Near-Infrared-II Fluorophores for In Vivo Multichannel Biosensing
Abstract
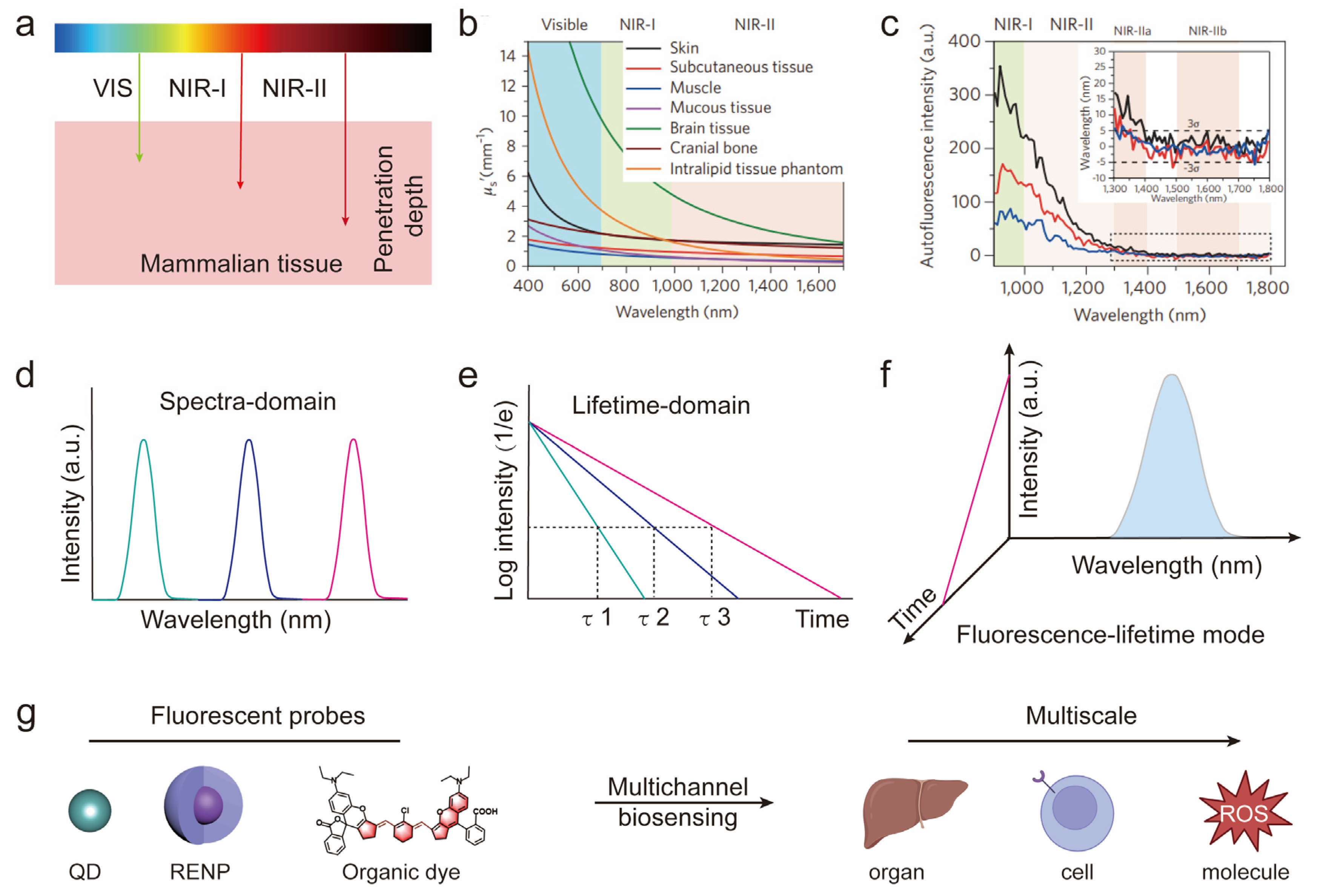
:1. Introduction
2. Near-Infrared-II Fluorophores
2.1. QDs
2.2. RENPs
2.3. Organic Dyes
3. Spectra-Domain Multichannel Biosensing
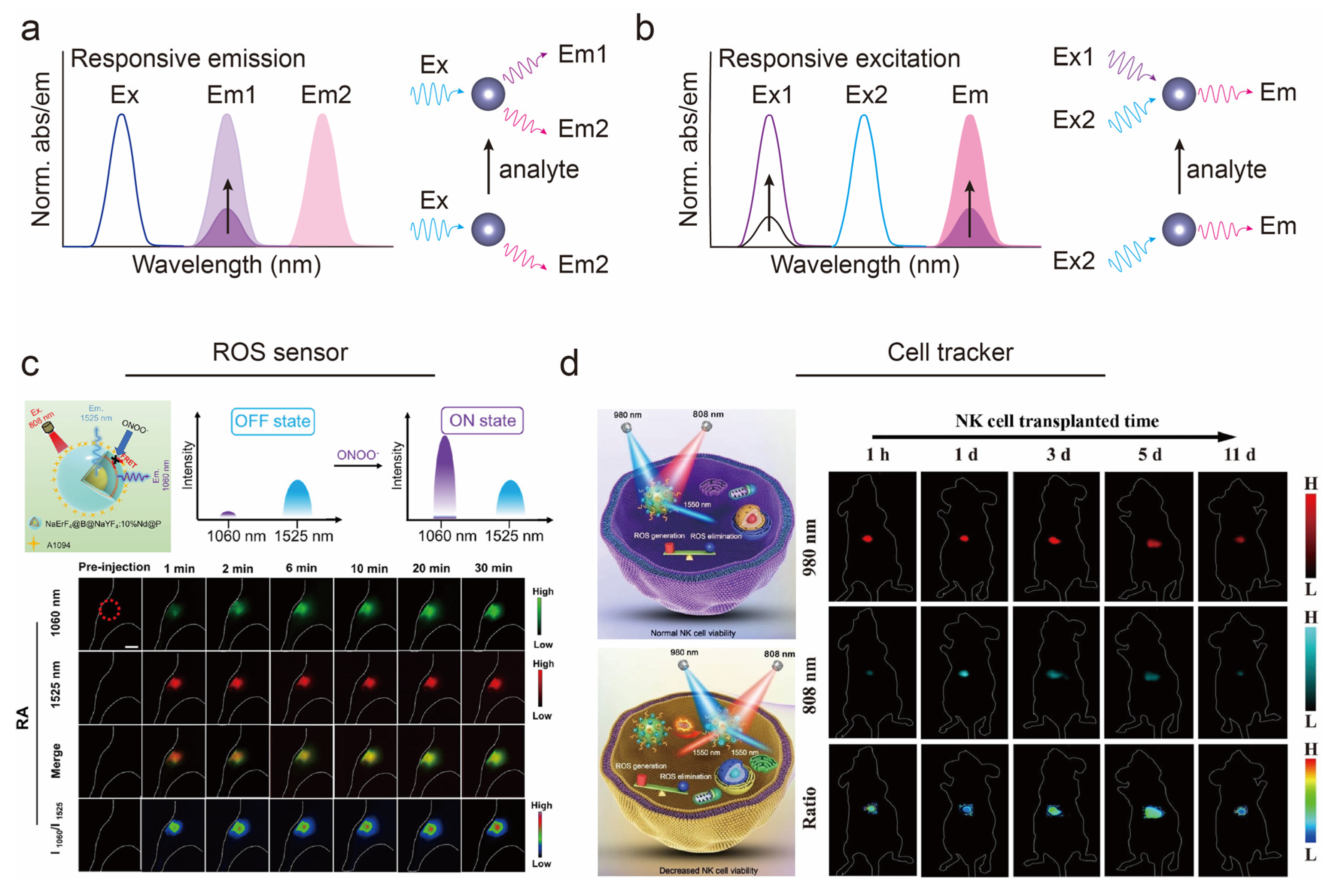
3.1. Excitation–Emission Multiplexed Biosensing

3.2. Ratiometric Fluorescence Biosensing
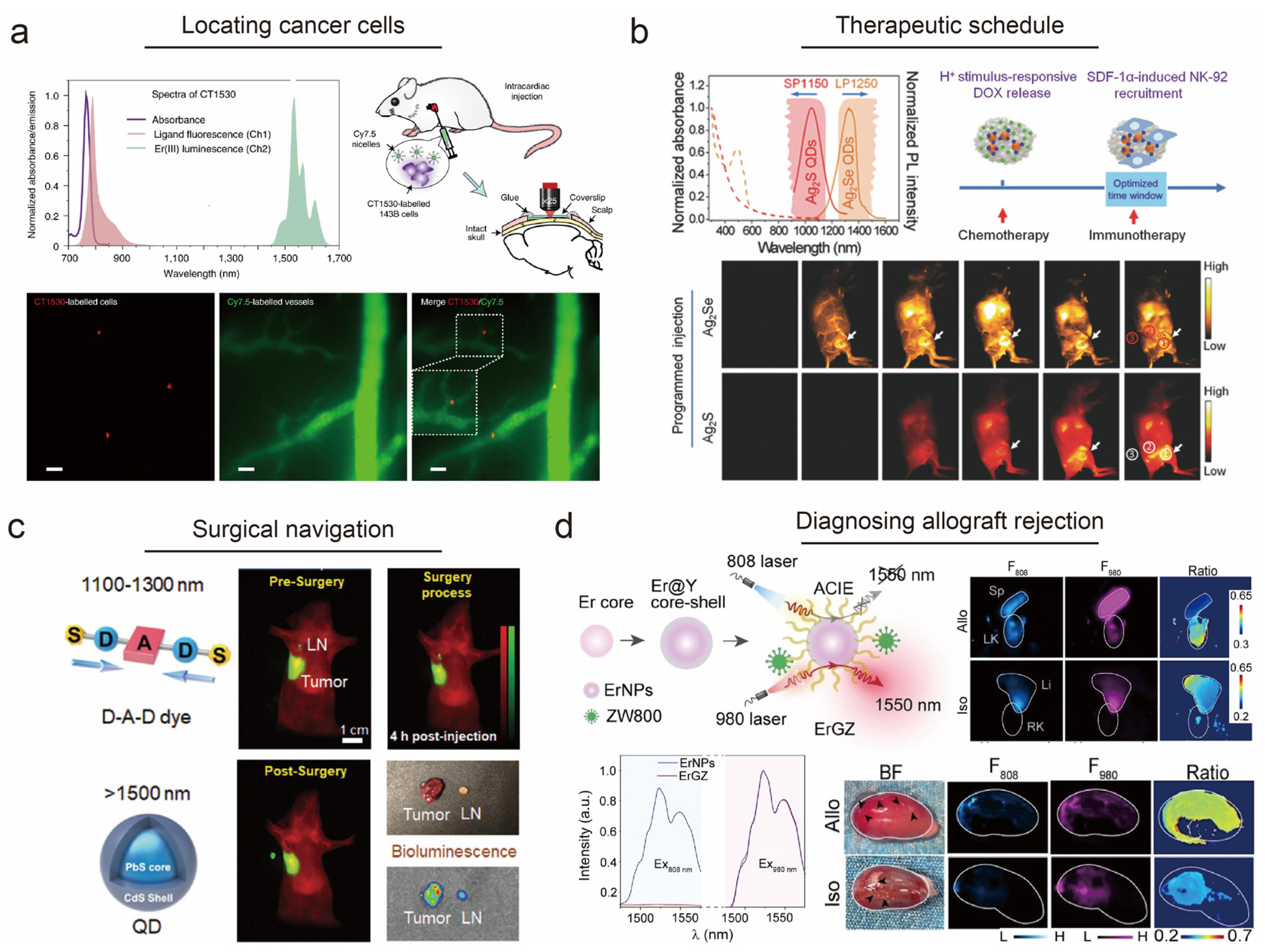
3.3. Spectra-Domain Multichannel Biosensing in Various Scenarios
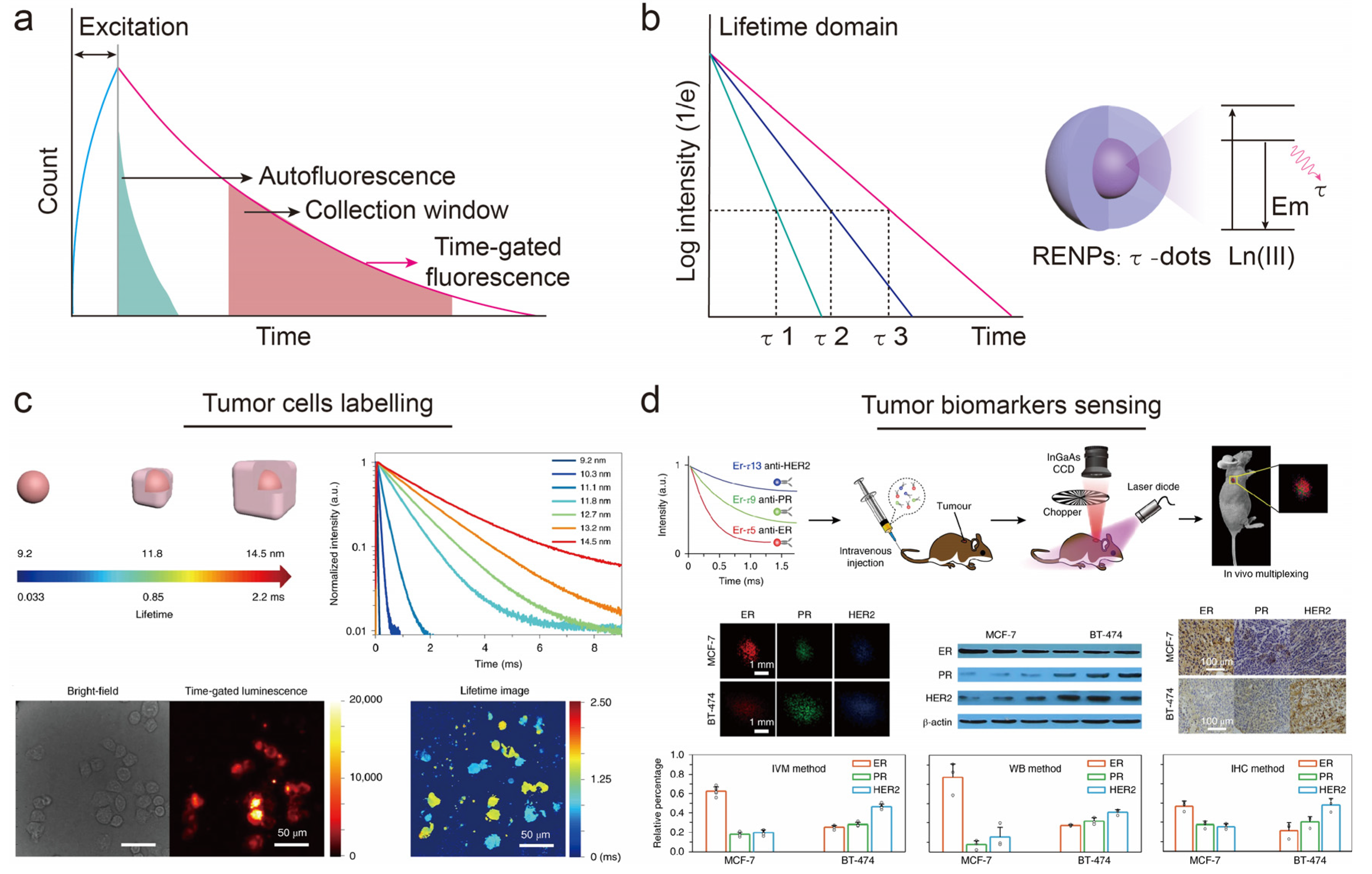
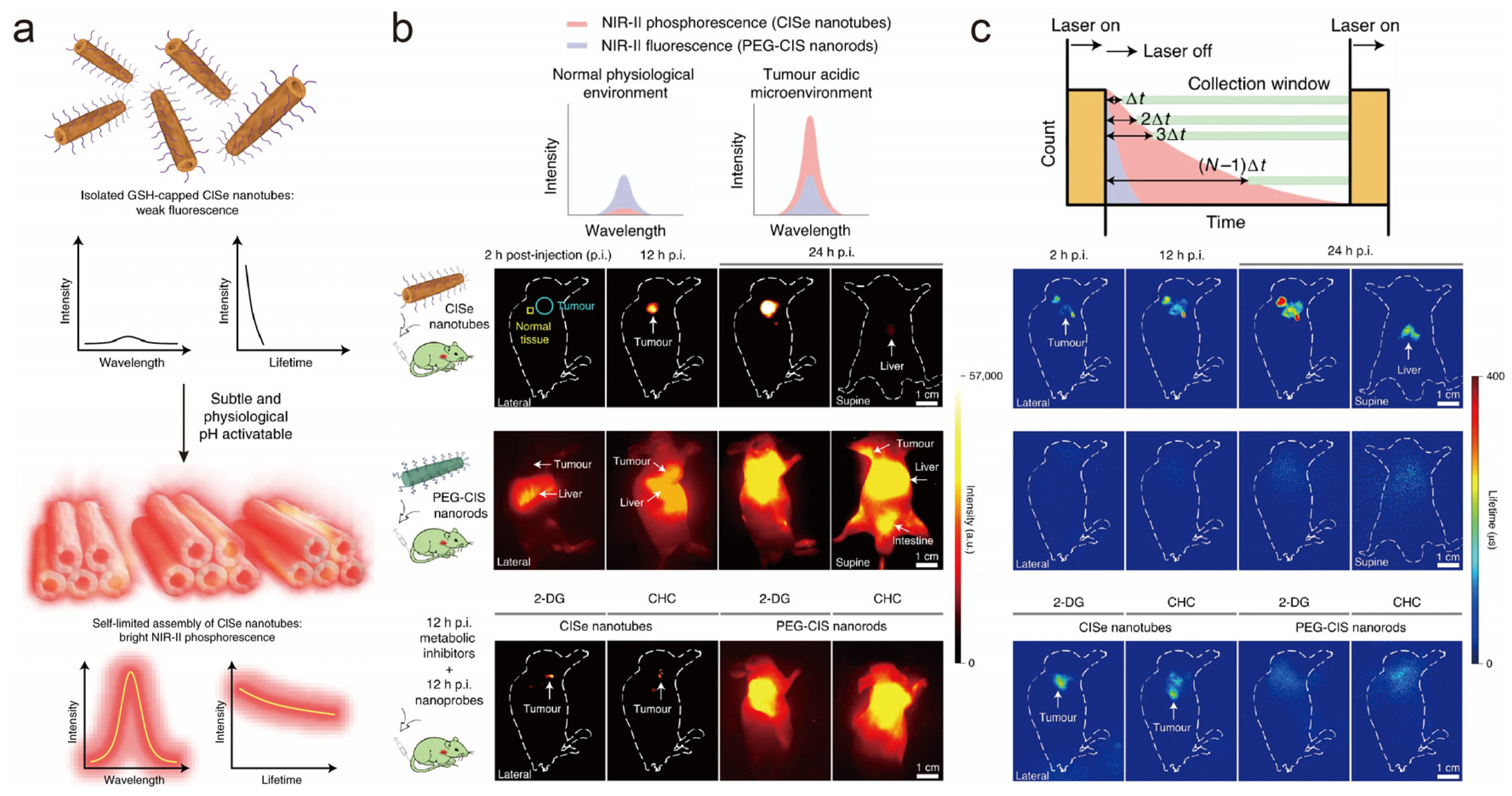
4. Lifetime-Domain Multichannel Biosensing
5. Fluorescence-Lifetime Multichannel Biosensing
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, C.; Wang, Q. Challenges and opportunities for intravital near-infrared fluorescence imaging technology in the second transparency window. ACS Nano 2018, 12, 9654–9659. [Google Scholar] [CrossRef]
- Weissleder, R.; Pittet, M.J. Imaging in the era of molecular oncology. Nature 2008, 452, 580–589. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Du, Y.; Zhang, Z.; He, K.; Cheng, Z.; Yin, L.; Dong, D.; Li, C.; Li, W.; Hu, Z.; et al. Fluorescence image-guided tumour surgery. Nat. Rev. Bioeng. 2023, 1, 161–179. [Google Scholar] [CrossRef]
- Andreou, C.; Weissleder, R.; Kircher, M.F. Multiplexed imaging in oncology. Nat. Biomed. Eng. 2022, 6, 527–540. [Google Scholar] [CrossRef]
- Hu, Z.; Fang, C.; Li, B.; Zhang, Z.; Cao, C.; Cai, M.; Su, S.; Sun, X.; Shi, X.; Li, C.; et al. First-in-human liver-tumour surgery guided by multispectral fluorescence imaging in the visible and near-infrared-I/II windows. Nat. Biomed. Eng. 2020, 4, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, S.; Zhang, F. Optical multiplexed bioassays for improved biomedical diagnostics. Angew. Chem. Int. Ed. 2019, 58, 13208–13219. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Hou, Y.; Zeng, J.; Liu, C.; Zhang, P.; Jing, L.; Shangguan, D.; Gao, M. Dual-ratiometric target-triggered fluorescent probe for simultaneous quantitative visualization of tumor microenvironment protease activity and pH in vivo. J. Am. Chem. Soc. 2018, 140, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.S.; Antaris, A.L.; Dai, H.J. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 2017, 1, 0010. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, S.; Zhang, F. Near-infrared luminescence high-contrast in vivo biomedical imaging. Nat. Rev. Bioeng. 2023, 1, 60–78. [Google Scholar] [CrossRef]
- Chen, G.; Cao, Y.; Tang, Y.; Yang, X.; Liu, Y.; Huang, D.; Zhang, Y.; Li, C.; Wang, Q. Advanced near-infrared light for monitoring and modulating the spatiotemporal dynamics of cell functions in living systems. Adv. Sci. 2020, 7, 1903783. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Huang, H.; Ma, X.; Zhang, Y.; Yang, X.; Yu, M.; Sun, Z.; Li, C.; Wu, F.; Wang, Q. Au-doped Ag2Te quantum dots with bright NIR-IIb fluorescence for in situ monitoring of angiogenesis and arteriogenesis in a hindlimb ischemic model. Adv. Mater. 2021, 33, e2103953. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.S.; Robinson, J.T.; Zhang, Y.J.; Diao, S.; Antaris, A.L.; Wang, Q.B.; Dai, H.J. In vivo fluorescence imaging with Ag2S quantum dots in the second near-infrared region. Angew. Chem. Int. Ed. 2012, 51, 9818–9821. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, G.; Zhang, Y.; Wu, F.; Wang, Q. Advanced fluorescence imaging technology in the near-infrared-II window for biomedical applications. J. Am. Chem. Soc. 2020, 142, 14789–14804. