Single-Atom Nanomaterials in Electrochemical Sensors Applications
Abstract
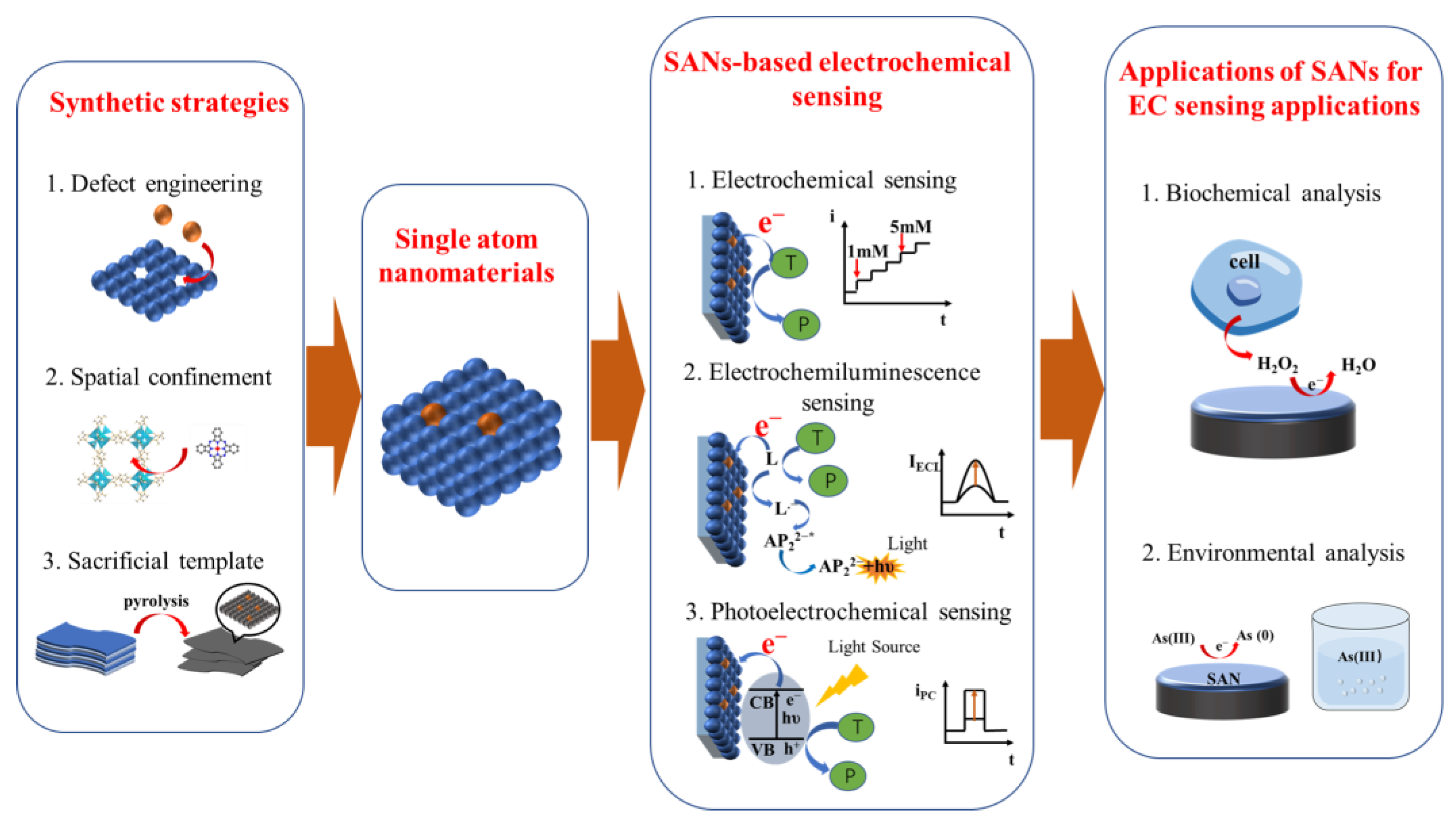
:1. Introduction
2. Active Centers Modulation of SANs
2.1. Noble Metal-Based SANs
2.2. Non-Noble Metal-Based SANs
3. Types of SANs-Based Electrochemical Sensing
3.1. SANs-Based Electrochemical Sensing
| SANs | Analyte | Sensitivity (µA mM−1) | Linear Range (µM) | LOD (µM) | Reference |
|---|---|---|---|---|---|
| Fe–SASC/NW | H2O2 | - | 5 × 10−4~5 × 105 | 0.04635 | [71] |
| Fe3C@C/Fe–N–C | H2O2 | 1225 cm−2 | 1~6000 | 0.26 | [69] |
| Cu1/C3N4 | H2O2 | 0.155 | - | - | [73] |
| Fe–SASC/G 1 | H2O2 | 3214.28 cm−2 1785.71 cm−2 | 10~920 920~7020 | 0.2 | [68] |
| Fe Sas–N/C | H2O2 | 86.99 32.66 19.91 | 1~54 54~764 764~9664 | 0.34 | [74] |
| Ni–SAC | H2O2 | - | 2 × 10−5~2.22 × 104 | 6.87 × 10−6 | [75] |
| Se SA/NC | H2O2 | 403.9 cm−2 | 40~1.11× 104 | 18 | [76] |
| Fe1Se1/NC | H2O2 | 1508.6 cm−2 | 20~1.3 × 104 | 11.5 | [77] |
| Co–N–C-800 | H2O2 DA | 943.9 cm−2 979.6 cm−2 | 0.3~1.0 × 106 0.06~1200 | 0.13 0.04 | [78] |
| Ni–MoS2 | DA | - | 1 × 10−6~1000 | 1 × 10−6 | [79] |
| Ru–Ala 2–C3N4 | DA UA | 0.083 0.033 | 0.06~490 0.5~2135 | 0.02 0.17 | [80] |
| Ru3/NC | DA UA | 58 24 | 0.01~200 0.05~1000 | 0.033 0.01 | [58] |
| Fe–N5–SAC | DA UA | 2150 2740 | 0.005~500 0.01~480 | 7 27 | [25] |
| Pt1/Cu@CuO NWs | glucose | 852.163 cm−2 | 0.01~5.18 | 3.6 | [54] |
| NCA 3–Co | glucose | 7.8 1 | 0.5~1000 1000~6000 | 0.1 | [27] |
| Pt1/Ni6Co1LDHs 4/NG 5 | glucose | 273.78 cm−2 | 100~2180 | 10 | [81] |
| Pt1/Ni(OH)2/NG | glucose | 220.75 cm−2 | 10~2180 | - | [82] |
| Ti–MOF 6–Pt | thrombin | - | 4 × 10−6~0.2 | 1.3 × 10−6 | [83] |
| SANb–BCN 7 | NB | 480.37 156.64 | 2~100 100~600 | 0.7 | [84] |
3.2. SANs-Based Electrochemiluminescence Sensing
3.3. SANs-Based Photoelectrochemical Sensing
4. Applications of SANs in Analytical Chemistry
4.1. Applications of SANs in Biochemical Analysis
4.2. Applications of SANs in Environmental Analysis
5. Summary and Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement:
Conflicts of Interest
References
- Zheng, W. Single-Atom Materials as Electrochemical Sensors: Sensitivity, Selectivity, and Stability. Anal. Sens. 2022, 3, e20220007. [Google Scholar] [CrossRef]
- Li, H.Y.; Qi, H.J.; Chang, J.F.; Gai, P.P.; Li, F. Recent progress in homogeneous electrochemical sensors and their designs and applications. Trac-Trends Anal. Chem. 2022, 156, 116712. [Google Scholar] [CrossRef]
- Chen, Y.; Jiao, L.; Yan, H.; Xu, W.; Wu, Y.; Zheng, L.; Gu, W.; Zhu, C. Fe-N-C Single-Atom Catalyst Coupling with Pt Clusters Boosts Peroxidase-like Activity for Cascade-Amplified Colorimetric Immunoassay. Anal. Chem. 2021, 93, 12353–12359. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Xu, W.; Wu, Y.; Wang, H.; Hu, L.; Gu, W.; Zhu, C. On the Road from Single-Atom Materials to Highly Sensitive Electrochemical Sensing and Biosensing. Anal. Chem. 2023, 95, 433–443. [Google Scholar] [CrossRef]
- Chang, B.; Zhang, L.; Wu, S.; Sun, Z.; Cheng, Z. Engineering single-atom catalysts toward biomedical applications. Chem. Soc. Rev. 2022, 51, 3688–3734. [Google Scholar] [CrossRef] [PubMed]
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Ji, S.F.; Chen, C.; Peng, Q.; Wang, D.S.; Li, Y.D. Single-Atom Catalysts: Synthetic Strategies and Electrochemical Applications. Joule 2018, 2, 1242–1264. [Google Scholar] [CrossRef]
- Huang, Z.; Sun, X.; Wang, P.; Wan, H. Emerging single-atom catalysts in electrochemical biosensing. View 2023, 4, 20220058. [Google Scholar] [CrossRef]
- Tajik, S.; Dourandish, Z.; Garkani Nejad, F.; Beitollahi, H.; Afshar, A.A.; Jahani, P.M.; Di Bartolomeo, A. Review—Single-Atom Catalysts as Promising Candidates for Electrochemical Applications. J. Electrochem. Soc. 2022, 169, 046504. [Google Scholar] [CrossRef]
