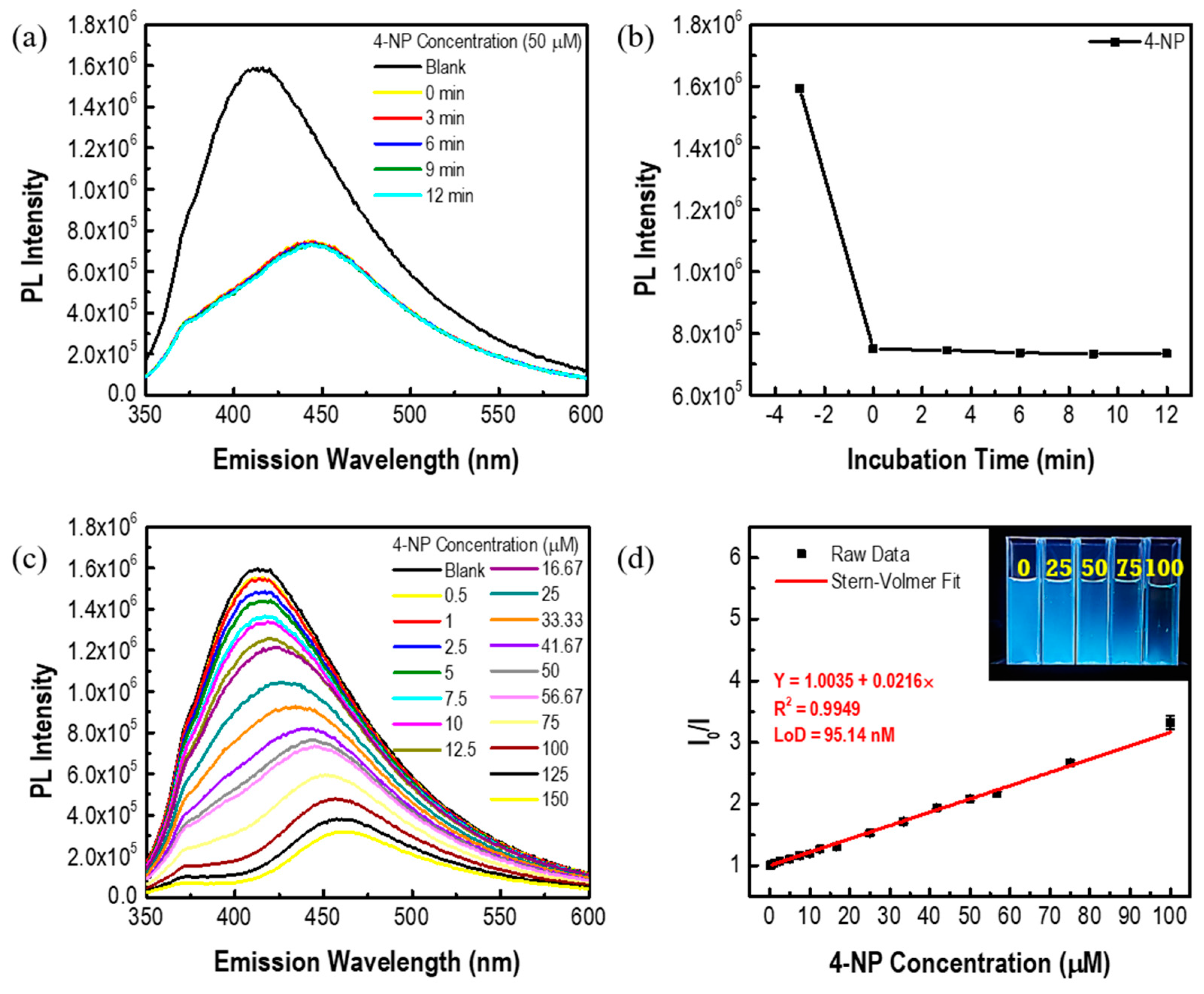
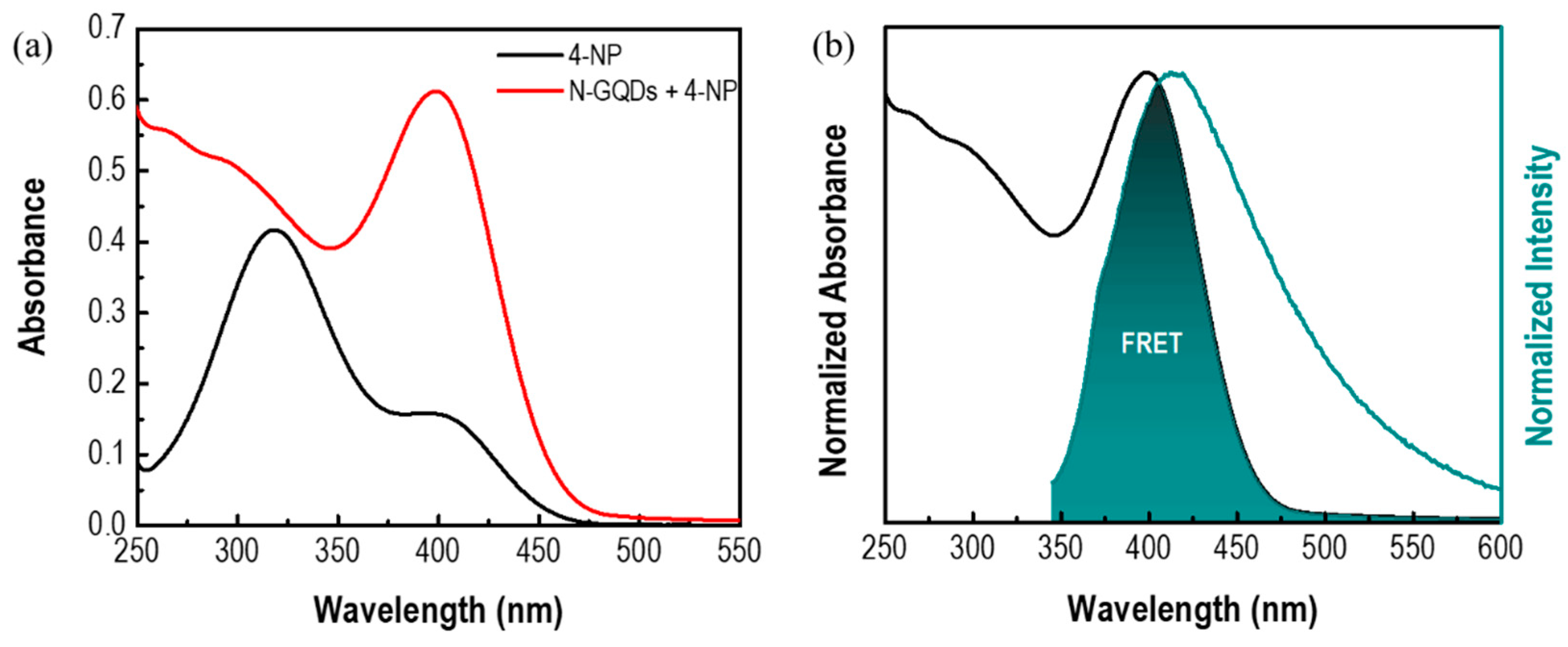
3.2. 4-NP Sensing with N-GQDs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, L.; Liu, S.G.; Liang, J.Y.; Ju, Y.J.; Li, N.B.; Luo, H.Q. pH-Mediated Reversible Fluorescence Nanoswitch Based on Inner Filter Effect Induced Fluorescence Quenching for Selective and Visual Detection of 4-Nitrophenol. J. Hazard. Mater. 2019, 362, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Bogireddy, N.K.R.; Cruz Silva, R.; Valenzuela, M.A.; Agarwal, V. 4-Nitrophenol Optical Sensing with N Doped Oxidized Carbon Dots. J. Hazard. Mater. 2020, 386, 121643. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Yu, J.; Feng, S.; Gong, Y. Highly Photoluminescent pH-Independent Nitrogen-Doped Carbon Dots for Sensitive and Selective Sensing of p-Nitrophenol. RSC Adv. 2016, 6, 15192–15200. [Google Scholar] [CrossRef]
- Bogireddy, N.K.R.; Kiran Kumar, H.A.; Mandal, B.K. Biofabricated Silver Nanoparticles as Green Catalyst in the Degradation of Different Textile Dyes. J. Environ. Chem. Eng. 2016, 4, 56–64. [Google Scholar] [CrossRef]
- Xiao, N.; Liu, S.G.; Mo, S.; Li, N.; Ju, Y.J.; Ling, Y.; Li, N.B.; Luo, H.Q. Highly Selective Detection of p-Nitrophenol Using Fluorescence Assay Based on Boron, Nitrogen Co-Doped Carbon Dots. Talanta 2018, 184, 184–192. [Google Scholar] [CrossRef]
- Gudipati, N.S.; Vanjari, S.; Korutla, S.; Tammineni, R.R.; Challapalli, S. Electrochemical Detection of 4-Nitrophenol on Nanostructured CuBi2O4 with Plausible Mechanism Supported by DFT Calculations. J. Environ. Chem. Eng. 2022, 10, 108758. [Google Scholar] [CrossRef]
- Mejia, Y.R.; Reddy Bogireddy, N.K. Reduction of 4-Nitrophenol Using Green-Fabricated Metal Nanoparticles. RSC Adv. 2022, 12, 18661–18675. [Google Scholar] [CrossRef]
- Galeano-Díaz, T.; Guiberteau-Cabanillas, A.; Díez, N.M.; Vázquez, P.P.; López, F.S. Rapid and Sensitive Determination of 4-Nitrophenol Fenitrothion and Parathion-Ethyl by Liquid Chromatography with Electrochemical Detection. J. Agric. Food Chem. 2000, 48, 4508–4513. [Google Scholar] [CrossRef]
- Herterich, R. Gas Chromatographic Determination of Nitrophenols in Atmospheric Liquid Water and Airborne Particulates. J. Chromatogr. A 1991, 549, 313–324. [Google Scholar] [CrossRef]
- Kitanovski, Z.; Grgic, I.; Vermeylen, R.; Claeys, M.; Maenhaut, W. Liquid Chromatography Tandem Mass Spectrometry Method for Characterization of Monoaromatic Nitro-Compounds in Atmospheric Particulate Matter. J. Chromatogr. A 2012, 1268, 35–43. [Google Scholar] [CrossRef]
- Deng, P.; Xu, Z.; Feng, Y.; Li, J. Electrocatalytic Reduction and Determination of p-Nitrophenol on Acetylene Black Paste Electrode Coated with Salicylaldehyde-Modified Chitosan. Sens. Actuators B Chem. 2012, 168, 381–389. [Google Scholar] [CrossRef]