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.X.; Li, B.H.; Zhao, M.Y.; Wang, S.F.; Lei, Z.H.; Lu, L.F.; Zhang, H.X.; Feng, L.S.; Dou, C.R.; Yin, D.R.; et al. J-aggregates of cyanine dye for NIR-II in vivo dynamic vascular imaging beyond 1500 nm. J. Am. Chem. Soc. 2019, 141, 19221–19225. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, C.; Wang, S.; Yan, K.; Zhao, M.; Wu, B.; Zhang, F. Counterion-paired bright heptamethine fluorophores with NIR-II excitation and emission enable multiplexed biomedical imaging. Angew. Chem. Int. Ed. 2022, 61, e202117436. [Google Scholar]
- Lu, L.; Li, B.; Ding, S.; Fan, Y.; Wang, S.; Sun, C.; Zhao, M.; Zhao, C.X.; Zhang, F. NIR-II bioluminescence for in vivo high contrast imaging and in situ ATP-mediated metastases tracing. Nat. Commun. 2020, 11, 4192. [Google Scholar] [CrossRef]
- Bruns, O.T.; Bischof, T.S.; Harris, D.K.; Franke, D.; Shi, Y.; Riedemann, L.; Bartelt, A.; Jaworski, F.B.; Carr, J.A.; Rowlands, C.J.; et al. Next-generation in vivo optical imaging with short-wave infrared quantum dots. Nat. Biomed. Eng. 2017, 1, 0056. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.P.; Xu, B.; Fu, T.; Cai, M.; Li, F.; Zhang, Y.; Wang, Q.B. Near-infrared photoluminescent Ag2S quantum dots from a single source precursor. J. Am. Chem. Soc. 2010, 132, 1470–1471. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, X.; Zhang, H.; Zhao, M.; Pei, P.; Chen, Y.; Yang, Y.; Lu, L.; Yu, P.; Sun, C.; et al. High-fidelity NIR-II multiplexed lifetime bioimaging with bright double interfaced lanthanide nanoparticles. Angew. Chem. Int. Ed. 2021, 60, 23545–23551. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, F.; Zhong, Y.; Salazar, F.; Li, J.; Zhang, M.; Ren, F.; Wu, A.M.; Dai, H. Cross-link-functionalized nanoparticles for rapid excretion in nanotheranostic applications. Angew. Chem. Int. Ed. 2020, 59, 20552–20560. [Google Scholar] [CrossRef]
- Yu, G.T.; Luo, M.Y.; Li, H.; Chen, S.; Huang, B.; Sun, Z.J.; Cui, R.; Zhang, M. Molecular targeting nanoprobes with non-Overlap emission in the second near-infrared window for in vivo two-color colocalization of immune cells. ACS Nano 2019, 13, 12830–12839. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, X.; Zhang, H.; Zhang, F. Luminescence lifetime imaging based on lanthanide nanoparticles. Angew. Chem. Int. Ed. 2022, 61, e202209378. [Google Scholar] [CrossRef]
- Li, T.; Cao, K.; Yang, X.; Liu, Y.; Wang, X.; Wu, F.; Chen, G.; Wang, Q. An oral ratiometric NIR-II fluorescent probe for reliable monitoring of gastrointestinal diseases in vivo. Biomaterials 2023, 293, 121956. [Google Scholar] [CrossRef] [PubMed]
- Lan, Q.; Yu, P.; Yan, K.; Li, X.; Zhang, F.; Lei, Z. Polymethine molecular platform for ratiometric fluorescent probes in the second near-infrared window. J. Am. Chem. Soc. 2022, 144, 21010–21015. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Yang, X.; Zhang, Y.; Yang, H.; Huang, H.; Wang, Z.; Dong, J.; Zhang, R.; Sun, Z.; Li, C.; et al. Pb-doped Ag2Se quantum dots with enhanced photoluminescence in the NIR-II window. Small 2021, 17, e2006111. [Google Scholar] [CrossRef]
- Ortgies, D.H.; Tan, M.; Ximendes, E.C.; Del Rosal, B.; Hu, J.; Xu, L.; Wang, X.; Martin Rodriguez, E.; Jacinto, C.; Fernandez, N.; et al. Lifetime-encoded infrared-emitting nanoparticles for in vivo multiplexed imaging. ACS Nano 2018, 12, 4362–4368. [Google Scholar] [CrossRef]
