Sub-ppm NO2 Detection through Chipless RFID Sensor Functionalized with Reduced SnO2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chipless RFID Resonator Structure
2.2. Sensing Material Synthesis, Characterization and Deposition
2.3. Test Bench Setup and RF Measurements
3. Results and Discussion
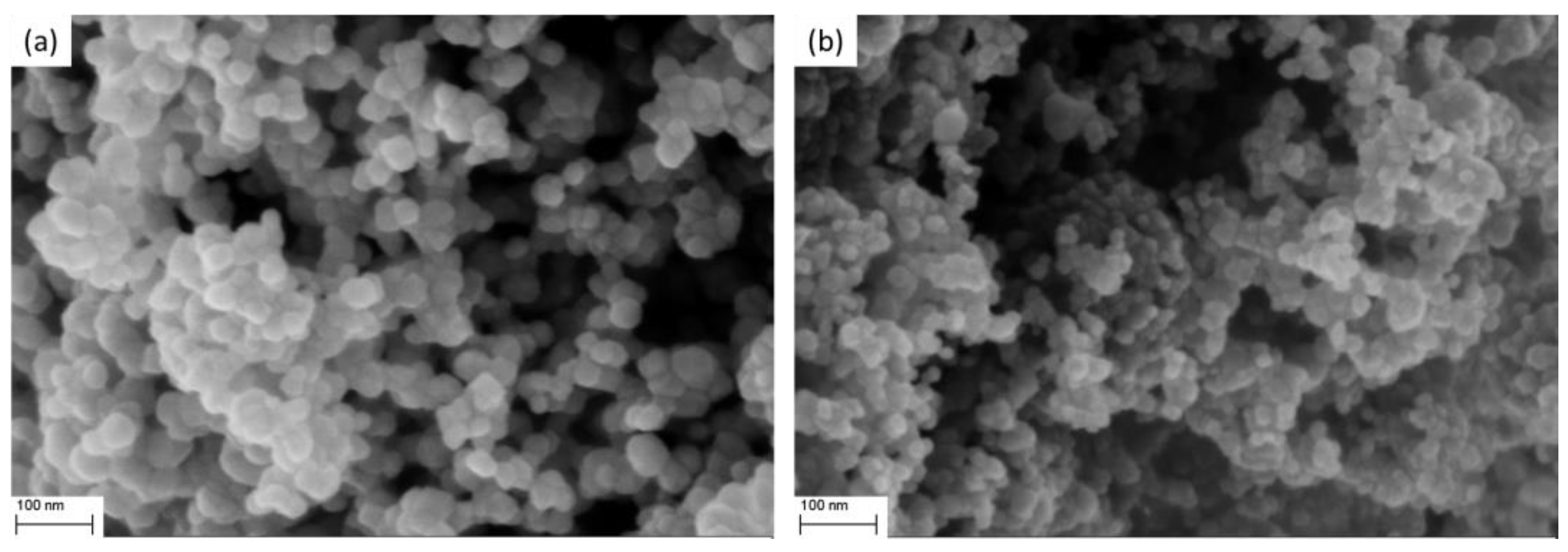