- Wang, A.Q.; Li, J.; Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2018, 2, 65–81. [Google Scholar] [CrossRef]
- Wang, P.; Ren, Y.; Wang, R.; Zhang, P.; Ding, M.; Li, C.; Zhao, D.; Qian, Z.; Zhang, Z.; Zhang, L.; et al. Atomically dispersed cobalt catalyst anchored on nitrogen-doped carbon nanosheets for lithium-oxygen batteries. Nat. Commun. 2020, 11, 1576. [Google Scholar] [CrossRef]
- Xia, C.; Qiu, Y.; Xia, Y.; Zhu, P.; King, G.; Zhang, X.; Wu, Z.; Kim, J.Y.T.; Cullen, D.A.; Zheng, D.; et al. General synthesis of single-atom catalysts with high metal loading using graphene quantum dots. Nat. Chem. 2021, 13, 887–894. [Google Scholar] [CrossRef]
- Ran, L.; Li, Z.; Ran, B.; Cao, J.; Zhao, Y.; Shao, T.; Song, Y.; Leung, M.K.H.; Sun, L.; Hou, J. Engineering Single-Atom Active Sites on Covalent Organic Frameworks for Boosting CO2 Photoreduction. J. Am. Chem. Soc. 2022, 144, 17097–17109. [Google Scholar] [CrossRef]
- Qin, L.; Gan, J.; Niu, D.; Cao, Y.; Duan, X.; Qin, X.; Zhang, H.; Jiang, Z.; Jiang, Y.; Dai, S.; et al. Interfacial-confined coordination to single-atom nanotherapeutics. Nat. Commun. 2022, 13, 91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Tao, L.; An, J.; Zhang, J.; Meng, L.; Zheng, X.; Wang, Y.; Li, N.; Du, S.; Zhang, J.; et al. Engineering the Local Atomic Environments of Indium Single-Atom Catalysts for Efficient Electrochemical Production of Hydrogen Peroxide. Angew. Chem. Int. Ed. Engl. 2022, 61, e202117347. [Google Scholar] [CrossRef] [PubMed]
- Yanan, S.; Xiaoguang, D.; Shaobin, W.; Qinyan, Y.; Baoyu, G.; Xing, X. Carbon-based single atom catalyst: Synthesis, characterization, DFT calculations. Chin. Chem. Lett. 2021, 33, 663–673. [Google Scholar] [CrossRef]
- Chen, J.; Kang, Y.; Zhang, W.; Zhang, Z.; Chen, Y.; Yang, Y.; Duan, L.; Li, Y.; Li, W. Lattice-Confined Single-Atom Fe1Sx on Mesoporous TiO2 for Boosting Ambient Electrocatalytic N2 Reduction Reaction. Angew. Chem. Int. Ed. Engl. 2022, 61, e202203022. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Tian, M.; Jing, M.; Chai, S.; Jian, Y.; Chen, C.; Douthwaite, M.; Zheng, L.; Ma, M.; Song, W.; et al. Modulating the Electronic Metal-Support Interactions in Single-Atom Pt1-CuO Catalyst for Boosting Acetone Oxidation. Angew. Chem. Int. Ed. Engl. 2022, 61, e202200763. [Google Scholar] [CrossRef] [PubMed]
- Kuai, L.; Liu, L.; Tao, Q.; Yu, N.; Kan, E.; Sun, N.; Liu, S.; Geng, B. High-Areal Density Single-Atoms/Metal Oxide Nanosheets: A Micro-Gas Blasting Synthesis and Superior Catalytic Properties. Angew. Chem. Int. Ed. Engl. 2022, 61, e202212338. [Google Scholar] [CrossRef] [PubMed]
- Meza, E.; Diaz, R.E.; Li, C.W. Solution-Phase Activation and Functionalization of Colloidal WS2 Nanosheets with Ni Single Atoms. ACS Nano 2020, 14, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, R.; Fan, X.; Liu, Y.; Kong, N.; Lin, H.; Li, Y. A mechanistic study of selective propane dehydrogenations on MoS2 supported single Fe atoms. Chin. Chem. Lett. 2022, 34, 107257. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, X.; Yang, L.; Cheng, D.; Cao, D. Single-Atom Ru Doping Induced Phase Transition of MoS2 and S Vacancy for Hydrogen Evolution Reaction. Small Methods 2019, 3, 1900653. [Google Scholar] [CrossRef]
- Peng, Y.; Geng, Z.; Zhao, S.; Wang, L.; Li, H.; Wang, X.; Zheng, X.; Zhu, J.; Li, Z.; Si, R.; et al. Pt Single Atoms Embedded in the Surface of Ni Nanocrystals as Highly Active Catalysts for Selective Hydrogenation of Nitro Compounds. Nano Lett. 2018, 18, 3785–3791. [Google Scholar] [CrossRef] [PubMed]