- Welsher, K.; Liu, Z.; Sherlock, S.P.; Robinson, J.T.; Chen, Z.; Daranciang, D.; Dai, H. A route to brightly fluorescent carbon nanotubes for near-infrared imaging in mice. Nat. Nanotechnol. 2009, 4, 773–780. [Google Scholar] [CrossRef]
- Weidman, M.C.; Beck, M.E.; Hoffman, R.S.; Prins, F.; Tisdale, W.A. Monodisperse, air-stable PbS nanocrystals via precursor stoichiometry control. ACS Nano 2014, 8, 6363–6371. [Google Scholar] [CrossRef] [Green Version]
- Hines, M.A.; Scholes, G.D. Colloidal PbS nanocrystals with size-tunable near-infrared emission: Observation of post-synthesis self-narrowing of the particle size distribution. Adv. Mater. 2003, 15, 1844–1849. [Google Scholar] [CrossRef]
- Yang, H.; Li, R.; Zhang, Y.; Yu, M.; Wang, Z.; Liu, X.; You, W.; Tu, D.; Sun, Z.; Zhang, R.; et al. Colloidal alloyed quantum dots with enhanced photoluminescence quantum yield in the NIR-II window. J. Am. Chem. Soc. 2021, 143, 2601–2607. [Google Scholar] [CrossRef]
- Naczynski, D.J.; Tan, M.C.; Zevon, M.; Wall, B.; Kohl, J.; Kulesa, A.; Chen, S.; Roth, C.M.; Riman, R.E.; Moghe, P.V. Rare-earth-doped biological composites as in vivo shortwave infrared reporters. Nat. Commun. 2013, 4, 2199. [Google Scholar] [CrossRef] [Green Version]
- Johnson, N.J.; He, S.; Diao, S.; Chan, E.M.; Dai, H.; Almutairi, A. Direct evidence for coupled surface and concentration quenching dynamics in lanthanide-doped nanocrystals. J. Am. Chem. Soc. 2017, 139, 3275–3282. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, P.; Lu, Y.; Wang, R.; Zhou, L.; Zheng, X.; Li, X.; Piper, J.A.; Zhang, F. Lifetime-engineered NIR-II nanoparticles unlock multiplexed in vivo imaging. Nat. Nanotechnol. 2018, 13, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Bricks, J.L.; Kachkovskii, A.D.; Slominskii, Y.L.; Gerasov, A.O.; Popov, S.V. Molecular design of near infrared polymethine dyes: A review. Dyes Pigments 2015, 121, 238–255. [Google Scholar] [CrossRef]
- Li, B.; Lu, L.; Zhao, M.; Lei, Z.; Zhang, F. An efficient 1064 nm NIR-II excitation fluorescent molecular dye for deep-tissue high-resolution dynamic bioimaging. Angew. Chem. Int. Ed. 2018, 57, 7483–7487. [Google Scholar] [CrossRef]
- Cosco, E.D.; Spearman, A.L.; Ramakrishnan, S.; Lingg, J.G.P.; Saccomano, M.; Pengshung, M.; Arus, B.A.; Wong, K.C.Y.; Glasl, S.; Ntziachristos, V.; et al. Shortwave infrared polymethine fluorophores matched to excitation lasers enable non-invasive, multicolour in vivo imaging in real time. Nat. Chem. 2020, 12, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Antaris, A.L.; Chen, H.; Cheng, K.; Sun, Y.; Hong, G.S.; Qu, C.R.; Diao, S.; Deng, Z.X.; Hu, X.M.; Zhang, B.; et al. A small-molecule dye for NIR-II imaging. Nat. Mater. 2016, 15, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Bouit, P.A.; Aronica, C.; Toupet, L.; Guennic, B.L.; Andraud, C.; Maury, O. Continuous symmetry breaking induced by ion pairing effect in heptamethine cyanine dyes: Beyond the cyanine limit. J. Am. Chem. Soc. 2010, 132, 4328–4335. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef]
- Li, Y.Y.; Liu, S.J.; Ni, H.W.; Zhang, H.; Zhang, H.Q.; Chuah, C.; Ma, C.; Wong, K.S.; Lam, J.W.Y.; Kwok, R.T.K.; et al. ACQ-to-AIE transformation: Tuning molecular packing by regioisomerization for two-photon NIR bioimaging. Angew. Chem. Int. Ed. 2020, 59, 12822–12826. [Google Scholar] [CrossRef]
- Sheng, Z.; Guo, B.; Hu, D.; Xu, S.; Wu, W.; Liew, W.H.; Yao, K.; Jiang, J.; Liu, C.; Zheng, H.; et al. Bright aggregation-induced-emission dots for targeted synergetic NIR-II fluorescence and NIR-I photoacoustic imaging of orthotopic brain tumors. Adv. Mater. 2018, 30, e1800766. [Google Scholar] [CrossRef]