- Li, J.; Kuang, D.; Feng, Y.; Zhang, F.; Xu, Z.; Liu, M. A Graphene Oxide-Based Electrochemical Sensor for Sensitive Determination of 4-Nitrophenol. J. Hazard. Mater. 2012, 201–202, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Vilian, A.T.E.; Choe, S.R.; Giribabu, K.; Jang, S.C.; Roh, C.; Huh, Y.S.; Han, Y.K. Pd Nanospheres Decorated Reduced Graphene Oxide with Multi-Functions: Highly Efficient Catalytic Reduction and Ultrasensitive Sensing of Hazardous 4-Nitrophenol Pollutant. J. Hazard. Mater. 2017, 333, 54–62. [Google Scholar] [CrossRef]
- Ma, X.; Wu, Y.; Devaramani, S.; Zhang, C.; Niu, Q.; Ibrahim Shinger, M.; Li, W.; Shan, D.; Lu, X. Preparation of GO-COOH/AuNPs/ZnAPTPP Nanocomposites Based on the π–π Conjugation: Efficient Interface for Low-Potential Photoelectrochemical Sensing of 4-Nitrophenol. Talanta 2018, 178, 962–969. [Google Scholar] [CrossRef]
- Mehdinia, A.; Dadkhah, S.; Baradaran Kayyal, T.; Jabbari, A. Design of a Surface-Immobilized 4-Nitrophenol Molecularly Imprinted Polymer via Pre-Grafting Amino Functional Materials on Magnetic Nanoparticles. J. Chromatogr. A 2014, 1364, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Niu, Y.; Zhao, S.; Khan, A.; Ling, Z.; Chen, Y.; Liu, P.; Li, X. A Novel Biosensor for p-Nitrophenol Based on an Aerobic Anode Microbial Fuel Cell. Biosens. Bioelectron. 2016, 85, 860–868. [Google Scholar] [CrossRef]
- Yan, K.; Yang, Y.; Zhu, Y.; Zhang, J. Highly Selective Self-Powered Sensing Platform for p-Nitrophenol Detection Constructed with a Photocathode-Based Photocatalytic Fuel Cell. Anal. Chem. 2017, 89, 8599–8603. [Google Scholar] [CrossRef]
- Ponomarenko, L.A.; Schedin, F.; Katsnelson, M.I.; Yang, R.; Hill, E.W.; Novoselov, K.S.; Geim, A.K. Chaotic Dirac Billiard in Graphene Quantum Dots. Science 2008, 320, 356–358. [Google Scholar] [CrossRef]
- Tshangana, C.S.; Muleja, A.A.; Kuvarega, A.T.; Malefetse, T.J.; Mamba, B.B. The Applications of Graphene Oxide Quantum Dots in the Removal of Emerging Pollutants in Water: An Overview. J. Water Process. Eng. 2021, 43, 102249. [Google Scholar] [CrossRef]
- Yan, Y.; Gong, J.; Chen, J.; Zeng, Z.; Huang, W.; Pu, K.; Liu, J.; Chen, P. Recent Advances on Graphene Quantum Dots: From Chemistry and Physics to Applications. Adv. Mater. 2019, 31, e1808283. [Google Scholar] [CrossRef]
- Kurniawan, D.; Weng, R.-J.; Chen, Y.-Y.; Rahardja, M.R.; Nanaricka, Z.C.; Chiang, W.-H. Recent Advances in the Graphene Quantum Dot-Based Biological and Environmental Sensors. Sens. Actuators Rep. 2022, 4, 100130. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Trivizas, G.; Karakassides, M.A.; Baikousi, M.; Kouloumpis, A.; Gournis, D.; Bakandritsos, A.; Hola, K.; Kozak, O.; Zboril, R.; et al. Green and Simple Route toward Boron Doped Carbon Dots with Significantly Enhanced Non-Linear Optical Properties. Carbon 2015, 83, 173–179. [Google Scholar] [CrossRef]
- Li, J.; Jiao, Y.; Feng, L.; Zhong, Y.; Zuo, G.; Xie, A.; Dong, W. Highly N,P-Doped Carbon Dots: Rational Design, Photoluminescence and Cellular Imaging. Mikrochim. Acta 2017, 184, 2933–2940. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Z.; Kong, B.; Feng, S.; Wang, J.; Wang, L.; Yang, J.; Zhang, F.; Wu, P.; Zhao, D. Simple and Green Synthesis of Nitrogen-Doped Photoluminescent Carbonaceous Nanospheres for Bioimaging. Angew. Chem. Int. Ed. 2013, 52, 8151–8155. [Google Scholar] [CrossRef]