- Pei, G.X.; Liu, X.Y.; Yang, X.F.; Zhang, L.L.; Wang, A.Q.; Li, L.; Wang, H.; Wang, X.D.; Zhang, T. Performance of Cu-Alloyed Pd Single-Atom Catalyst for Semihydrogenation of Acetylene under Simulated Front-End Conditions. Acs Catal. 2017, 7, 1491–1500. [Google Scholar] [CrossRef]
- Bushira, F.A.; Kitte, S.A.; Li, H.; Zheng, L.; Wang, P.; Jin, Y. Enzyme-like Fe-N5 single atom catalyst for simultaneous electrochemical detection of dopamine and uric acid. J. Electroanal. Chem. 2022, 904, 115956. [Google Scholar] [CrossRef]
- Liang, W.; Gao, M.; Li, Y.; Tong, Y.; Ye, B.C. Single-atom electrocatalysts templated by MOF for determination of levodopa. Talanta 2021, 225, 122042. [Google Scholar] [CrossRef]
- Song, Y.Y.; He, T.; Zhang, Y.L.; Yin, C.Y.; Chen, Y.; Liu, Q.M.; Zhang, Y.; Chen, S.W. Cobalt single atom sites in carbon aerogels for ultrasensitive enzyme-free electrochemical detection of glucose. J. Electroanal. Chem. 2022, 906, 116024. [Google Scholar] [CrossRef]
- Bushira, F.A.; Wang, P.; Wang, Y.; Hou, S.; Diao, X.; Li, H.; Zheng, L.; Jin, Y. Plasmon-Boosted Fe, Co Dual Single-Atom Catalysts for Ultrasensitive Luminol-Dissolved O2 Electrochemiluminescence Detection of Prostate-Specific Antigen. Anal. Chem. 2022, 94, 9758–9765. [Google Scholar] [CrossRef]
- Gu, W.; Wang, X.; Xi, M.; Wei, X.; Jiao, L.; Qin, Y.; Huang, J.; Cui, X.; Zheng, L.; Hu, L.; et al. Single-Atom Iron Enables Strong Low-Triggering-Potential Luminol Cathodic Electrochemiluminescence. Anal. Chem. 2022, 94, 9459–9465. [Google Scholar] [CrossRef]
- Bott-Neto, J.L.; Martins, T.S.; Buscaglia, L.A.; Machado, S.A.S.; Oliveira, O.N., Jr. Photocatalysis of TiO2 Sensitized with Graphitic Carbon Nitride and Electrodeposited Aryl Diazonium on Screen-Printed Electrodes to Detect Prostate Specific Antigen under Visible Light. ACS Appl. Mater. Interfaces 2022, 14, 22114–22121. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Wen, J.; Wang, X.; Jiao, L.; Wei, X.; Wang, H.; Li, J.; Liu, M.; Zheng, L.; Hu, L.; et al. Iron Single-Atom Catalysts Boost Photoelectrochemical Detection by Integrating Interfacial Oxygen Reduction and Enzyme-Mimicking Activity. ACS Nano 2022, 16, 2997–3007. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Qin, X.; Zhang, X.; Cai, X.; Huang, F.; Jia, Z.; Diao, J.; Xiao, D.; Jiang, Z.; Lu, R.; et al. A Magnetically Separable Pd Single-Atom Catalyst for Efficient Selective Hydrogenation of Phenylacetylene. Adv. Mater. 2022, 34, e2110455. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Xu, M.; Xu, S.M.; Wang, Y.; Ge, R.; Hu, X.; Sun, X.; Duan, H. Electrocatalytic Hydrogenation of 5-Hydroxymethylfurfural Promoted by a Ru1Cu Single-Atom Alloy Catalyst. Angew. Chem. Int. Ed. Engl. 2022, 61, e202209849. [Google Scholar] [CrossRef]
- Zheng, K.; Li, Y.; Liu, B.; Jiang, F.; Xu, Y.; Liu, X. Ti-doped CeO2 Stabilized Single-Atom Rhodium Catalyst for Selective and Stable CO2 Hydrogenation to Ethanol. Angew. Chem. Int. Ed. Engl. 2022, 61, e202210991. [Google Scholar] [CrossRef]
- Zou, R.; Xie, R.; Peng, Y.; Guan, W.; Lin, Y.; Lu, C. Ag-O-Co Interface Modulation-Amplified Luminol Cathodic Electrogenerated Chemiluminescence. Anal. Chem. 2022, 94, 4813–4820. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Z.; Tian, Y.; Li, X.; Cai, Q.; Zhao, J. Single-atom rhodium anchored on S-doped black phosphorene as a promising bifunctional electrocatalyst for overall water splitting. Chin. Chem. Lett. 2022, 354, 107812. [Google Scholar] [CrossRef]
- Xu, J.; Xu, H.; Dong, A.; Zhang, H.; Zhou, Y.; Dong, H.; Tang, B.; Liu, Y.; Zhang, L.; Liu, X.; et al. Strong Electronic Metal-Support Interaction between Iridium Single Atoms and a WO3 Support Promotes Highly Efficient and Robust CO2 Cycloaddition. Adv. Mater. 2022, 34, e2206991. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, Y.; Zhou, Y.; Zheng, R.; Feng, X.; Ge, Q.; Wang, R.; Yang, D.; Wei, W.; Wu, X.; Lin, J.; et al. Bandgap engineering of tetragonal phase CuFeS2 quantum dots via mixed-valence single-atomic Ag decoration for synergistic Cr(VI) reduction and RhB degradation. Chin. Chem. Lett. 2021, 32, 3450–3456. [Google Scholar] [CrossRef]