- Li, Y.; Cai, Z.; Liu, S.; Zhang, H.; Wong, S.T.H.; Lam, J.W.Y.; Kwok, R.T.K.; Qian, J.; Tang, B.Z. Design of AIEgens for near-infrared IIb imaging through structural modulation at molecular and morphological levels. Nat. Commun. 2020, 11, 1255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, Y.; Geng, W.; Zhang, T.; Gong, G.; Li, C.; Zheng, C.; Liu, F.; Qian, J.; Chen, M.; Tang, B.Z. Facile access to far-red fluorescent probes with through-space charge-transfer effects for in vivo two-photon microscopy of the mouse cerebrovascular system. Angew. Chem. Int. Ed. 2022, 61, e202209590. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Alifu, N.; Lam, J.W.Y.; Cui, Y.; Su, H.; Liang, G.; Qian, J.; Tang, B.Z. Facile synthesis of efficient luminogens with AIE features for three-photon fluorescence imaging of the brain through the intact skull. Adv. Mater. 2020, 32, e2000364. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Zhu, Z.; Leung, C.W.; Xi, W.; Su, L.; Chen, G.; Qin, A.; Tang, B.Z.; He, S. Long-term two-photon neuroimaging with a photostable AIE luminogen. Biomed. Opt. Express 2015, 6, 1477–1486. [Google Scholar] [CrossRef] [Green Version]
- Mandal, A.K.; Sreejith, S.; He, T.H.; Maji, S.K.; Wang, X.J.; Ong, S.L.; Joseph, J.; Sun, H.D.; Zhao, Y.L. Three-photon-excited luminescence from unsymmetrical cyanostilbene aggregates: Morphology tuning and targeted bioimaging. ACS Nano 2015, 9, 4796–4805. [Google Scholar] [CrossRef]
- Yao, C.; Chen, Y.; Zhao, M.; Wang, S.; Wu, B.; Yang, Y.; Yin, D.; Yu, P.; Zhang, H.; Zhang, F. A bright, renal-clearable NIR-II brush macromolecular probe with long blood circulation time for kidney disease bioimaging. Angew. Chem. Int. Ed. 2022, 61, e202114273. [Google Scholar] [CrossRef]
- Wang, F.; Qu, L.; Ren, F.; Baghdasaryan, A.; Jiang, Y.; Hsu, R.; Liang, P.; Li, J.; Zhu, G.; Ma, Z.; et al. High-precision tumor resection down to few-cell level guided by NIR-IIb molecular fluorescence imaging. Proc. Natl. Acad. Sci. USA 2022, 119, e2123111119. [Google Scholar] [CrossRef]
- Zhang, M.X.; Yue, J.Y.; Cui, R.; Ma, Z.R.; Wan, H.; Wang, F.F.; Zhu, S.J.; Zhou, Y.; Kuang, Y.; Zhong, Y.T.; et al. Bright quantum dots emitting at ∼1600 nm in the NIR-IIb window for deep tissue fluorescence imaging. Proc. Natl. Acad. Sci. USA 2018, 115, 6590–6595. [Google Scholar] [CrossRef] [Green Version]
- Ren, F.; Liu, H.H.; Zhang, H.; Jiang, Z.L.; Xia, B.; Genevois, C.; He, T.; Allix, M.; Sun, Q.; Li, Z.; et al. Engineering NIR-IIb fluorescence of Er-based lanthanide nanoparticles for through-skull targeted imaging and imaging-guided surgery of orthotopic glioma. Nano Today 2020, 34, 100905. [Google Scholar] [CrossRef]
- Zhang, H.; Fu, P.; Liu, Y.; Zheng, Z.; Zhu, L.; Wang, M.; Abdellah, M.; He, M.; Qian, J.; Roe, A.W.; et al. Large-depth three-photon fluorescence microscopy imaging of cortical microvasculature on nonhuman primates with bright AIE probe in vivo. Biomaterials 2022, 289, 121809. [Google Scholar] [CrossRef]