- Qian, Z.; Shan, X.; Chai, L.; Ma, J.; Chen, J.; Feng, H. Si-Doped Carbon Quantum Dots: A Facile and General Preparation Strategy, Bioimaging Application, and Multifunctional Sensor. ACS Appl. Mater. Interfaces 2014, 6, 6797–6805. [Google Scholar] [CrossRef]
- Xu, Q.; Pu, P.; Zhao, J.; Dong, C.; Gao, C.; Chen, Y.; Chen, J.; Liu, Y.; Zhou, H. Preparation of Highly Photoluminescent Sulfur-Doped Carbon Dots for Fe(iii) Detection. J. Mater. Chem. A 2015, 3, 542–546. [Google Scholar] [CrossRef]
- Yang, S.; Sun, J.; He, P.; Deng, X.; Wang, Z.; Hu, C.; Ding, G.; Xie, X. Selenium Doped Graphene Quantum Dots as an Ultrasensitive Redox Fluorescent Switch. Chem. Mater. 2015, 27, 2004–2011. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, A. Carbon Quantum Dots: Synthesis, Properties and Applications. J. Mater. Chem. C 2014, 2, 6921–6939. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Chakradhar, D.; Perumal, S.; Shim, J.-J.; Lee, Y.R. Facile Green Synthesis of Nitrogen-Doped Carbon Dots using Chionanthus Retusus Fruit Extract and Investigation of Their Suitability for Metal Ion Sensing and Biological Applications. Sens. Actuators B Chem. 2017, 246, 497–509. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.; Lee, Y.R. Nitrogen-Doped Carbon Dots Originating from Unripe Peach for Fluorescent Bioimaging and Electrocatalytic Oxygen Reduction Reaction. J. Colloid. Interface Sci. 2016, 482, 8–18. [Google Scholar] [CrossRef]
- Edison, T.N.; Atchudan, R.; Sethuraman, M.G.; Shim, J.J.; Lee, Y.R. Microwave Assisted Green Synthesis of Fluorescent N-Doped Carbon Dots: Cytotoxicity and Bio-Imaging Applications. J. Photochem. Photobiol. B Biol. 2016, 161, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Edison, T.N.; Atchudan, R.; Shim, J.J.; Kalimuthu, S.; Ahn, B.C.; Lee, Y.R. Turn-Off Fluorescence Sensor for the Detection of Ferric Ion in Water Using Green Synthesized N-Doped Carbon Dots and Its Bio-Imaging. J. Photochem. Photobiol. B Biol. 2016, 158, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Vinodh, R.; Lee, Y.R. In-situ Green Synthesis of Nitrogen-Doped Carbon Dots for Bioimaging and TiO2 Nanoparticles@Nitrogen-doped Carbon Composite for Photocatalytic Degradation of Organic Pollutants. J. Alloys Compd. 2018, 766, 12–24. [Google Scholar] [CrossRef]
- Du, Y.; Guo, S. Chemically Doped Fluorescent Carbon and Graphene Quantum Dots for Bioimaging, Sensor, Catalytic and Photoelectronic Applications. Nanoscale 2016, 8, 2532–2543. [Google Scholar] [CrossRef]
- Ghaffarkhah, A.; Hosseini, E.; Kamkar, M.; Sehat, A.A.; Dordanihaghighi, S.; Allahbakhsh, A.; van der Kuur, C.; Arjmand, M. Synthesis, Applications, and Prospects of Graphene Quantum Dots: A Comprehensive Review. Small 2022, 18, e2102683. [Google Scholar] [CrossRef]