- Yan, P.; Shu, S.; Shi, X.; Li, J. Promotion effect of Au single-atom support graphene for CO oxidation. Chin. Chem. Lett. 2022, 33, 4822–4827. [Google Scholar] [CrossRef]
- Peng, L.; Yang, J.; Yang, Y.; Qian, F.; Wang, Q.; Sun-Waterhouse, D.; Shang, L.; Zhang, T.; Waterhouse, G.I.N. Mesopore-Rich Fe-N-C Catalyst with FeN4-O-NC Single-Atom Sites Delivers Remarkable Oxygen Reduction Reaction Performance in Alkaline Media. Adv. Mater. 2022, 34, e2202544. [Google Scholar] [CrossRef]
- Cai, X.; Ma, F.; Jiang, J.; Yang, X.; Zhang, Z.; Jian, Z.; Liang, M.; Li, P.; Yu, L. Fe-N-C single-atom nanozyme for ultrasensitive, on-site and multiplex detection of mycotoxins using lateral flow immunoassay. J. Hazard. Mater. 2023, 441, 129853. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Chen, X.; Hu, W.; Chuang, C.; Xie, S.; Hu, A.; Yan, W.; Kong, X.; Wu, X.; Ji, H.; et al. Cobalt in Nitrogen-Doped Graphene as Single-Atom Catalyst for High-Sulfur Content Lithium-Sulfur Batteries. J. Am. Chem. Soc. 2019, 141, 3977–3985. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liu, J. Carbon-supported CoS4-C single-atom nanozyme for dramatic improvement in CO2 electroreduction to HCOOH: A DFT study combined with hybrid solvation model. Chin. Chem. Lett. 2022, 34, 108018. [Google Scholar] [CrossRef]
- Cai, S.; Liu, J.; Ding, J.; Fu, Z.; Li, H.; Xiong, Y.; Lian, Z.; Yang, R.; Chen, C. Tumor-Microenvironment-Responsive Cascade Reactions by a Cobalt-Single-Atom Nanozyme for Synergistic Nanocatalytic Chemotherapy. Angew. Chem. Int. Ed. Engl. 2022, 61, e202204502. [Google Scholar] [CrossRef]
- Wu, W.; Wang, Y.; Luo, L.; Wang, M.; Li, Z.; Chen, Y.; Wang, Z.; Chai, J.; Cen, Z.; Shi, Y.; et al. CO2 Hydrogenation over Copper/ZnO Single-Atom Catalysts: Water-Promoted Transient Synthesis of Methanol. Angew. Chem. Int. Ed. Engl. 2022, 61, e202213024. [Google Scholar] [CrossRef]
- Yitao, Z.; Lei, T. Towards catalytic reactions of Cu single-atom catalysts: Recent progress and future perspective. Chin. Chem. Lett. 2023, 108571. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, W.; Cheng, J.; Qu, Y.; Dai, Y.; Liu, M.; Yu, J.; Wang, C.; Wang, H.; Wang, S.; et al. Stimuli-Responsive Manganese Single-Atom Nanozyme for Tumor Therapy via Integrated Cascade Reactions. Angew. Chem. Int. Ed. Engl. 2021, 60, 9480–9488. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Sung, Y.; Choi, C.; Joo Bang, G.; Hong, S.; Tan, X.; Wu, T.S.; Soo, Y.L.; Xiong, P.; Meng-Jung Li, M.; et al. Single-Atom Molybdenum-N3 Sites for Selective Hydrogenation of CO2 to CO. Angew. Chem. Int. Ed. Engl. 2022, 61, e202203836. [Google Scholar] [CrossRef]
- Xu, B.; Wang, H.; Wang, W.; Gao, L.; Li, S.; Pan, X.; Wang, H.; Yang, H.; Meng, X.; Wu, Q.; et al. A Single-Atom Nanozyme for Wound Disinfection Applications. Angew. Chem. Int. Ed. Engl. 2019, 58, 4911–4916. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhao, Y.; Shi, R.; Wang, Y.; Ashok, A.; Heraly, F.; Zhang, T.; Yuan, J. Vacancy-Rich MXene-Immobilized Ni Single Atoms as a High-Performance Electrocatalyst for the Hydrazine Oxidation Reaction. Adv. Mater. 2022, 34, e2204388. [Google Scholar] [CrossRef]
- Quanguo, J.; Yushuai, Q.; Yuqing, L.; Min, H.; Zhimin, A. Strain boosts CO oxidation on Ni single-atom-catalyst supported by defective graphene. Chin. Chem. Lett. 2022, 34, 107395. [Google Scholar] [CrossRef]