- He, M.; Li, D.; Zheng, Z.; Zhang, H.; Wu, T.; Geng, W.; Hu, Z.; Feng, Z.; Peng, S.; Zhu, L.; et al. Aggregation-induced emission nanoprobe assisted ultra-deep through-skull three-photon mouse brain imaging. Nano Today 2022, 45, 101536. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, H.; Cao, H.; Gong, J.; He, M.; Gou, X.; Yang, T.; Wei, P.; Qian, J.; Xi, W.; et al. Intra- and intermolecular synergistic engineering of aggregation-induced emission luminogens to boost three-photon absorption for through-skull brain imaging. ACS Nano 2022, 16, 6444–6454. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, Y.; He, Z.; Wang, X.; Wang, S.; Zhang, F. Molecular-based FRET nanosensor with dynamic ratiometric NIR-IIb fluorescence for real-time in vivo imaging and sensing. Nano Lett. 2023, 23, 4548–4556. [Google Scholar] [CrossRef]
- Wang, F.; Wan, H.; Ma, Z.; Zhong, Y.; Sun, Q.; Tian, Y.; Qu, L.; Du, H.; Zhang, M.; Li, L.; et al. Light-sheet microscopy in the near-infrared II window. Nat. Methods 2019, 16, 545–552. [Google Scholar] [CrossRef]
- Dong, H.; Sun, L.D.; Yan, C.H. Local structure engineering in lanthanide-doped nanocrystals for tunable upconversion emissions. J. Am. Chem. Soc. 2021, 143, 20546–20561. [Google Scholar] [CrossRef]
- Zhong, Y.; Ma, Z.; Zhu, S.; Yue, J.; Zhang, M.; Antaris, A.L.; Yuan, J.; Cui, R.; Wan, H.; Zhou, Y.; et al. Boosting the down-shifting luminescence of rare-earth nanocrystals for biological imaging beyond 1500 nm. Nat. Commun. 2017, 8, 737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pei, P.; Chen, Y.; Sun, C.; Fan, Y.; Yang, Y.; Liu, X.; Lu, L.; Zhao, M.; Zhang, H.; Zhao, D.; et al. X-ray-activated persistent luminescence nanomaterials for NIR-II imaging. Nat. Nanotechnol. 2021, 16, 1011–1018. [Google Scholar] [CrossRef]
- Wang, T.; Wang, S.; Liu, Z.; He, Z.; Yu, P.; Zhao, M.; Zhang, H.; Lu, L.; Wang, Z.; Wang, Z.; et al. A hybrid erbium(III)-bacteriochlorin near-infrared probe for multiplexed biomedical imaging. Nat. Mater. 2021, 20, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Bakueva, L.; Gorelikov, I.; Musikhin, S.; Zhao, X.S.; Sargent, E.H.; Kumacheva, E. PbS quantum dots with stable efficient luminescence in the NIR spectral range. Adv. Mater. 2004, 16, 926–929. [Google Scholar] [CrossRef]
- McDonald, S.A.; Konstantatos, G.; Zhang, S.; Cyr, P.W.; Klem, E.J.; Levina, L.; Sargent, E.H. Solution-processed PbS quantum dot infrared photodetectors and photovoltaics. Nat. Mater. 2005, 4, 138–142. [Google Scholar] [CrossRef]
- Yu, P.; Yan, K.; Wang, S.; Yao, C.; Lei, Z.; Tang, Y.; Zhang, F. NIR-II dyad-doped ratiometric nanosensor with enhanced spectral fidelity in biological media for in vivo biosensing. Nano Lett. 2022, 22, 9732–9740. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Huang, H.; Zhang, R.; Yang, X.; Yang, H.; Li, C.; Zhang, Y.; Wang, Q. Activatable rare earth near-infrared-II fluorescence ratiometric nanoprobes. Nano Lett. 2021, 21, 6576–6583. [Google Scholar] [CrossRef] [PubMed]
- Liao, N.; Su, L.; Zheng, Y.; Zhao, B.; Wu, M.; Zhang, D.; Yang, H.; Liu, X.; Song, J. In vivo tracking of cell viability for adoptive natural killer cell-based immunotherapy by ratiometric NIR-II fluorescence imaging. Angew. Chem. Int. Ed. 2021, 60, 20888–20896. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Li, J.; Zhao, X.; Pu, K.; Zhang, R. Semiconducting polymer nanoreporters for near-infrared chemiluminescence imaging of immunoactivation. Adv. Mater. 2020, 32, e1906314. [Google Scholar] [CrossRef]
- Ramesh, A.; Kumar, S.; Brouillard, A.; Nandi, D.; Kulkarni, A. A nitric oxide (NO) nanoreporter for noninvasive real-time imaging of macrophage immunotherapy. Adv. Mater. 2020, 32, e2000648. [Google Scholar] [CrossRef]