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesaro, A. “The Good, the Bad and the Ugly” of Chitosans. Mar. Drugs 2016, 14, 99. [Google Scholar] [CrossRef]
- Nilsen-Nygaard, J.; Strand, S.; Vårum, K.; Draget, K.; Nordgård, C. Chitosan: Gels and Interfacial Properties. Polymers 2015, 7, 552–579. [Google Scholar] [CrossRef]
- Bruggeman, P.J.; Kushner, M.J.; Locke, B.R.; Gardeniers, J.G.E.; Graham, W.G.; Graves, D.B.; Hofman-Caris, R.C.H.M.; Maric, D.; Reid, J.P.; Ceriani, E.; et al. Plasma–Liquid Interactions: A Review and Roadmap. Plasma Sources Sci. Technol. 2016, 25, 053002. [Google Scholar] [CrossRef]
- Chiang, W.-H.; Richmonds, C.; Sankaran, R.M. Continuous-Flow, Atmospheric-Pressure Microplasmas: A Versatile Source for Metal Nanoparticle Synthesis in the Gas or Liquid Phase. Plasma Sources Sci. Technol. 2010, 19, 034011. [Google Scholar] [CrossRef]
- Mariotti, D.; Patel, J.; Švrček, V.; Maguire, P. Plasma-Liquid Interactions at Atmospheric Pressure for Nanomaterials Synthesis and Surface Engineering. Plasma Process Polym. 2012, 9, 1074–1085. [Google Scholar] [CrossRef]
- Mariotti, D.a.R.M.S. Microplasmas for Nanomaterials Synthesis. J. Phys. D 2010, 43, 323001. [Google Scholar] [CrossRef]
- Yang, J.-S.; Pai, D.Z.; Chiang, W.-H. Microplasma-Enhanced Synthesis of Colloidal Graphene Quantum Dots at Ambient Conditions. Carbon 2019, 153, 315–319. [Google Scholar] [CrossRef]
- Chiang, W.-H.; Mariotti, D.; Sankaran, R.M.; Eden, J.G.; Ostrikov, K.K. Microplasmas for Advanced Materials and Devices. Adv. Mater. 2020, 32, e1905508. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, D.; Rahardja, M.R.; Fedotov, P.V.; Obraztsova, E.D.; Ostrikov, K.K.; Chiang, W.-H. Plasma-Bioresource-Derived Multifunctional Porous NGQD/AuNP Nanocomposites for Water Monitoring and Purification. Chem. Eng. J. 2023, 451, 139083. [Google Scholar] [CrossRef]
- Yeh, T.F.; Huang, W.L.; Chung, C.J.; Chiang, I.T.; Chen, L.C.; Chang, H.Y.; Su, W.C.; Cheng, C.; Chen, S.J.; Teng, H. Elucidating Quantum Confinement in Graphene Oxide Dots Based on Excitation-Wavelength-Independent Photoluminescence. J. Phys. Chem. Lett. 2016, 7, 2087–2092. [Google Scholar] [CrossRef]
- Jiao, Y.; Gong, X.; Han, H.; Gao, Y.; Lu, W.; Liu, Y.; Xian, M.; Shuang, S.; Dong, C. Facile Synthesis of Orange Fluorescence Carbon Dots with Excitation Independent Emission for pH Sensing and Cellular Imaging. Anal. Chim. Acta 2018, 1042, 125–132. [Google Scholar] [CrossRef]
- Kurniawan, D.; Sharma, N.; Rahardja, M.R.; Cheng, Y.Y.; Chen, Y.T.; Wu, G.X.; Yeh, Y.Y.; Yeh, P.C.; Ostrikov, K.K.; Chiang, W.H. Plasma Nanoengineering of Bioresource-Derived Graphene Quantum Dots as Ultrasensitive Environmental Nanoprobes. ACS Appl. Mater. Interfaces 2022, 14, 52289–52300. [Google Scholar] [CrossRef]
- Kurniawan, D.; Chiang, W.-H. Microplasma-Enabled Colloidal Nitrogen-Doped Graphene Quantum Dots for Broad-Range Fluorescent pH Sensors. Carbon 2020, 167, 675–684. [Google Scholar] [CrossRef]