- Ai, Y.; Hu, Z.N.; Liang, X.; Sun, H.b.; Xin, H.; Liang, Q. Recent Advances in Nanozymes: From Matters to Bioapplications. Adv. Funct. Mater. 2021, 32, 107395. [Google Scholar] [CrossRef]
- Fan, Y.; Gan, X.; Zhao, H.; Zeng, Z.; You, W.; Quan, X. Multiple application of SAzyme based on carbon nitride nanorod-supported Pt single-atom for H2O2 detection, antibiotic detection and antibacterial therapy. Chem. Eng. J. 2022, 427, 131572. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, Y.; Mo, Y.; Zhai, Y.; Liu, J.; Strzelecki, A.C.; Guo, X.; Shan, C. Boosting Electrochemical Catalysis and Nonenzymatic Sensing Toward Glucose by Single-Atom Pt Supported on Cu@CuO Core-Shell Nanowires. Small 2023, 19, 2207240. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, P.; Hao, H.; Hong, J.; Li, H.; Ji, S.; Li, A.; Gao, R.; Dong, J.; Han, X.; et al. Thermal Atomization of Platinum Nanoparticles into Single Atoms: An Effective Strategy for Engineering High-Performance Nanozymes. J. Am. Chem. Soc. 2021, 143, 18643–18651. [Google Scholar] [CrossRef]
- Gu, W.; Wang, H.; Jiao, L.; Wu, Y.; Chen, Y.; Hu, L.; Gong, J.; Du, D.; Zhu, C. Single-Atom Iron Boosts Electrochemiluminescence. Angew. Chem. Int. Ed. Engl. 2020, 59, 3534–3538. [Google Scholar] [CrossRef]
- Qin, J.; Liu, H.; Zou, P.; Zhang, R.; Wang, C.; Xin, H.L. Altering Ligand Fields in Single-Atom Sites through Second-Shell Anion Modulation Boosts the Oxygen Reduction Reaction. J. Am. Chem. Soc. 2022, 144, 2197–2207. [Google Scholar] [CrossRef]
- Wu, N.; Zhong, H.; Zhang, Y.; Wei, X.; Jiao, L.; Wu, Z.; Huang, J.; Wang, H.; Beckman, S.P.; Gu, W.; et al. Atomically dispersed Ru3 site catalysts for electrochemical sensing of small molecules. Biosens. Bioelectron. 2022, 216, 114609. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Li, J.C.; Liu, D.; Lin, Y.; Du, D. Single-Atom Nanozyme Based on Nanoengineered Fe-N-C Catalyst with Superior Peroxidase-Like Activity for Ultrasensitive Bioassays. Small 2019, 15, e1901485. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Feng, M.; Deng, D.; Xie, X.; Deng, C.; Khattak, K.N.; Yang, X. Dual-active-site Fe/Cu single-atom nanozymes with multifunctional specific peroxidase-like properties for S2− detection and dye degradation. Chin. Chem. Lett. 2022, 34, 107969. [Google Scholar] [CrossRef]
- Ma, C.B.; Xu, Y.; Wu, L.; Wang, Q.; Zheng, J.J.; Ren, G.; Wang, X.; Gao, X.; Zhou, M.; Wang, M.; et al. Guided Synthesis of a Mo/Zn Dual Single-Atom Nanozyme with Synergistic Effect and Peroxidase-like Activity. Angew. Chem. Int. Ed. Engl. 2022, 61, e202116170. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, J.; Park, O.K.; Kim, J.; Kim, J.; Lee, D.; Paidi, V.K.; Jung, E.; Lee, H.S.; Lee, B.; et al. Geometric Tuning of Single-Atom FeN4 Sites via Edge-Generation Enhances Multi-Enzymatic Properties. Adv. Mater. 2023, 35, 2207666. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.F.; Jiang, B.; Hao, H.G.; Chen, Y.J.; Dong, J.C.; Mao, Y.; Zhang, Z.D.; Gao, R.; Chen, W.X.; Zhang, R.F.; et al. Matching the kinetics of natural enzymes with a single-atom iron nanozyme. Nat. Catal. 2021, 4, 407–417. [Google Scholar] [CrossRef]
- Bian, W.; Shen, X.; Tan, H.; Fan, X.; Liu, Y.; Lin, H.; Li, Y. The triggering of catalysis via structural engineering at atomic level: Direct propane dehydrogenation on Fe-N3P-C. Chin. Chem. Lett. 2023, 34, 107289. [Google Scholar] [CrossRef]
- Jiao, L.; Xu, W.; Zhang, Y.; Wu, Y.; Gu, W.; Ge, X.; Chen, B.; Zhu, C.; Guo, S. Boron-doped Fe-N-C single-atom nanozymes specifically boost peroxidase-like activity. Nano Today 2020, 35, 100971. [Google Scholar] [CrossRef]
