Green and Sensitive Analysis of the Antihistaminic Drug Pheniramine Maleate and Its Main Toxic Impurity Using UPLC and TLC Methods, Blueness Assessment, and Greenness Assessments
Abstract
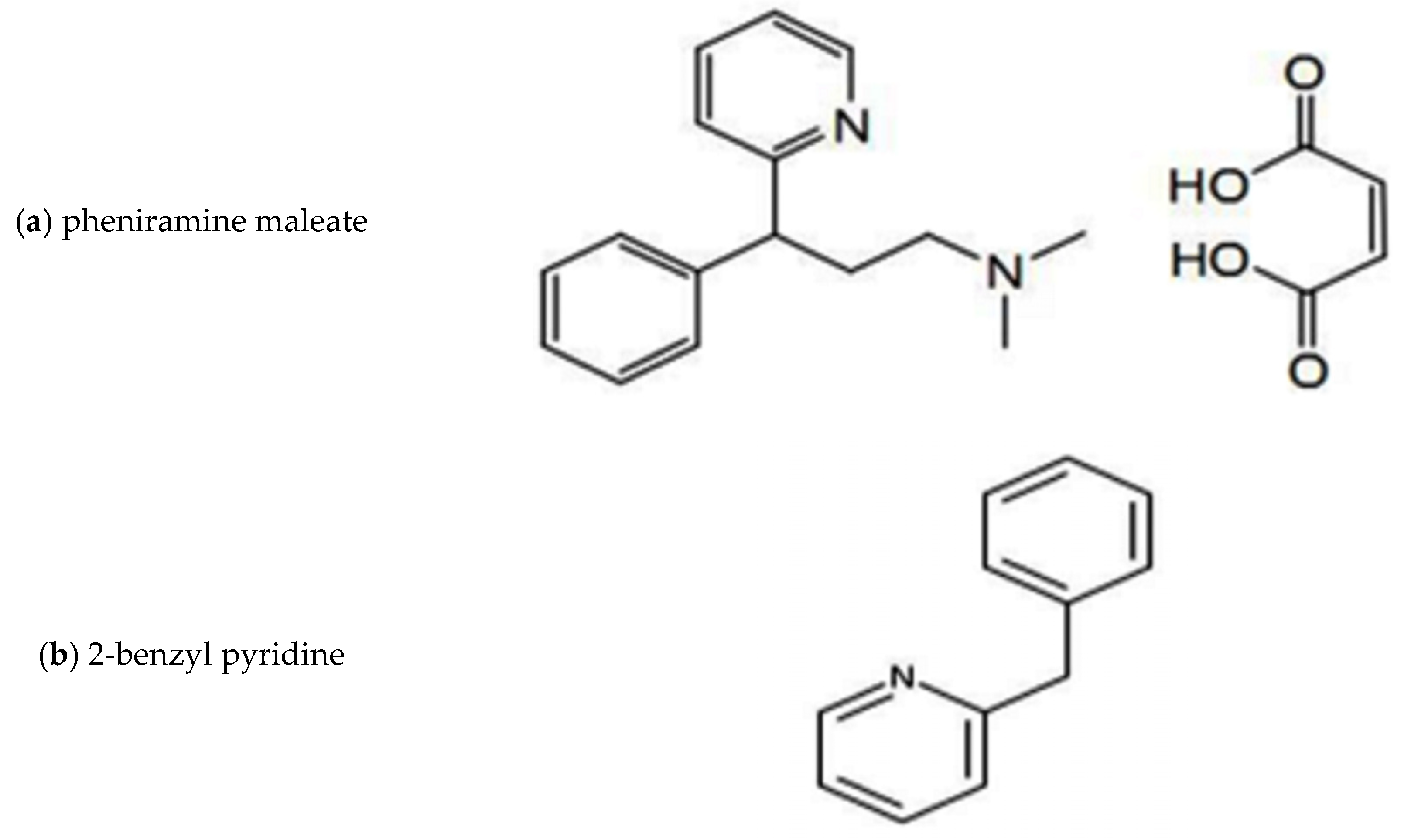
:1. Introduction
2. Materials and Methods Descriptions
2.1. Apparatuses
2.2. Pure Standard Samples
2.3. Pharmaceutical Formulation
2.4. Solvents and Reagents
2.5. Parent and Working Solutions
2.5.1. PAM and BNZ Stock Typical Solutions (1 mg/mL)
2.5.2. PAM and BNZ Secondary Standard Solutions (100 µg/mL)