- Das, R.; Parveen, S.; Bora, A.; Giri, P.K. Origin of High Photoluminescence Yield and High SERS Sensitivity of Nitrogen-Doped Graphene Quantum Dots. Carbon 2020, 160, 273–286. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, Z.; Wang, M.; Zhu, L.; Cheng, X.; Cao, D.; Guan, R.; Zhou, C. Application of Graphene Quantum Dots in the Detection of Hg2+ and ClO− and Analysis of Detection Mechanism. Diam. Relat. Mater. 2021, 117, 108454. [Google Scholar] [CrossRef]
- Soni, H.; Pamidimukkala, P. Liquid Crystalline Multilayer Graphene Quantum Dots with Hackelite Structures: Characterisation and Application for Sensing Nitrophenols. Sens. Actuators B Chem. 2018, 268, 100–107. [Google Scholar] [CrossRef]
- Chen, C.; Hildebrandt, N. Resonance Energy Transfer to Gold Nanoparticles: NSET Defeats FRET. Trends Anal. Chem. 2020, 123, 115748. [Google Scholar] [CrossRef]
- Zu, F.; Yan, F.; Bai, Z.; Xu, J.; Wang, Y.; Huang, Y.; Zhou, X. The Quenching of the Fluorescence of Carbon Dots: A Review on Mechanisms and Applications. Microchim. Acta 2017, 184, 1899–1914. [Google Scholar] [CrossRef]
- Anh, N.T.N.; Chang, P.-Y.; Doong, R.-A. Sulfur-Doped Graphene Quantum Dot-Based Paper Sensor for Highly Sensitive and Selective Detection of 4-Nitrophenol in Contaminated Water and Wastewater. RSC Adv. 2019, 9, 26588–26597. [Google Scholar]
- Sun, P.P.; Araud, E.M.; Huang, C.; Shen, Y.; Monroy, G.L.; Zhong, S.; Tong, Z.; Boppart, S.A.; Eden, J.G.; Nguyen, T.H. Disintegration of Simulated Drinking Water Biofilms with Arrays of Microchannel Plasma Jets. NPJ Biofilms Microbiomes 2018, 4, 24. [Google Scholar] [CrossRef]
- Lin, L.; Pho, H.Q.; Zong, L.; Li, S.; Pourali, N.; Rebrov, E.; Tran, N.N.; Ostrikov, K.K.; Hessel, V. Microfluidic Plasmas: Novel Technique for Chemistry and Chemical Engineering. Chem. Eng. J. 2021, 417, 129355. [Google Scholar] [CrossRef]
- Chatzimarkou, A.; Chatzimitakos, T.G.; Kasouni, A.; Sygellou, L.; Avgeropoulos, A.; Stalikas, C.D. Selective FRET-Based Sensing of 4-Nitrophenol and Cell Imaging Capitalizing on the Fluorescent Properties of Carbon Nanodots from Apple Seeds. Sens. Actuators B Chem. 2018, 258, 1152–1160. [Google Scholar] [CrossRef]
- Dai, H.; Deng, Z.; Zeng, Y.; Zhang, J.; Yang, Y.; Ma, Q.; Hu, W.; Guo, L.; Li, L.; Wan, S.; et al. Highly Sensitive Determination of 4-Nitrophenol with Coumarin-Based Fluorescent Molecularly Imprinted Poly (Ionic Liquid). J. Hazard. Mater. 2020, 398, 122854. [Google Scholar] [CrossRef]
- Dang, D.K.; Sundaram, C.; Ngo, Y.-L.T.; Choi, W.M.; Chung, J.S.; Kim, E.J.; Hur, S.H. Pyromellitic Acid-Derived Highly Fluorescent N-Doped Carbon Dots for the Sensitive and Selective Determination of 4-Nitrophenol. Dye. Pigm. 2019, 165, 327–334. [Google Scholar] [CrossRef]
- Shu, T.; Wang, J.; Lin, X.; Zhou, Z.; Liang, F.; Su, L.; Zhang, X. Dual-Emissive Gold Nanoclusters for Label-Free and Separation-Free Ratiometric Fluorescence Sensing of 4-Nitrophenol Based on the Inner Filter Effect. J. Mater. Chem. C 2018, 6, 5033–5038. [Google Scholar] [CrossRef]