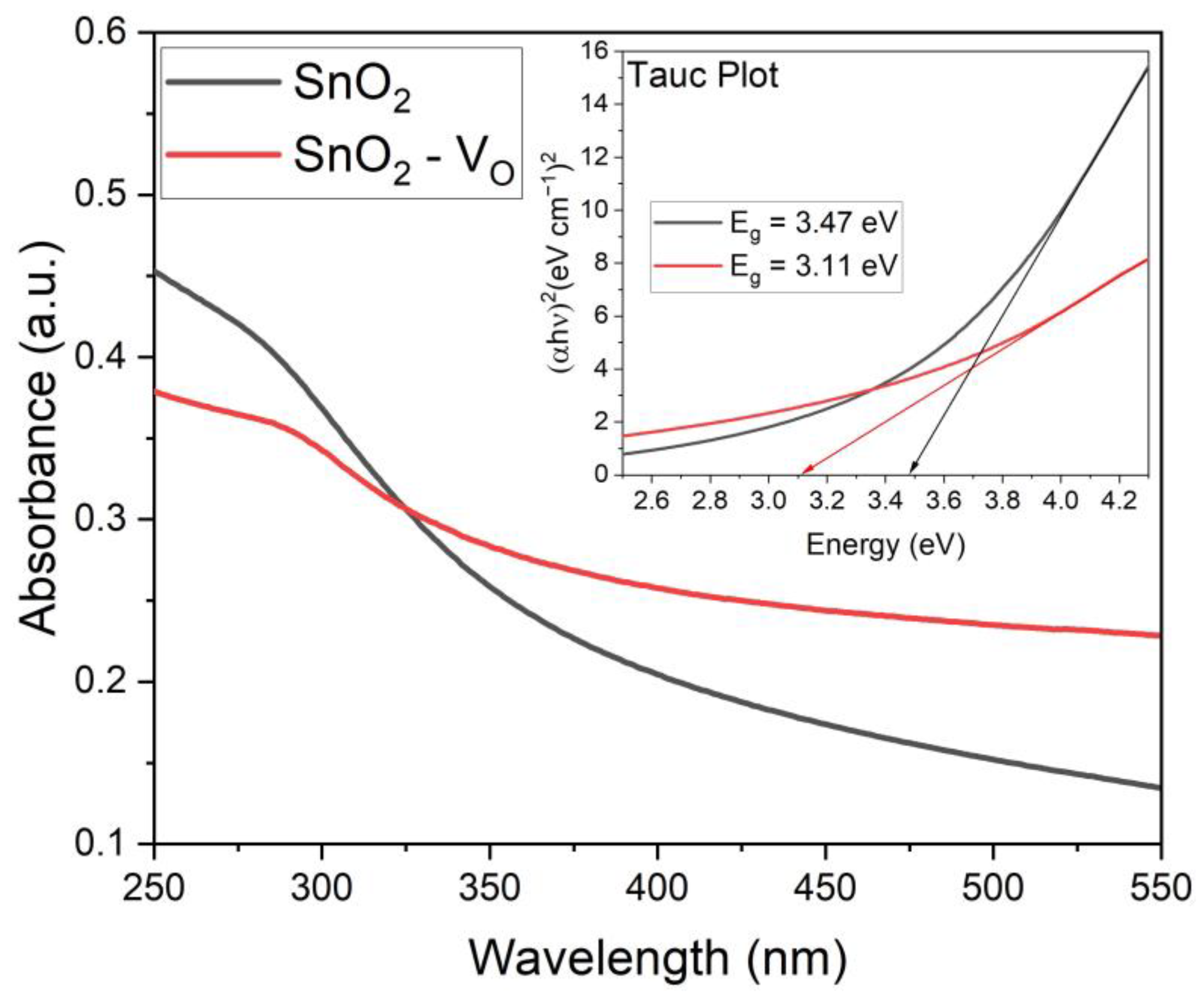
3.1. Sensing Material Characterization
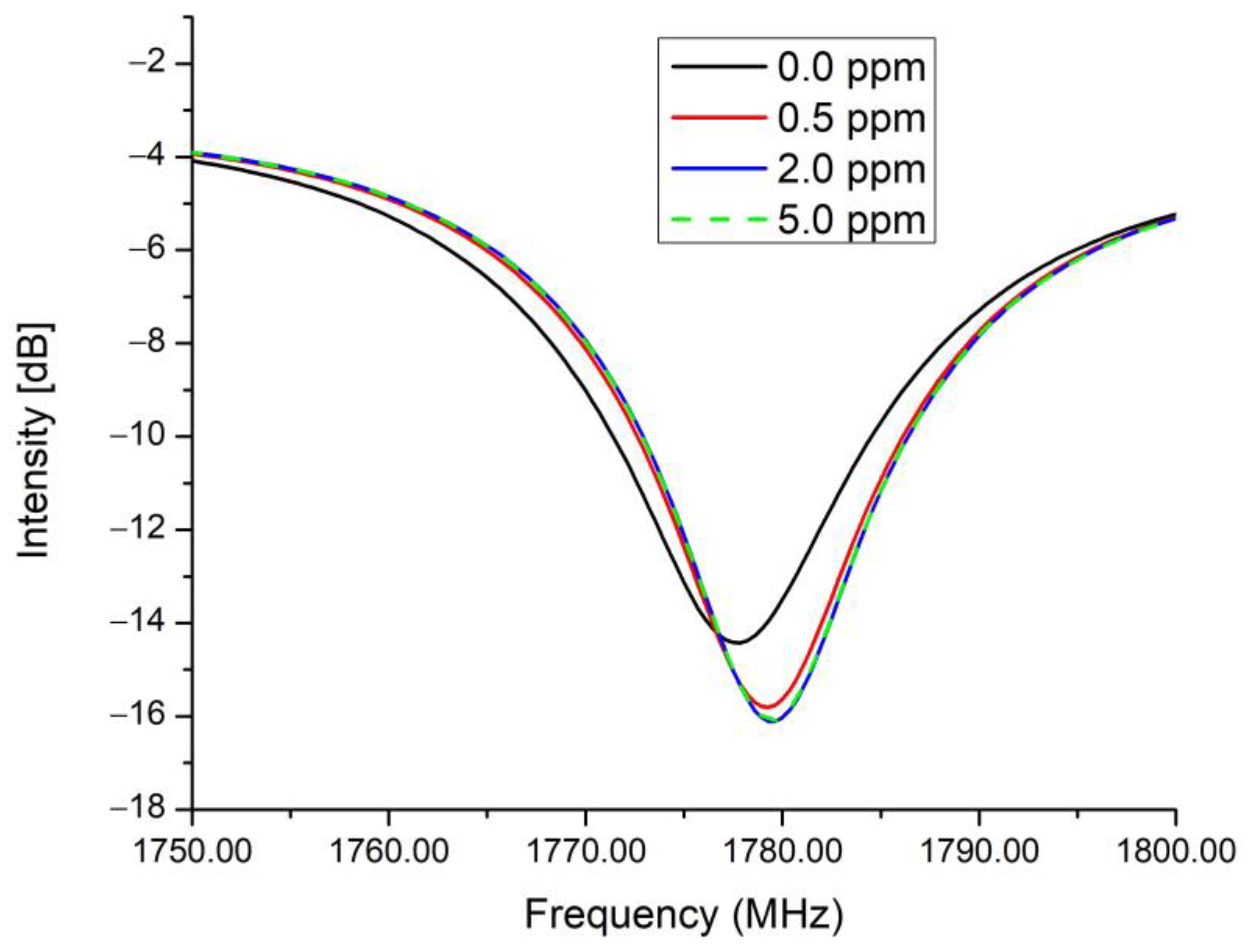
3.2. Performance of Chipless RFID Sensor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NITROGEN DIOXIDE|Occupational Safety and Health Administration. Available online: https://www.osha.gov/chemicaldata/21 (accessed on 20 March 2023).