2.6. Pharmaceutical Formulation Solutions
2.7. Analytical Procedures
2.7.1. Circumstances for the Chromatographic Techniques
- -
- RP-UPLC Chromatographic Approach
- -
- TLC–Densitometry Method
2.7.2. Development of Measurement Curves
- -
- RP-UPLC Approach
- -
- TLC Spectrodensitometric Approach
2.8. Application to Avil® Ampoules
3. Results and Discussion
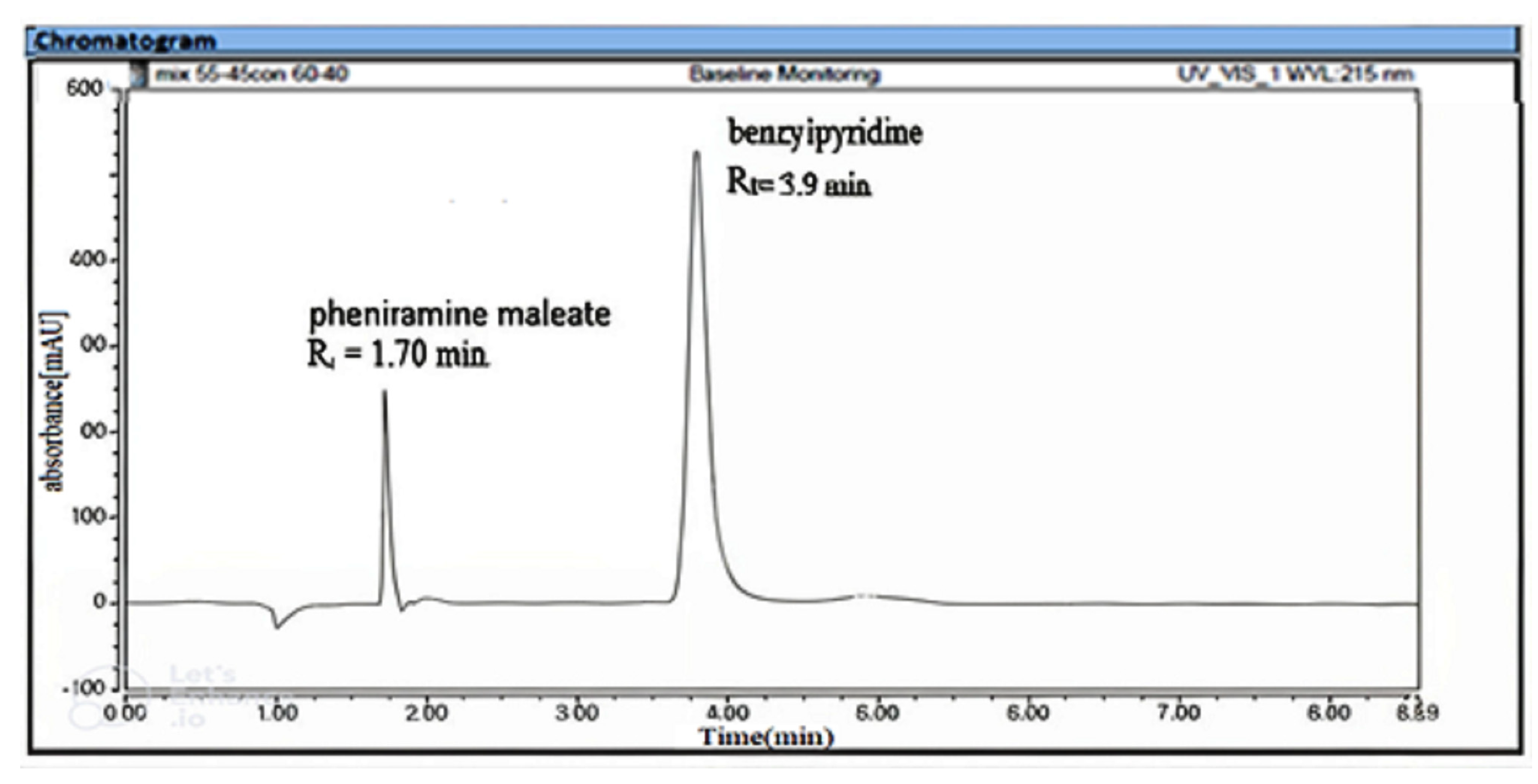
3.1. Developments of the Chromatographic Methods and Their Optimization
3.1.1. For RP- UPLC Method
- A.