- Wang, F.; Fu, X.; Chai, X.; Han, Q.; Wang, H.; Hao, Q. Highly Selective Fluorometric Detection of para-Nitrophenol from Its Isomers by Nitrogen-Doped Graphene Quantum Dots. Microchem. J. 2021, 168, 106389. [Google Scholar] [CrossRef]
- Wang, X.; Zuo, Y.; Feng, S. Ultrasensitive Polysiloxane-Based Fluorescent Probes for Selectively Detecting of 4-Nitrophenol and Their Application in Paper Sensors. Mater. Today Commun. 2020, 25, 101570. [Google Scholar] [CrossRef]
- Yang, J.-M.; Hu, X.-W.; Liu, Y.-X.; Zhang, W. Fabrication of a Carbon Quantum Dots-Immobilized Zirconium-Based Metal-Organic Framework Composite Fluorescence Sensor for Highly Sensitive Detection of 4-Nitrophenol. Microporous Mesoporous Mater. 2019, 274, 149–154. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, D.; Ding, Y.; Hua, J.; Tang, B.; Ji, X.; Zhang, Q.; Wei, Y.; Qin, K.; Li, B. Bacteria-Derived Fluorescent Carbon Dots for Highly Selective Detection of p-Nitrophenol and Bioimaging. Analyst 2019, 144, 5497–5503. [Google Scholar] [CrossRef] [PubMed]
- Venugopalan, P.; Vidya, N. Microwave Assisted Green Synthesis of Carbon Dots from Sweet Flag (Acorus calamus) for Fluorescent Sensing of 4-Nitrophenol. J. Photochem. Photobiol. A 2023, 439, 114625. [Google Scholar] [CrossRef]
- Chen, J.; Xia, X.; Li, P.; Yu, H.; Xie, Y.; Guo, Y.; Yao, W.; Qian, H.; Cheng, Y. Crayfish Shells-Derived Carbon Dots as a Fluorescence Sensor for the Selective Detection of 4-Nitrophenol. Food Agric. Immunol. 2023, 34, 36–47. [Google Scholar] [CrossRef]
- Wang, K.; Tan, L.; Zhang, Y.; Zhang, D.; Wang, N.; Wang, J. A Molecular Imprinted Fluorescence Sensor Based on Carbon Quantum Dots for Selective Detection of 4-Nitrophenol in Aqueous Environments. Mar. Pollut. Bull. 2023, 187, 114587. [Google Scholar] [CrossRef]
- Wang, H.; Ma, S.; Sun, Y.; Gao, M.; Wang, X. Detection of 4-Nitrophenol by a Naphthene Carboxylic Acid-Based Fluorescent Dicationic Ionic Liquid in Environmental Waters and Soils. Microchem. J. 2023, 190, 108720. [Google Scholar] [CrossRef]
- Luo, K.; Luo, X.; Wu, Y.; Liang, Z.; Kang, X.; Wen, Y. Synthesis of Graphene Quantum Dots with Temperature-Sensitive Properties from Sea Rice for Rapid and Highly Selective Detection of 4-Nitrophenol. Diam. Relat. Mater. 2023, 135, 109849. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, R.; Wang, X.; Liu, F.; Gao, Y.; Guan, R.; Chen, Y. Flexible and Washable CDs@Eu-MOFs/PVDF Multifunctional Thin Films as Highly Selective Sensing for Nitrobenzene and 4-Nitrophenol. Inorg. Chem. Commun. 2023, 149, 110423. [Google Scholar] [CrossRef]
- Han, Y.; Wang, Y.; Zhang, H.; Zhao, L.; Qiu, H. Facile Synthesis of Yellow-Green Fluorescent Silicon Nanoparticles and Their Application in Detection of Nitrophenol Isomers. Talanta 2023, 257, 124347. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Shao, R.; Wang, Q.; Gao, Y.; Ma, Y.; Guan, R.; Yang, T. Eu Doped Zn-MOF Nanofiber Fluorescent Membrane and Its Multifunctional Detection of Nitroaromatic Compounds and Fe3+. Polyhedron 2023, 235, 116363. [Google Scholar] [CrossRef]