- Huang, Y.; Snuderl, M.; Jain, R.K. Polarization of tumor-associated macrophages: A novel strategy for vascular normalization and antitumor immunity. Cancer Cell 2011, 19, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Kim, B.Y.S.; Chan, C.K.; Hahn, S.M.; Weissman, I.L.; Jiang, W. Improving immune-vascular crosstalk for cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 195–203. [Google Scholar] [CrossRef]
- Pittet, M.J.; Garris, C.S.; Arlauckas, S.P.; Weissleder, R. Recording the wild lives of immune cells. Sci. Immunol. 2018, 3, eaaq0491. [Google Scholar] [CrossRef]
- Hao, X.; Li, C.; Zhang, Y.; Wang, H.; Chen, G.; Wang, M.; Wang, Q. Programmable chemotherapy and immunotherapy against breast cancer guided by multiplexed fluorescence imaging in the second near-infrared window. Adv. Mater. 2018, 30, e1804437. [Google Scholar] [CrossRef]
- Lucero, M.Y.; Chan, J. Photoacoustic imaging of elevated glutathione in models of lung cancer for companion diagnostic applications. Nat. Chem. 2021, 13, 1248–1256. [Google Scholar] [CrossRef]
- Tian, R.; Ma, H.; Zhu, S.; Lau, J.; Ma, R.; Liu, Y.; Lin, L.; Chandra, S.; Wang, S.; Zhu, X.; et al. Multiplexed N IR-II probes for lymph node-invaded cancer detection and imaging-guided surgery. Adv. Mater. 2020, 32, e1907365. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pei, P.; Yang, Y.; Zhang, H.; Zhang, F. Noninvasive early diagnosis of allograft rejection by a granzyme B protease responsive NIR-II bioimaging nanosensor. Angew. Chem. Int. Ed. 2023, 62, e202301696. [Google Scholar] [CrossRef] [PubMed]
- Kantamneni, H.; Zevon, M.; Donzanti, M.J.; Zhao, X.; Sheng, Y.; Barkund, S.R.; McCabe, L.H.; Banach-Petrosky, W.; Higgins, L.M.; Ganesan, S.; et al. Surveillance nanotechnology for multi-organ cancer metastases. Nat. Biomed. Eng. 2017, 1, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Duneton, C.; Winterberg, P.D.; Ford, M.L. Activation and regulation of alloreactive T cell immunity in solid organ transplantation. Nat. Rev. Nephrol. 2022, 18, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Cherry, C.; Maestas, D.R.; Han, J.; Andorko, J.I.; Cahan, P.; Fertig, E.J.; Garmire, L.X.; Elisseeff, J.H. Computational reconstruction of the signalling networks surrounding implanted biomaterials from single-cell transcriptomics. Nat. Biomed. Eng. 2021, 5, 1228–1238. [Google Scholar] [CrossRef]
- Pei, P.; Hu, H.; Chen, Y.; Wang, S.; Chen, J.; Ming, J.; Yang, Y.; Sun, C.; Zhao, S.; Zhang, F. NIR-II ratiometric lanthanide-dye hybrid nanoprobes doped bioscaffolds for in situ bone repair monitoring. Nano Lett. 2022, 22, 783–791. [Google Scholar] [CrossRef]
- Del Rosal, B.; Benayas, A. Strategies to overcome autofluorescence in nanoprobe-driven in vivo fluorescence imaging. Small Methods 2018, 2, 1800075. [Google Scholar] [CrossRef]
- Zhao, M.; Li, B.; Wu, Y.; He, H.; Zhu, X.; Zhang, H.; Dou, C.; Feng, L.; Fan, Y.; Zhang, F. A tumor-microenvironment-responsive lanthanide-cyanine FRET sensor for NIR-II luminescence-lifetime in situ Imaging of hepatocellular carcinoma. Adv. Mater. 2020, 32, e2001172. [Google Scholar] [CrossRef]
- Becker, W. Fluorescence lifetime imaging—Techniques and applications. J. Microsc. 2012, 247, 119–136. [Google Scholar] [CrossRef]
- Del Rosal, B.; Ortgies, D.H.; Fernandez, N.; Sanz-Rodriguez, F.; Jaque, D.; Rodriguez, E.M. Overcoming autofluorescence: Long-lifetime infrared nanoparticles for time-gated in vivo imaging. Adv. Mater. 2016, 28, 10188–10193. [Google Scholar] [CrossRef]
- Gu, Y.; Guo, Z.; Yuan, W.; Kong, M.; Liu, Y.; Liu, Y.; Gao, Y.; Feng, W.; Wang, F.; Zhou, J.; et al. High-sensitivity imaging of time-domain near-infrared light transducer. Nat. Photon. 2019, 13, 525–531. [Google Scholar] [CrossRef]