- Rydosz, A.; Brudnik, A.; Staszek, K. Metal Oxide Thin Films Prepared by Magnetron Sputtering Technology for Volatile Organic Compound Detection in the Microwave Frequency Range. Materials 2019, 12, 877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malik, R.; Tomer, V.K.; Mishra, Y.K.; Lin, L. Functional Gas Sensing Nanomaterials: A Panoramic View. Appl. Phys. Rev. 2020, 7, 021301. [Google Scholar] [CrossRef] [Green Version]
- Paleczek, A.; Grochala, D.; Staszek, K.; Wincza, K.; Gruszczynski, S.; Rydosz, A. Microwave-Based Nitrogen Dioxide Gas Sensor For Automotive Applications. In Proceedings of the 2021 International Conference on Electrical, Computer, Communications and Mechatronics Engineering (ICECCME), Réduit, Mauritius, 7–8 October 2021; pp. 1–4. [Google Scholar]
- Spagnoli, E.; Krik, S.; Fabbri, B.; Valt, M.; Ardit, M.; Gaiardo, A.; Vanzetti, L.; Della Ciana, M.; Cristino, V.; Vola, G.; et al. Development and Characterization of WO3 Nanoflakes for Selective Ethanol Sensing. Sens. Actuators B Chem. 2021, 347, 130593. [Google Scholar] [CrossRef]
- Gaiardo, A.; Bellutti, P.; Fabbri, B.; Gherardi, S.; Giberti, A.; Guidi, V.; Landini, N.; Malagù, C.; Pepponi, G.; Valt, M.; et al. Chemoresistive Gas Sensor Based on SiC Thick Film: Possible Distinctive Sensing Properties between H2S and SO2. Procedia Eng. 2016, 168, 276–279. [Google Scholar] [CrossRef]
- Neri, G. First Fifty Years of Chemoresistive Gas Sensors. Chemosensors 2015, 3, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Xuan, W.; Bermak, A.; Boussaid, F.; Tsui, C.-Y.; Luo, J. Dual Transduction on a Single Sensor for Gas Identification. Sens. Actuators B Chem. 2019, 278, 21–27. [Google Scholar] [CrossRef]
- Elnemr, Y.E.; Abu-Libdeh, A.; Raj, G.C.A.; Birjis, Y.; Nazemi, H.; Munirathinam, P.; Emadi, A. Multi-Transduction-Mechanism Technology, an Emerging Approach to Enhance Sensor Performance. Sensors 2023, 23, 4457. [Google Scholar] [CrossRef]
- Dey, S.; Saha, J.K.; Karmakar, N.C. Smart Sensing: Chipless RFID Solutions for the Internet of Everything. IEEE Microw. 2015, 16, 26–39. [Google Scholar] [CrossRef]
- Muñoz-Enano, J.; Vélez, P.; Gil, M.; Martín, F. Planar Microwave Resonant Sensors: A Review and Recent Developments. Appl. Sci. 2020, 10, 2615. [Google Scholar] [CrossRef]
- Schueler, M.; Mandel, C.; Puentes, M.; Jakoby, R. Metamaterial Inspired Microwave Sensors. IEEE Microw. 2012, 13, 57–68. [Google Scholar] [CrossRef]
- Singh, R.; Singh, E.; Nalwa, H.S. Inkjet Printed Nanomaterial Based Flexible Radio Frequency Identification (RFID) Tag Sensors for the Internet of Nano Things. RSC Adv. 2017, 7, 48597–48630. [Google Scholar] [CrossRef] [Green Version]
- Mulloni, V.; Donelli, M. Chipless RFID Sensors for the Internet of Things: Challenges and Opportunities. Sensors 2020, 20, 2135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patre, S.R. Passive Chipless RFID Sensors: Concept to Applications—A Review. IEEE J. Radio Freq. Identif. 2022, 6, 64–76. [Google Scholar] [CrossRef]
- Devkota, J.; Ohodnicki, P.R.; Greve, D.W. SAW Sensors for Chemical Vapors and Gases. Sensor 2017, 17, 801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadihaji, A.; Izquierdo, R.; Shih, A. From Chip-Based to Chipless RFID Sensors: A Review. IEEE Sens. J. 2023, 23, 11356–11373. [Google Scholar] [CrossRef]
- Herrojo, C.; Paredes, F.; Mata-Contreras, J.; Martín, F. Chipless-RFID: A Review and Recent Developments. Sensors 2019, 19, 3385. [Google Scholar] [CrossRef] [Green Version]
- Karmakar, N.C.; Amin, E.M.; Saha, J.K. Chipless RFID Sensors; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- El Matbouly, H.; Tedjini, S.; Zannas, K.; Duroc, Y. Chipless RFID Threshold Temperature Sensor Compliant with UHF and ISM Radio Frequency. In Proceedings of the 2018 2nd URSI Atlantic Radio Science Meeting (AT-RASC), Gran Canaria, Spain, 28 May–1 June 2018; pp. 1–4. [Google Scholar]
- Marchi, G.; Mulloni, V.; Manekiya, M.; Donelli, M.; Lorenzelli, L. A Preliminary Microwave Frequency Characterization of a Nafion-Based Chipless Sensor for Humidity Monitoring. In Proceedings of the 2020 IEEE SENSORS, Rotterdam, The Netherlands, 25–28 October 2020; pp. 1–4. [Google Scholar]
- Tao, B.; Feng, L.; Miao, F.; Zang, Y. High Sensitivity Chipless RFID Humidity Sensor Tags Are Based on SnO2/G Nanomaterials. Vacuum 2022, 202, 111126. [Google Scholar] [CrossRef]