- Fu, J.; Zhou, S.; Zhao, P.; Wu, X.; Tang, S.; Chen, S.; Yang, Z.; Zhang, Z. A Dual-Response Ratiometric Fluorescence Imprinted Sensor Based on Metal-Organic Frameworks for Ultrasensitive Visual Detection of 4-Nitrophenol in Environments. Biosens. Bioelectron. 2022, 198, 113848. [Google Scholar] [CrossRef] [PubMed]
- El-Shaheny, R.; Yoshida, S.; Fuchigami, T. Graphene Quantum Dots as a Nanoprobe for Analysis of o- and p-Nitrophenols in Environmental Water Adopting Conventional Fluorometry and Smartphone Image Processing-Assisted Paper-Based Analytical Device. In-Depth Study of Sensing Mechanisms. Microchem. J. 2020, 158, 105241. [Google Scholar] [CrossRef]

| Added Amounts (mL) | Final Concentrations | |||
|---|---|---|---|---|
| 0.3 mg/mL N-GQD Mother Solution | DI Water | 300 μM 4-NP Mother Solution | N-GQDs (mg/mL) | 4-NP (μM) |
| 1.000 | 1.995 | 0.005 | 0.1 | 0.5 |
| 1.990 | 0.01 | 1 | ||
| 1.975 | 0.025 | 2.5 | ||
| 1.950 | 0.05 | 5 | ||
| 1.925 | 0.075 | 7.5 | ||
| 1.900 | 0.1 | 10 | ||
| 1.875 | 0.125 | 12.5 | ||
| 1.833 | 0.1667 | 16.67 | ||
| 1.750 | 0.25 | 25 | ||
| 1.667 | 0.3333 | 33.33 | ||
| 1.583 | 0.4167 | 41.67 | ||
| 1.500 | 0.5 | 50 | ||
| 1.433 | 0.5667 | 56.67 | ||
| 1.250 | 0.75 | 75 | ||
| 1.000 | 1 | 100 | ||
| 0.750 | 1.25 | 125 | ||
| 0.500 | 1.5 | 150 | ||
| No. | Precursor | Synthesis Method | Reaction Parameters | Materials | Linear Range (µM) | R2 | LoD (nM) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Chitosan | Microplasma | 1 h 9.6 mA | N-GQDs | 0.5–100 | 0.995 | 95.14 | This study |
| 2 | Hydroquinone and EDA | Hydrothermal | 2 h 50 °C | PCDs | 0.5–60 | 0.991 | 260 | [1] |
| 3 | Apple seeds | Pyrolysis | 1 h 300 °C | CNDs | 0.05–53 | 0.994 | 13 | [57] |
| 4 | Coumarin-FL-IL, EGDMA, IL [V2C4(mim)2][(PF6)2] | Polymerization | 15 min | Coumarin-based FL-MIPIL | 0.001–7.5 | 0.992 | 0.5 | [58] |
| FL-MIPIL and EGDMA | Polymerization | 15 min | Coumarin-based FL-EGDMA-MIP | 0.05–7.5 | 0.991 | 10 | ||
| 5 | PA and EDA | One Pot hydrothermal | 6 h 200 °C | N-CDs | 0.1–100 | 0.999 | 17 | [59] |
| 6 | CA and 3-MPA | Pyrolysis | 45 min 200 °C | S-GQDs | 0.01–−1 1–200 | 0.979 0.984 | 0.7 | [54] |
| 7 | BSA and HAuCl4 | Incubation | 12 h 37 °C | Dual-emissive GNCs | 0.05–5 | 0.991 | 13.8 | [60] |
| 8 | H4BF4N and C6H5Na3O7 | Hydrothermal | 5 h 200 °C | N-GQDs | 0–20 | 0.999 | 290 | [61] |
| 9 | Polymethylvinylsiloxane P500 and 4-Bromotriphenylamine | Heck reaction | 48 h | BpaP | 0–50 | 0.940 | 600 | [62] |
| 2,4,6,8-tetramethyltetravinylcyclotetra-siloxane and 4-Bromotriphenylamine | Heck reaction | 48 h | BpaD | 0–50 | 0.935 | 230 | ||
| 10 | UiO-66 nanocrystals and amine-CQDs | Immersion | 12 h Room temperature | Amine-CQDs@UiO-66 | 0.01–20 | 0.990 | 3.5 | [63] |
| 11 | Bacillus cereus MYB41-22 | Hydrothermal | 12 h 200 °C | CDs-BC | 0.3–6.5 6.5–30 | 0.999 0.991 | 110 | [64] |