- Li, H.; Tan, M.; Wang, X.; Li, F.; Zhang, Y.; Zhao, L.; Yang, C.; Chen, G. Temporal multiplexed in vivo upconversion imaging. J. Am. Chem. Soc. 2020, 142, 2023–2030. [Google Scholar] [CrossRef]
- Wu, L.; Jia, M.; Li, D.; Chen, G. Shell engineering on thermal sensitivity of lifetime-based NIR nanothermometers for accurate temperature measurement in murine internal liver organ. Nano Lett. 2023, 23, 2862–2869. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhao, J.; Zhang, R.; Liu, Y.; Liu, D.; Goldys, E.M.; Yang, X.; Xi, P.; Sunna, A.; Lu, J.; et al. Tunable lifetime multiplexing using luminescent nanocrystals. Nat. Photon. 2013, 8, 32–36. [Google Scholar] [CrossRef] [Green Version]
- Qiu, X.; Zhou, Q.; Zhu, X.; Wu, Z.; Feng, W.; Li, F. Ratiometric upconversion nanothermometry with dual emission at the same wavelength decoded via a time-resolved technique. Nat. Commun. 2020, 11, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribas, J.D.W. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [Green Version]
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T cell immunotherapy for human cancer. Science 2018, 359, 1361–1365. [Google Scholar] [CrossRef] [Green Version]
- Bensch, F.; van der Veen, E.L.; Lub-de Hooge, M.N.; Jorritsma-Smit, A.; Boellaard, R.; Kok, I.C.; Oosting, S.F.; Schroder, C.P.; Hiltermann, T.J.N.; van der Wekken, A.J.; et al. (89)Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat. Med. 2018, 24, 1852–1858. [Google Scholar] [CrossRef]
- Zou, W.; Wolchok, J.D.; Chen, L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci. Transl. Med. 2016, 8, 328rv4. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Ma, Z.; Wang, F.; Wang, X.; Yang, Y.; Liu, Y.; Zhao, X.; Li, J.; Du, H.; Zhang, M.; et al. In vivo molecular imaging for immunotherapy using ultra-bright near-infrared-IIb rare-earth nanoparticles. Nat. Biotechnol. 2019, 37, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, M.J.; Wang, J.; Zhang, T.; Xue, P.; Kang, Y.; Sun, Z.J.; Xu, Z. Emerging biomaterials imaging antitumor immune response. Adv. Mater. 2022, 34, e2204034. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhuang, H.; Zhang, H.; Li, B.; Ming, J.; Chen, X.; Chen, M. A LRET nanoplatform consisting of lanthanide and amorphous manganese oxide for NIR-II luminescence lifetime imaging of tumor redox status. Angew. Chem. Int. Ed. 2022, 61, e202209592. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Li, D.; Ren, Y.; Qu, C.; Shi, X.; Liu, R.; Liu, H.; Tian, J.; Hu, Z.; Sun, T.; et al. A phosphorescent probe for in vivo imaging in the second near-infrared window. Nat. Biomed. Eng. 2021, 6, 629–639. [Google Scholar] [CrossRef]
- Liu, J.; Wang, P.; Zhang, X.; Wang, L.; Wang, D.; Gu, Z.; Tang, J.; Guo, M.; Cao, M.; Zhou, H.; et al. Rapid degradation and high renal clearance of Cu3BiS3 nanodots for efficient cancer diagnosis and photothermal therapy in vivo. ACS Nano 2016, 10, 4587–4598. [Google Scholar] [CrossRef]
- He, S.; Cheng, P.; Pu, K. Activatable near-infrared probes for the detection of specific populations of tumour-infiltrating leukocytes in vivo and in urine. Nat. Biomed. Eng. 2023, 7, 281–297. [Google Scholar] [CrossRef]
- Huang, J.; Chen, X.; Jiang, Y.; Zhang, C.; He, S.; Wang, H.; Pu, K. Renal clearable polyfluorophore nanosensors for early diagnosis of cancer and allograft rejection. Nat. Mater. 2022, 21, 598–607. [Google Scholar] [CrossRef]