- Xu, B.; Li, S.; Zheng, L.; Liu, Y.; Han, A.; Zhang, J.; Huang, Z.; Xie, H.; Fan, K.; Gao, L.; et al. A Bioinspired Five-Coordinated Single-Atom Iron Nanozyme for Tumor Catalytic Therapy. Adv. Mater. 2022, 34, e2107088. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Pan, C.; He, C.T.; Han, Y.; Ma, W.; Wei, H.; Ji, W.; Chen, W.; Mao, J.; Yu, P.; et al. Single-Atom Co-N4 Electrocatalyst Enabling Four-Electron Oxygen Reduction with Enhanced Hydrogen Peroxide Tolerance for Selective Sensing. J. Am. Chem. Soc. 2020, 142, 16861–16867. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, C.; Yuan, C.; Shi, Z.; Zhang, K.; Zou, Z.; Xiong, L.; Chen, J.; Jiang, Y.; Sun, W.; et al. Single-Atom Iron Anchored on 2-D Graphene Carbon to Realize Bridge-Adsorption of O-O as Biomimetic Enzyme for Remarkably Sensitive Electrochemical Detection of H2O2. Anal. Chem. 2022, 94, 14109–14117. [Google Scholar] [CrossRef]
- Wei, X.; Song, S.; Song, W.; Xu, W.; Jiao, L.; Luo, X.; Wu, N.; Yan, H.; Wang, X.; Gu, W.; et al. Fe3C-Assisted Single Atomic Fe Sites for Sensitive Electrochemical Biosensing. Anal. Chem. 2021, 93, 5334–5342. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Butler, D.; Lucking, M.C.; Zhang, F.; Xia, T.; Fujisawa, K.; Nakajima, T.G.; Silva, R.C.; Endo, M.; Terrones, H.; et al. Single-atom doping of MoS2 with manganese enables ultrasensitive detection of dopamine: Experimental and computational approach. Sci. Adv. 2020, 6, eabc4250. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Lyu, Z.; Fang, L.; Li, T.; Zhu, W.; Li, S.; Li, X.; Li, J.C.; Du, D.; Lin, Y. Single-Atomic Site Catalyst with Heme Enzymes-Like Active Sites for Electrochemical Sensing of Hydrogen Peroxide. Small 2021, 17, e2100664. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Wu, F.; Mao, J.; Wu, W.; Zhao, G.; Ji, W.; Ma, W.; Yu, P.; Mao, L. Highly Stable and Selective Sensing of Hydrogen Sulfide in Living Mouse Brain with NiN4 Single-Atom Catalyst-Based Galvanic Redox Potentiometry. J. Am. Chem. Soc. 2022, 144, 14678–14686. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Ma, W.; Mao, J.; He, C.T.; Ji, W.; Chen, Z.; Chen, W.; Wu, W.; Yu, P.; Mao, L. A single-atom Cu-N2 catalyst eliminates oxygen interference for electrochemical sensing of hydrogen peroxide in a living animal brain. Chem. Sci. 2021, 12, 15045–15053. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhao, P.; Zheng, J.L.; Chen, Y.Y.; Liu, Y.Y.; Zheng, J.; Luo, X.G.; Huo, D.Q.; Hou, C.J. Fe Single-Atom Electrochemical Sensors for H2O2 Produced by Living Cells. Acs Appl. Nano Mater. 2022, 5, 11852–11863. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Chen, S.; Zha, Q.; Zhu, M. In-situ monitoring of hydrogen peroxide production at nickel single-atom electrocatalyst. Chem. Eng. J. 2023, 460, 141657. [Google Scholar] [CrossRef]
- Qi, C.; Wang, W.; Dong, Y. Synthesis of Se single atoms on nitrogen-doped carbon as novel electrocatalyst for sensitive nonenzymatic sensing of hydrogen peroxide. Anal. Bioanal. Chem. 2023, 415, 5391–5401. [Google Scholar] [CrossRef]
- Qi, C.; Luo, Y.; Dong, Y. Synergistic effects of Fe-Se dual single-atom sites for boosting electrochemical nonenzymatic H2O2 sensing. Appl. Surf. Sci. 2023, 637, 157900. [Google Scholar] [CrossRef]
- Shu, Y.J.; Li, Z.J.; Yang, Y.; Tan, J.W.; Liu, Z.Y.; Shi, Y.H.; Ye, C.X.; Gao, Q.S. Isolated Cobalt Atoms on N-Doped Carbon as Nanozymes for Hydrogen Peroxide and Dopamine Detection. Acs Appl. Nano Mater. 2021, 4, 7954–7962. [Google Scholar] [CrossRef]
- Sun, X.J.; Chen, C.; Xiong, C.; Zhang, C.M.; Zheng, X.S.; Wang, J.; Gao, X.P.; Yu, Z.Q.; Wu, Y.E. Surface modification of MoS2 nanosheets by single Ni atom for ultrasensitive dopamine detection. Nano Res. 2022, 16, 917–924. [Google Scholar] [CrossRef]