- Amin, E.M.; Saha, J.K.; Karmakar, N.C. Smart Sensing Materials for Low-Cost Chipless RFID Sensor. IEEE Sens. J. 2014, 14, 2198–2207. [Google Scholar] [CrossRef]
- Lee, J.S.; Oh, J.; Jun, J.; Jang, J. Wireless Hydrogen Smart Sensor Based on Pt/Graphene-Immobilized Radio-Frequency Identification Tag. ACS Nano 2015, 9, 7783–7790. [Google Scholar] [CrossRef]
- Haiduk, Y.S.; Khort, A.A.; Lapchuk, N.M.; Savitsky, A.A. Study of WO3–In2O3 Nanocomposites for Highly Sensitive CO and NO2 Gas Sensors. J. Solid State Chem. 2019, 273, 25–31. [Google Scholar] [CrossRef]
- Morais, P.V.; Suman, P.H.; Silva, R.A.; Orlandi, M.O. High Gas Sensor Performance of WO3 Nanofibers Prepared by Electrospinning. J. Alloys Compd. 2021, 864, 158745. [Google Scholar] [CrossRef]
- Gaiardo, A.; Fabbri, B.; Giberti, A.; Guidi, V.; Bellutti, P.; Malagù, C.; Valt, M.; Pepponi, G.; Gherardi, S.; Zonta, G.; et al. ZnO and Au/ZnO Thin Films: Room-Temperature Chemoresistive Properties for Gas Sensing Applications. Sens. Actuators B Chem. 2016, 237, 1085–1094. [Google Scholar] [CrossRef]
- Su, P.-G.; Li, M.-C. Recognition of Binary Mixture of NO2 and NO Gases Using a Chemiresistive Sensors Array Combined with Principal Component Analysis. Sens. Actuators A Phys. 2021, 331, 112980. [Google Scholar] [CrossRef]
- Lou, C.; Wang, K.; Mei, H.; Xie, J.; Zheng, W.; Liu, X.; Zhang, J. ZnO Nanoarrays via a Thermal Decomposition–Deposition Method for Sensitive and Selective NO2 Detection. CrystEngComm 2021, 23, 3654–3663. [Google Scholar] [CrossRef]
- Zhou, L.; Hu, Z.; Wang, P.; Gao, N.; Zhai, B.; Ouyang, M.; Zhang, G.; Chen, B.; Luo, J.; Jiang, S.; et al. Enhanced NO2 Sensitivity of SnO2 SAW Gas Sensors by Facet Engineering. Sens. Actuators B Chem. 2022, 361, 131735. [Google Scholar] [CrossRef]
- Jeong, H.-S.; Park, M.-J.; Kwon, S.-H.; Joo, H.-J.; Song, S.-H.; Kwon, H.-I. Low Temperature NO2 Sensing Properties of RF-Sputtered SnO-SnO2 Heterojunction Thin-Film with p-Type Semiconducting Behavior. Ceram. Int. 2018, 44, 17283–17289. [Google Scholar] [CrossRef]
- Devabharathi, N.; Umarji, A.M.; Dasgupta, S. Fully Inkjet-Printed Mesoporous SnO2-Based Ultrasensitive Gas Sensors for Trace Amount NO2 Detection. ACS Appl. Mater. Interfaces 2020, 12, 57207–57217. [Google Scholar] [CrossRef]
- Feng, Z.; Gaiardo, A.; Valt, M.; Fabbri, B.; Casotti, D.; Krik, S.; Vanzetti, L.; Ciana, M.D.; Fioravanti, S.; Caramori, S.; et al. Investigation on Sensing Performance of Highly Doped Sb/SnO2. Sensors 2022, 22, 1233. [Google Scholar] [CrossRef]
- Krik, S.; Gaiardo, A.; Valt, M.; Fabbri, B.; Malagù, C.; Pepponi, G.; Casotti, D.; Cruciani, G.; Guidi, V.; Bellutti, P. Influence of Oxygen Vacancies in Gas Sensors Based on Metal-Oxide Semiconductors: A First-Principles Study. In Sensors and Microsystems, Proceedings of the 20th AISEM 2019 National Conference, Naples, Italy, 11–13 February 2019; Di Francia, G., Di Natale, C., Alfano, B., De Vito, S., Esposito, E., Fattoruso, G., Formisano, F., Massera, E., Miglietta, M.L., Polichetti, T., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 309–314. [Google Scholar]
- Al-Hashem, M.; Akbar, S.; Morris, P. Role of Oxygen Vacancies in Nanostructured Metal-Oxide Gas Sensors: A Review. Sens. Actuators B Chem. 2019, 301, 126845. [Google Scholar] [CrossRef]
- Pushkar, P.; Gupta, V.R. A Design Rule for an ELC Resonator. In Proceedings of the 2015 International Conference on Innovations in Information, Embedded and Communication Systems (ICIIECS), Coimbatore, India, 19–20 March 2015; pp. 1–4. [Google Scholar]
- Marchi, G.; Mulloni, V.; Acerbi, F.; Donelli, M.; Lorenzelli, L. Tailoring the Performance of a Nafion 117 Humidity Chipless RFID Sensor: The Choice of the Substrate. Sensors 2023, 23, 1430. [Google Scholar] [CrossRef]
- Mulloni, V.; Marchi, G.; Lorenzelli, L.; Donelli, M. Chipless RFID Sensing System for Precise Ethanol Determination in Alcoholic Solutions. Electronics 2022, 11, 735. [Google Scholar] [CrossRef]
- Marchi, G.; Zanazzi, E.; Mulloni, V.; Donelli, M.; Lorenzelli, L. Electromagnetic Modeling Strategy Supporting the Fabrication of Inkjet-Printed Chipless RFID Sensors. IEEE J. Flex. Electron. 2023, 2, 145–152. [Google Scholar] [CrossRef]
- EMC Near-Field Probe Set with 40 DB Wideband Amplifier. Signal Hound. Available online: https://signalhound.com/products/emc-near-field-probe-set-40-db-wideband-amplifier/ (accessed on 17 June 2023).