- Loynachan, C.N.; Soleimany, A.P.; Dudani, J.S.; Lin, Y.; Najer, A.; Bekdemir, A.; Chen, Q.; Bhatia, S.N.; Stevens, M.M. Renal clearable catalytic gold nanoclusters for in vivo disease monitoring. Nat. Nanotechnol. 2019, 14, 883–890. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Ren, F.; Ma, Z.; Qu, L.; Gourgues, R.; Xu, C.; Baghdasaryan, A.; Li, J.; Zadeh, I.E.; Los, J.W.N.; et al. In vivo non-invasive confocal fluorescence imaging beyond 1,700 nm using superconducting nanowire single-photon detectors. Nat. Nanotechnol. 2022, 17, 653–660. [Google Scholar] [CrossRef]
- Chang, Y.; Chen, H.; Xie, X.; Wan, Y.; Li, Q.; Wu, F.; Yang, R.; Wang, W.; Kong, X. Bright Tm3+-based downshifting luminescence nanoprobe operating around 1800 nm for NIR-IIb and c bioimaging. Nat. Commun. 2023, 14, 1079. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, J.; Lei, Z.; Lu, L.; Wang, S.; Zhang, H.; Li, B.; Zhang, F. NIR-II pH sensor with a FRET adjustable transition point for in situ dynamic tumor microenvironment visualization. Angew. Chem. Int. Ed. 2021, 60, 5091–5095. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, H.; Huang, Z.; Jia, Q. Spatially confining copper nanoclusters in porous ZrO2 for fluorescence/colorimetry/smartphone triple-mode detection of metoprolol tartrate. Biosens. Bioelectron. 2023, 231, 115290. [Google Scholar] [CrossRef] [PubMed]

| NIR-II Sensor | Ex. (nm) | Em. (nm) | Mode | Application | Ref |
|---|---|---|---|---|---|
| Er(III)–bacteriochlorin complexes | 766 | 1530 | Spectra domain | Multiplexing of tissues and tumor cells | [59] |
| PbS/CdS QDs | 808 | 1100&1600 | Spectra domain | Multiplexing of MDSCs | [21] |
| NaErF4@NaYF4@NaYF4:10%Nd @NaYF4@A1094 | 808 | 1060&1525 | Spectra domain | Ratiometric imaging of RNS | [63] |
| DCNP@IR760s | 808&980 | 1550 | Spectra domain | Ratiometric imaging of ROS to track NK cell viability | [64] |
| Ag2S&Ag2Se QDs | 808 | 1050&1350 | Spectra domain | Multiplexing-guided therapeutic schedule | [70] |
| PbS/CdS QDs &IR-FD dye | 808 | 1100&1600 | Spectra domain | Multiplexing-guided surgical navigation | [72] |
| NaErF4@NaYF4 @ZW800 | 808&980 | 1550 | Spectra domain | Ratiometric imaging of GzB to diagnose allograft rejection | [73] |
| NaYbF4@CaF2 | 920 | 980 | Lifetime domain | Tumor cells labeling | [82] |
| NaGdF4@NaGdF4:Yb,Er@NaYF4:Yb@NaNdF4:Yb | 808 | 1525 | Lifetime domain | Tumor biomarkers sensing | [33] |
| NaYbF4:Er@NaYF4& PbS QDs | 980&808 | 1530&1600 | Fluorescence lifetime | Tumor cells labeling | [91] |
| NaYF4@NaYF4:Nd@MY-1057 | 808 | 1060 | Fluorescence lifetime | HCC tumor detection | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, F.; Li, T.; Yao, T.; Chen, G.; Li, C.; Wang, Q. Near-Infrared-II Fluorophores for In Vivo Multichannel Biosensing. Chemosensors 2023, 11, 433. https://doi.org/10.3390/chemosensors11080433
Ren F, Li T, Yao T, Chen G, Li C, Wang Q. Near-Infrared-II Fluorophores for In Vivo Multichannel Biosensing. Chemosensors. 2023; 11(8):433. https://doi.org/10.3390/chemosensors11080433
Chicago/Turabian StyleRen, Feng, Tuanwei Li, Tingfeng Yao, Guangcun Chen, Chunyan Li, and Qiangbin Wang. 2023. "Near-Infrared-II Fluorophores for In Vivo Multichannel Biosensing" Chemosensors 11, no. 8: 433. https://doi.org/10.3390/chemosensors11080433
APA StyleRen, F., Li, T., Yao, T., Chen, G., Li, C., & Wang, Q. (2023). Near-Infrared-II Fluorophores for In Vivo Multichannel Biosensing. Chemosensors, 11(8), 433. https://doi.org/10.3390/chemosensors11080433