- Xie, X.; Wang, D.P.; Guo, C.; Liu, Y.; Rao, Q.; Lou, F.; Li, Q.; Dong, Y.; Li, Q.; Yang, H.B.; et al. Single-Atom Ruthenium Biomimetic Enzyme for Simultaneous Electrochemical Detection of Dopamine and Uric Acid. Anal. Chem. 2021, 93, 4916–4923. [Google Scholar] [CrossRef] [PubMed]
- Long, B.; Cao, P.; Zhao, Y.; Fu, Q.; Mo, Y.; Zhai, Y.; Liu, J.; Lyu, X.; Li, T.; Guo, X.; et al. Pt1/Ni6Co1 layered double hydroxides/N-doped graphene for electrochemical non-enzymatic glucose sensing by synergistic enhancement of single atoms and doping. Nano Res. 2022, 16, 318–324. [Google Scholar] [CrossRef]
- Long, B.; Zhao, Y.; Cao, P.; Wei, W.; Mo, Y.; Liu, J.; Sun, C.J.; Guo, X.; Shan, C.; Zeng, M.H. Single-Atom Pt Boosting Electrochemical Nonenzymatic Glucose Sensing on Ni(OH)2/N-Doped Graphene. Anal. Chem. 2022, 94, 1919–1924. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Cai, Q.; Deng, M. Construction of Electrochemical Aptamer Sensor Based on Pt-Coordinated Titanium-Based Porphyrin MOF for Thrombin Detection. Front. Chem. 2021, 9, 812983. [Google Scholar] [CrossRef]
- Li, M.; Peng, X.; Liu, X.; Wang, H.; Zhang, S.; Hu, G. Single-atom niobium doped BCN nanotubes for highly sensitive electrochemical detection of nitrobenzene. RSC Adv. 2021, 11, 28988–28995. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Wang, X.; Wen, J.; Cao, S.; Jiao, L.; Wu, Y.; Wei, X.; Zheng, L.; Hu, L.; Zhang, L.; et al. Modulating Oxygen Reduction Behaviors on Nickel Single-Atom Catalysts to Probe the Electrochemiluminescence Mechanism at the Atomic Level. Anal. Chem. 2021, 93, 8663–8670. [Google Scholar] [CrossRef]
- Bushira, F.A.; Kitte, S.A.; Xu, C.; Li, H.; Zheng, L.; Wang, P.; Jin, Y. Two-Dimensional-Plasmon-Boosted Iron Single-Atom Electrochemiluminescence for the Ultrasensitive Detection of Dopamine, Hemin, and Mercury. Anal. Chem. 2021, 93, 9949–9957. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Zheng, X.; Li, J.; Wang, L.; Xue, Y.; Peng, C.; Han, Y.; Wang, Y.; Guo, S.; Wang, J.; et al. Identifying Luminol Electrochemiluminescence at the Cathode via Single-Atom Catalysts Tuned Oxygen Reduction Reaction. J. Am. Chem. Soc. 2022, 144, 7741–7749. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Xu, W.; Wu, Z.; Jiao, L.; Luo, X.; Xi, M.; Su, R.; Hu, L.; Gu, W.; Zhu, C. Iron Single-Atom Catalyst-Enabled Peroxydisulfate Activation Enhances Cathodic Electrochemiluminescence of Tris(bipyridine)ruthenium(II). Anal. Chem. 2023, 95, 10762–10768. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, Y.; Gao, J.; Ouyang, H.; He, Y.; Fu, Z. PEGylated Ni Single-Atom Catalysts as Ultrasensitive Electrochemiluminescent Probes with Favorable Aqueous Dispersibility for Assaying Drug-Resistant Pathogens. Anal. Chem. 2022, 94, 14047–14053. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, Y.; Xia, Y.; Zhao, F.; Zeng, B. Simultaneous Detection of Ovarian Cancer-Concerned HE4 and CA125 Markers Based on Cu Single-Atom-Triggered CdS QDs and Eu MOF@Isoluminol ECL. Anal. Chem. 2023, 95, 4795–4802. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wan, X.; Kong, T.; Han, W.; Xiong, Y. Single-atom-based catalysts for photoelectrocatalysis: Challenges and opportunities. J. Mater. Chem. A 2022, 10, 5878–5888. [Google Scholar] [CrossRef]
- Zeng, R.J.; Wang, W.J.; Cai, G.N.; Huang, Z.L.; Tao, J.M.; Tang, D.P.; Zhu, C.Z. Single-atom platinum nanocatalyst-improved catalytic efficiency with enzyme-DNA supermolecular architectures. Nano Energy 2020, 74, 104931. [Google Scholar] [CrossRef]