- Speranza, G.; Canteri, R. RxpsG a New Open Project for Photoelectron and Electron Spectroscopy Data Processing. SoftwareX 2019, 10, 100282. [Google Scholar] [CrossRef]
- Shao, M.; Liu, J.; Ding, W.; Wang, J.; Dong, F.; Zhang, J. Oxygen Vacancy Engineering of Self-Doped SnO2−x Nanocrystals for Ultrasensitive NO2 Detection. J. Mater. Chem. C 2020, 8, 487–494. [Google Scholar] [CrossRef]
- De, A.K.; Sinha, I. Synergistic Effect of Ni Doping and Oxygen Vacancies on the Visible Light Photocatalytic Properties of Ag2O Nanoparticles. J. Phys. Chem. Solids 2022, 167, 110733. [Google Scholar] [CrossRef]
- Dangi, R.; Basnet, B.; Pandey, M.; Bhusal, S.; Budhathoki, B.; Parajuli, K.; Tiwari, S.K.; Kafle, B.P. Effect of Oxygen Vacancy on the Crystallinity and Optical Band Gap in Tin Oxide Thin Film. Energies 2023, 16, 2653. [Google Scholar] [CrossRef]
- Mesoudy, A.E.; Machon, D.; Ruediger, A.; Jaouad, A.; Alibart, F.; Ecoffey, S.; Drouin, D. Band Gap Narrowing Induced by Oxygen Vacancies in Reactively Sputtered TiO2 Thin Films. Thin Solid Film. 2023, 769, 139737. [Google Scholar] [CrossRef]
- Suzuki, T.; Watanabe, H.; Ueno, T.; Oaki, Y.; Imai, H. Significant Increase in Band Gap and Emission Efficiency of In2O3 Quantum Dots by Size-Tuning around 1 Nm in Supermicroporous Silicas. Langmuir 2017, 33, 3014–3017. [Google Scholar] [CrossRef]