| 12 | Sweet flag (Acorus calamus) | Microwave irradiation | 25 min | CDs | 0–14.28 | 0.997 | 207 | [65] |
| 13 | Crayfish shells | Hydrothermal | 8 h 180 °C | CDs | 0–50 | 0.996 | 160 | [66] |
| 14 | CA and O-PDA (CQDs); PTMS and TEOS (MIP@CQDs) | Hydrothermal (CQDs); Sol-gel imprinting (MIP@CQDs) | 8 h, 160 °C (CQDs); 12 h (MIP@CQDs) | MIP@CQDs | 0–144 | 0.995 | 410 | [67] |
| 15 | 1-propyl-3-methylimidazol and 1,3-dibromopropane | Mixing and heating | 20 h 60 °C | [C3(MIM)2] [NA]2 | 1–500 | 0.999 | 300 | [68] |
| 16 | Sea rice | Mild oxidation | 8 h 26 °C | GQDs | 0–1000 | 0.996 | 34 | [69] |
| 17 | Glucose (CDs); Eu(NO3)3⋅6H2O, 1,10-phen, H4btec, NaOH (Eu-MOF) | Hydrothermal carbonization (CDs); Hydrothermal (Eu-MOF) | 3 h, 160 °C (CDs); 48 h, 140 °C (Eu-MOF) | CDs@Eu-MOF | 0–68.96 | 0.996 | 40.04 | [70] |
| CDs@Eu-MOF/PVDF film | 0–129.3 | 0.998 | 72.41 | |||||
| 18 | DAPH and DAMO | One-step room temperature synthesis | 3 h Room temperature | SiNPs | 0.05–600 | 0.983 | 3.3 | [71] |
| 19 | Eu(NO3)3 · 6H2O, Zn(CH3COO)2·2H2O, and H3BTC | Solvothermal | 24 h 120 °C | Zn(Eu)-MOF | 0–129.3 | 0.977 | 3154.9 | [72] |
| Eu(NO3)3 · 6H2O, Zn(CH3COO)2·2H2O, H3BTC, and PAN | In-situ growth and mixed spinning | 10 h 60 °C | Zn(Eu)-MOF@PAN NFM | 0–129.3 | 0.989 | 2719.6 | ||
| 20 | CA and EDA (CDs); Zn(NO3)2·6H2O, CDs, and 2-methylimidazole (CDs@ZIF-8); Te, NaBH4, CdCl2, MPA (NIR CdTe QDs); CDs@ZIF-8, NIR CdTe QDs, TEOS, NH3·H2O, APTES (CDs@ZIF-8/CdTe@MIP) | Hydrothermal (CDs); Self-assembly (CDs@ZIF-8); Hydrothermal (NIR CdTe QDs); Mixing (CDs@ZIF-8/CdTe@MIP) | 5 h, 200 °C (CDs); 30 min (CDs@ZIF-8); 1 h, 100 °C (NIR CdTe QDs); 12 h, room temperature (CDs@ZIF-8/CdTe@MIP) | CDs@ZIF-8/CdTe@MIP | 1 × 10−7–3 × 10−6 | 0.995 | 8 × 10−5 | [73] |
| 0.05–30 | 0.996 | 50 | ||||||
| 21 | DTPA | Hydrothermal | 8 h 200 °C | GQDs | 0.5–350 | 0.962 | 154 | [51] |
| 22 | - | - | - | Commercial GQDs | 1.44–287.6 | 0.993 | 210 | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahardja, M.R.; Kurniawan, D.; Chiang, W.-H. Microplasma-Enabled Sustainable Synthesis of Nitrogen-Doped Graphene Quantum Dots for Sensitive Detection of 4-Nitrophenol. Chemosensors 2023, 11, 390. https://doi.org/10.3390/chemosensors11070390
Rahardja MR, Kurniawan D, Chiang W-H. Microplasma-Enabled Sustainable Synthesis of Nitrogen-Doped Graphene Quantum Dots for Sensitive Detection of 4-Nitrophenol. Chemosensors. 2023; 11(7):390. https://doi.org/10.3390/chemosensors11070390
Chicago/Turabian StyleRahardja, Michael Ryan, Darwin Kurniawan, and Wei-Hung Chiang. 2023. "Microplasma-Enabled Sustainable Synthesis of Nitrogen-Doped Graphene Quantum Dots for Sensitive Detection of 4-Nitrophenol" Chemosensors 11, no. 7: 390. https://doi.org/10.3390/chemosensors11070390
APA StyleRahardja, M. R., Kurniawan, D., & Chiang, W.-H. (2023). Microplasma-Enabled Sustainable Synthesis of Nitrogen-Doped Graphene Quantum Dots for Sensitive Detection of 4-Nitrophenol. Chemosensors, 11(7), 390. https://doi.org/10.3390/chemosensors11070390