- Qin, Y.; Wen, J.; Zheng, L.; Yan, H.; Jiao, L.; Wang, X.; Cai, X.; Wu, Y.; Chen, G.; Chen, L.; et al. Single-Atom-Based Heterojunction Coupling with Ion-Exchange Reaction for Sensitive Photoelectrochemical Immunoassay. Nano Lett. 2021, 21, 1879–1887. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Guo, L.; Chen, M.; Guo, Y.; Ge, L.; Kwok, H.F. Single-atom Pt-anchored Zn0.5Cd0.5S boosted photoelectrochemical immunoassay of prostate-specific antigen. Biosens. Bioelectron. 2022, 202, 114006. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Jiang, Y.; Wang, G.; Wu, W.; Chen, W.; Yu, P.; Lin, Y.; Mao, J.; Mao, L. Single-atom Ni-N4 provides a robust cellular NO sensor. Nat. Commun. 2020, 11, 3188. [Google Scholar] [CrossRef]
- Hou, H.F.; Mao, J.J.; Han, Y.H.; Wu, F.; Zhang, M.N.; Wang, D.S.; Mao, L.Q.; Li, Y.D. Single-atom electrocatalysis: A new approach to in vivo electrochemical biosensing. Sci. China-Chem. 2019, 62, 1720–1724. [Google Scholar] [CrossRef]
- Hu, F.X.; Hu, T.; Chen, S.; Wang, D.; Rao, Q.; Liu, Y.; Dai, F.; Guo, C.; Yang, H.B.; Li, C.M. Single-Atom Cobalt-Based Electrochemical Biomimetic Uric Acid Sensor with Wide Linear Range and Ultralow Detection Limit. Nanomicro Lett. 2020, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.Y.; Li, S.S.; Xiao, X.Y.; Chen, S.H.; Liu, J.H.; Huang, X.J. Defect- and phase-engineering of Mn-mediated MoS2 nanosheets for ultrahigh electrochemical sensing of heavy metal ions: Chemical interaction-driven in situ catalytic redox reactions. Chem. Commun. 2018, 54, 9329–9332. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Gao, S.; Liu, S.; Bi, Y.; Wang, R.; Qu, H.; Wu, Y.; Mao, Y.; Zheng, L. Single-Atom Enzyme-Functionalized Solution-Gated Graphene Transistor for Real-Time Detection of Mercury Ion. ACS Appl. Mater. Interfaces 2020, 12, 6268–6275. [Google Scholar] [CrossRef]
- Li, P.H.; Yang, M.; Li, Y.X.; Song, Z.Y.; Liu, J.H.; Lin, C.H.; Zeng, J.; Huang, X.J. Ultra-Sensitive and Selective Detection of Arsenic (III) via Electroanalysis over Cobalt Single-Atom Catalysts. Anal. Chem. 2020, 92, 6128–6135. [Google Scholar] [CrossRef] [PubMed]
- Cong, W.; Song, P.; Zhang, Y.; Yang, S.; Liu, W.; Zhang, T.; Zhou, J.; Wang, M.; Liu, X. Supramolecular confinement pyrolysis to carbon-supported Mo nanostructures spanning four scales for hydroquinone determination. J. Hazard. Mater. 2022, 437, 129327. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Luo, Z.; Wei, X.; Jiao, L.; Fang, Q.; Wang, H.; Wang, J.; Gu, W.; Hu, L.; Zhu, C. Iridium Single-Atomic Site Catalysts with Superior Oxygen Reduction Reaction Activity for Sensitive Monitoring of Organophosphorus Pesticides. Anal. Chem. 2022, 94, 1390–1396. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Yang, M.; Song, W.; Fang, Q.; Wei, X.; Jiao, L.; Xu, W.; Kang, Y.; Wang, H.; Wu, N.; et al. Neutral Zn-Air Battery Assembled with Single-Atom Iridium Catalysts for Sensitive Self-Powered Sensing System. Adv. Funct. Mater. 2021, 31, 2101193. [Google Scholar] [CrossRef]
- Tan, R.; Qin, Y.; Liu, M.; Wang, H.; Su, R.; Xiao, R.; Li, J.; Hu, L.; Gu, W.; Zhu, C. Bifunctional Single-Atom Iron Cocatalysts Enable an Efficient Photoelectrochemical Fuel Cell for Sensitive Biosensing. Adv. Funct. Mater. 2023, 2305673. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, J.; Liu, Y. Single-Atom Nanomaterials in Electrochemical Sensors Applications. Chemosensors 2023, 11, 486. https://doi.org/10.3390/chemosensors11090486
Fu J, Liu Y. Single-Atom Nanomaterials in Electrochemical Sensors Applications. Chemosensors. 2023; 11(9):486. https://doi.org/10.3390/chemosensors11090486
Chicago/Turabian StyleFu, Jinglin, and Yang Liu. 2023. "Single-Atom Nanomaterials in Electrochemical Sensors Applications" Chemosensors 11, no. 9: 486. https://doi.org/10.3390/chemosensors11090486
APA StyleFu, J., & Liu, Y. (2023). Single-Atom Nanomaterials in Electrochemical Sensors Applications. Chemosensors, 11(9), 486. https://doi.org/10.3390/chemosensors11090486