- Kang, Y.-G.; Kim, H.-J.; Park, H.-G.; Kim, B.-Y.; Seo, D.-S. Tin Dioxide Inorganic Nanolevel Films with Different Liquid Crystal Molecular Orientations for Application in Liquid Crystal Displays (LCDs). J. Mater. Chem. 2012, 22, 15969–15975. [Google Scholar] [CrossRef]
- Kwoka, M.; Krzywiecki, M. Impact of Air Exposure and Annealing on the Chemical and Electronic Properties of the Surface of SnO2 Nanolayers Deposited by Rheotaxial Growth and Vacuum Oxidation. Beilstein J. Nanotechnol. 2017, 8, 514–521. [Google Scholar] [CrossRef] [Green Version]
- Babu, B.; Neelakanta Reddy, I.; Yoo, K.; Kim, D.; Shim, J. Bandgap tuning and XPS study of SnO2 quantum dots. Mater. Lett. 2018, 221, 211–215. [Google Scholar] [CrossRef]
- Idriss, H. On the wrong assignment of the XPS O1s signal at 531–532 eV attributed to oxygen vacancies in photo- and electro-catalysts for water splitting and other materials applications. Surf. Sci. 2021, 12, 121894. [Google Scholar] [CrossRef]
- Amin, E.M.; Karmakar, N.C.; Jensen, B.W. Fully Printable Chipless RFID Multi-Parameter Sensor. Sens. Actuators A Phys. 2016, 248, 223–232. [Google Scholar] [CrossRef]
- Maeng, S.; Kim, S.-W.; Lee, D.-H.; Moon, S.-E.; Kim, K.-C.; Maiti, A. SnO2 Nanoslab as NO2 Sensor: Identification of the NO2 Sensing Mechanism on a SnO2 Surface. ACS Appl. Mater. Interfaces 2014, 6, 357–363. [Google Scholar] [CrossRef]
- He, K.; Jin, Z.; Chu, X.; Bi, W.; Wang, W.; Wang, C.; Liu, S. Fast Response–Recovery Time toward Acetone by a Sensor Prepared with Pd Doped WO3 Nanosheets. RSC Adv. 2019, 9, 28439–28450. [Google Scholar] [CrossRef]
- Arafat, M.M.; Dinan, B.; Akbar, S.A.; Haseeb, A.S.M.A. Gas Sensors Based on One Dimensional Nanostructured Metal-Oxides: A Review. Sensors 2012, 12, 7207–7258. [Google Scholar] [CrossRef]
- Chizhov, A.S.; Rumyantseva, M.N.; Vasiliev, R.B.; Filatova, D.G.; Drozdov, K.A.; Krylov, I.V.; Marchevsky, A.V.; Karakulina, O.M.; Abakumov, A.M.; Gaskov, A.M. Visible Light Activation of Room Temperature NO2 Gas Sensors Based on ZnO, SnO2 and In2O3 Sensitized with CdSe Quantum Dots. Thin Solid Film 2016, 618, 253–262. [Google Scholar] [CrossRef]




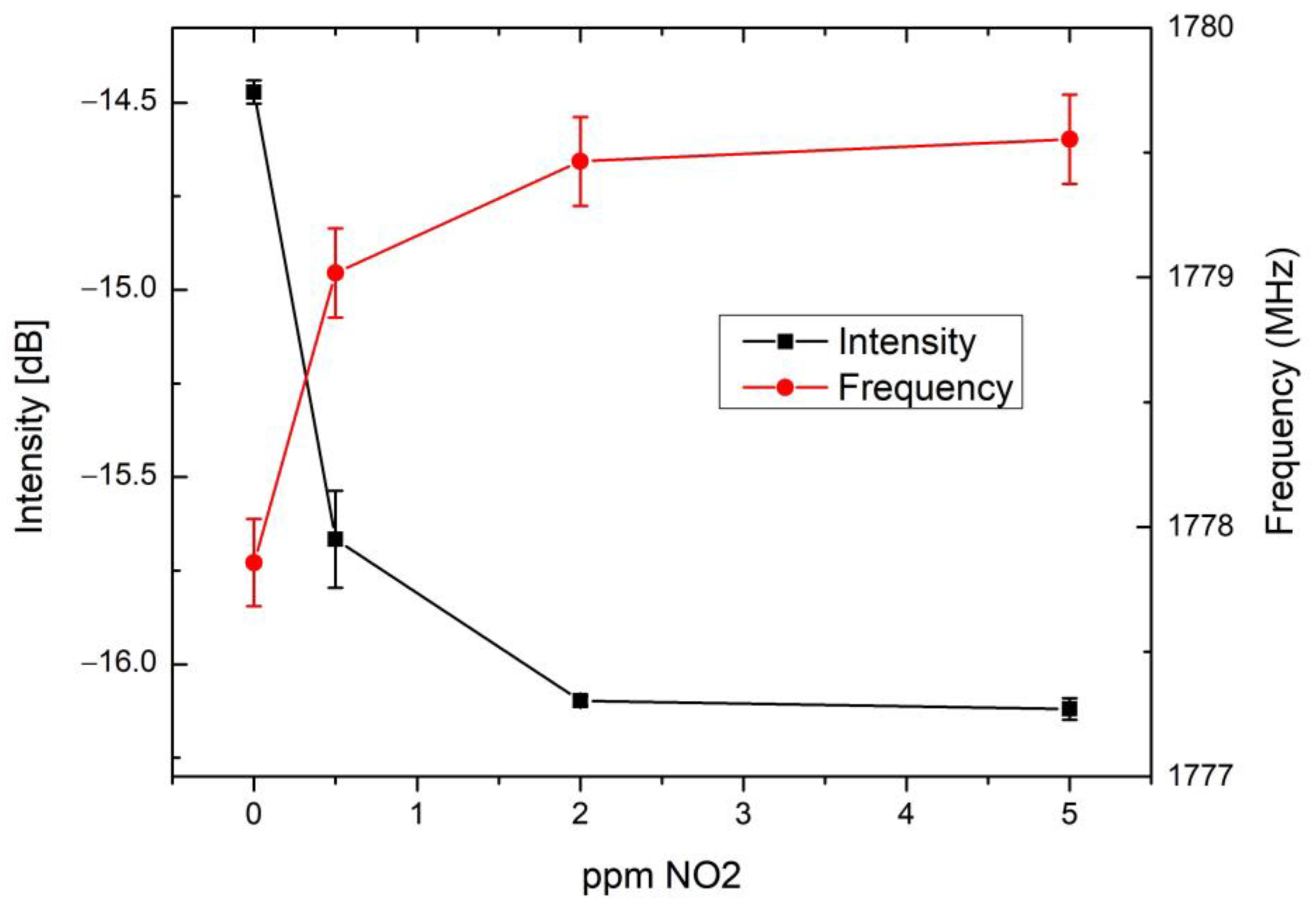
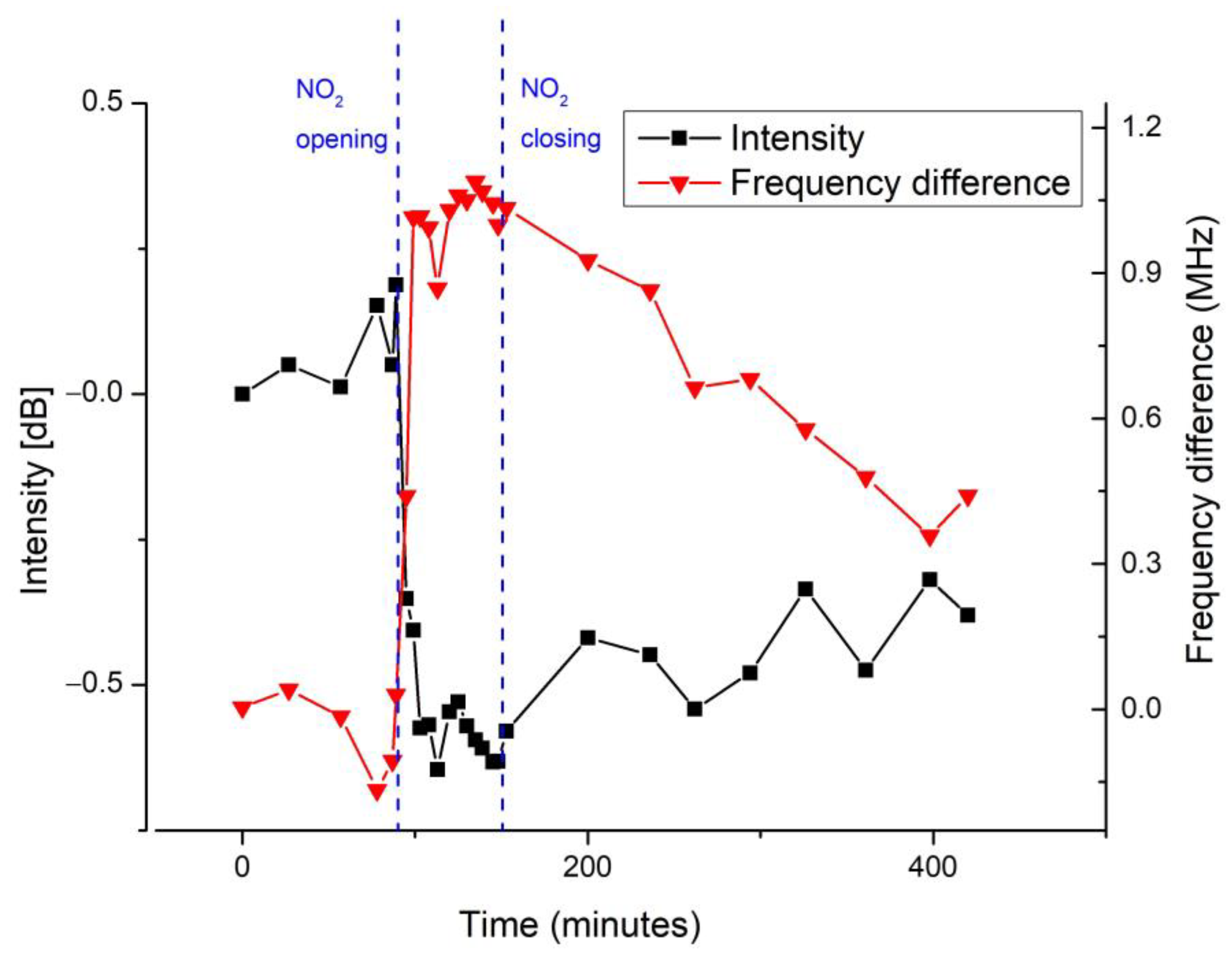
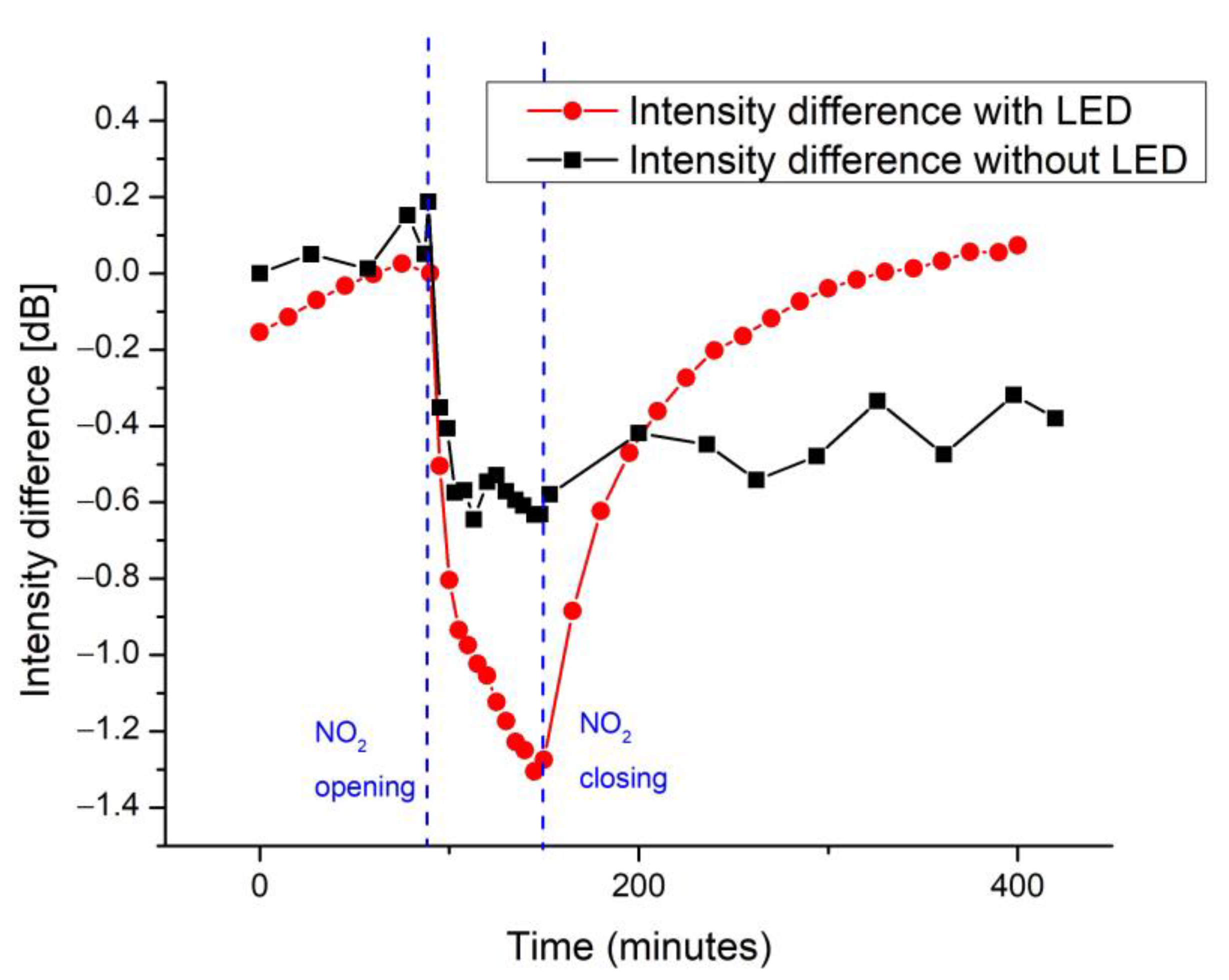

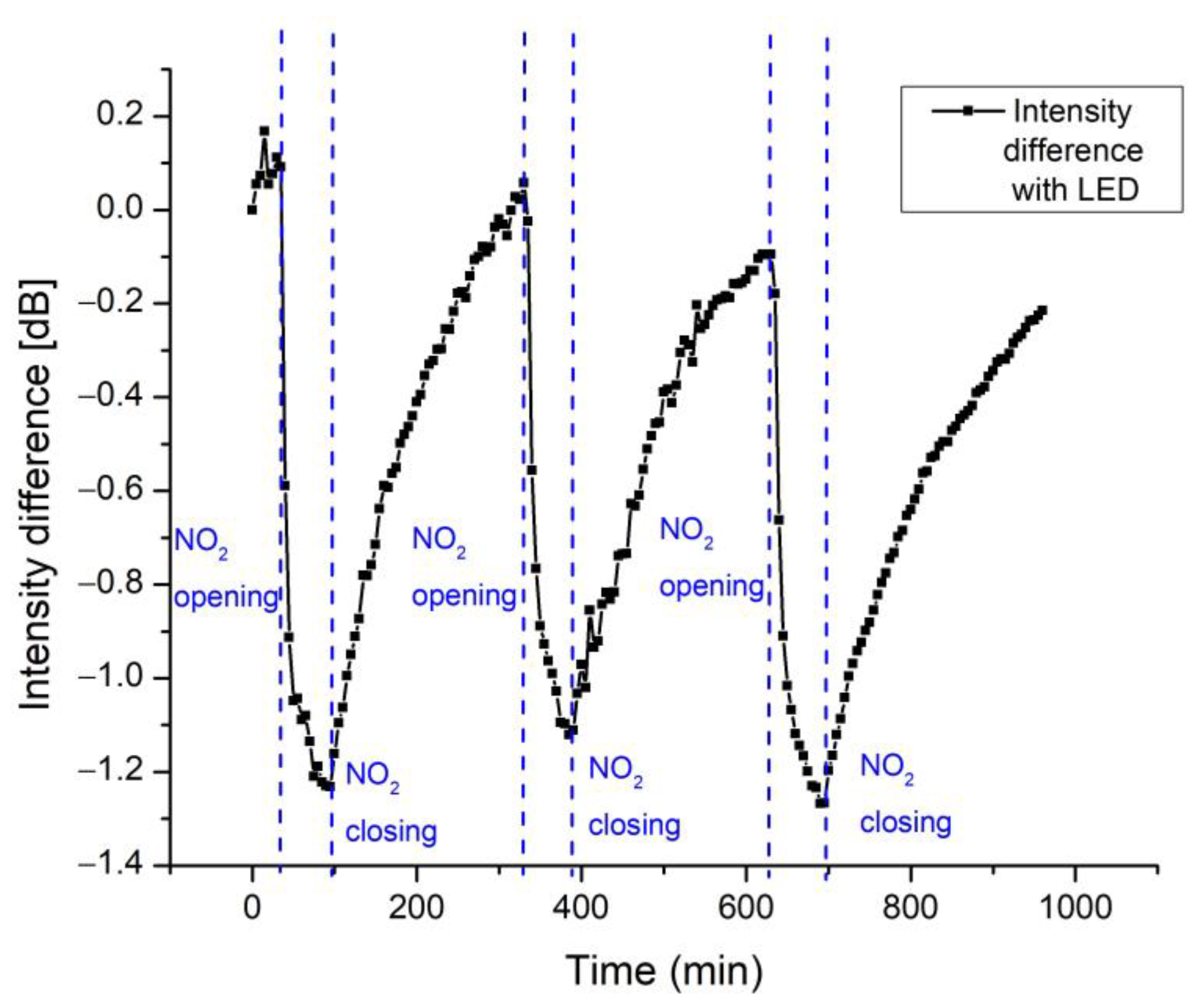
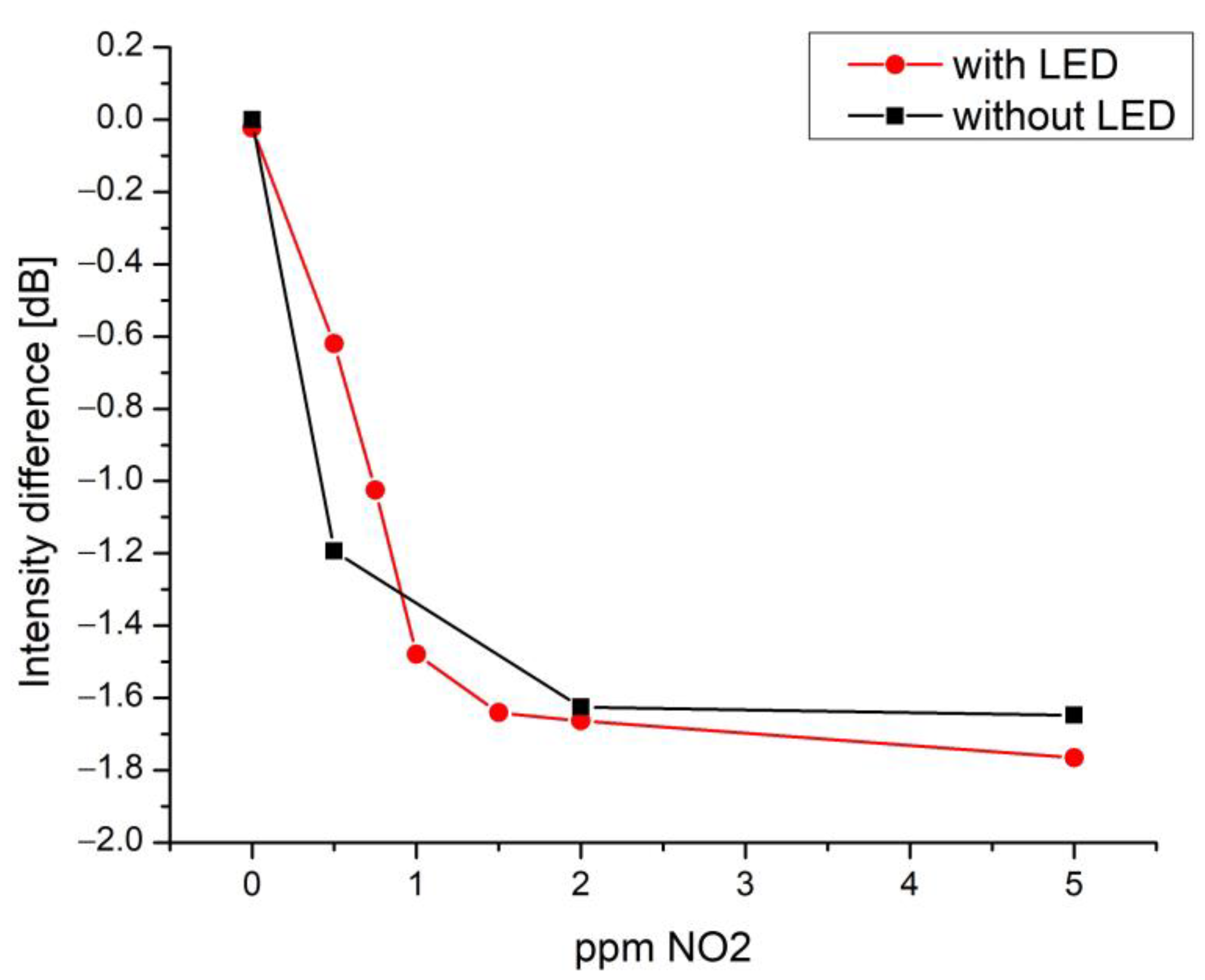
| Response Time (min) | Recovery Time (min) | |
|---|---|---|
| Intensity | ~17 | ~650 |
| Frequency | ~28 | ~1000 |
| Response Time (min) | Recovery Time (min) | |
|---|---|---|
| Intensity | ~60 | ~120 |
| Frequency | ~60 | ~200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulloni, V.; Gaiardo, A.; Marchi, G.; Valt, M.; Vanzetti, L.; Donelli, M.; Lorenzelli, L. Sub-ppm NO2 Detection through Chipless RFID Sensor Functionalized with Reduced SnO2. Chemosensors 2023, 11, 408. https://doi.org/10.3390/chemosensors11070408
Mulloni V, Gaiardo A, Marchi G, Valt M, Vanzetti L, Donelli M, Lorenzelli L. Sub-ppm NO2 Detection through Chipless RFID Sensor Functionalized with Reduced SnO2. Chemosensors. 2023; 11(7):408. https://doi.org/10.3390/chemosensors11070408
Chicago/Turabian StyleMulloni, Viviana, Andrea Gaiardo, Giada Marchi, Matteo Valt, Lia Vanzetti, Massimo Donelli, and Leandro Lorenzelli. 2023. "Sub-ppm NO2 Detection through Chipless RFID Sensor Functionalized with Reduced SnO2" Chemosensors 11, no. 7: 408. https://doi.org/10.3390/chemosensors11070408
APA StyleMulloni, V., Gaiardo, A., Marchi, G., Valt, M., Vanzetti, L., Donelli, M., & Lorenzelli, L. (2023). Sub-ppm NO2 Detection through Chipless RFID Sensor Functionalized with Reduced SnO2. Chemosensors, 11(7), 408. https://doi.org/10.3390/chemosensors11070408