- Mobile Phase Composition
- B.
- Scanning Wavelengths
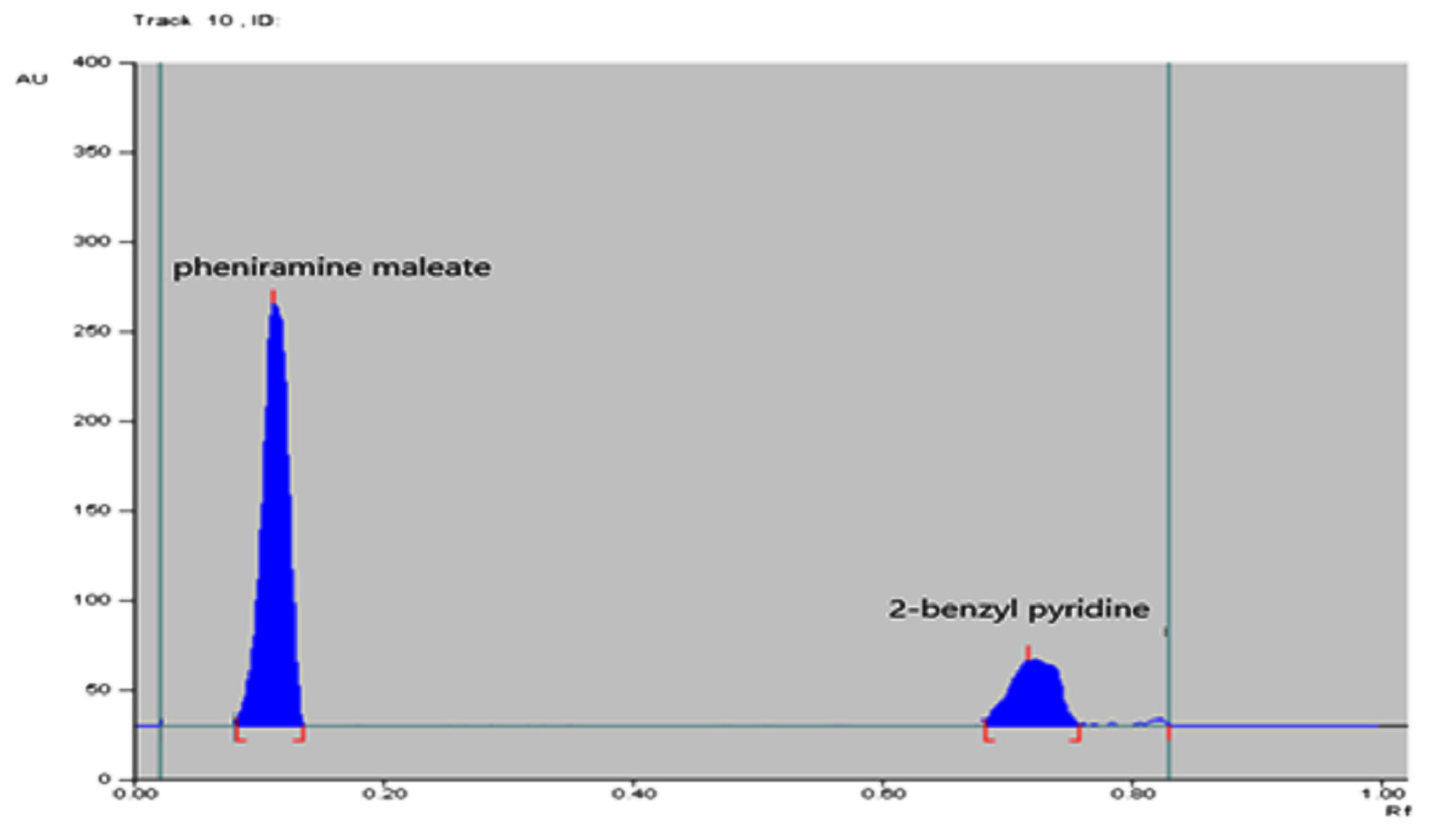
3.1.2. For TLC Method
- A.
- Developing System
- B.
- Investigation of Optimal Scanning Wavelength
- C.
- Dimensions of Scanning Beam’s Slit and Band
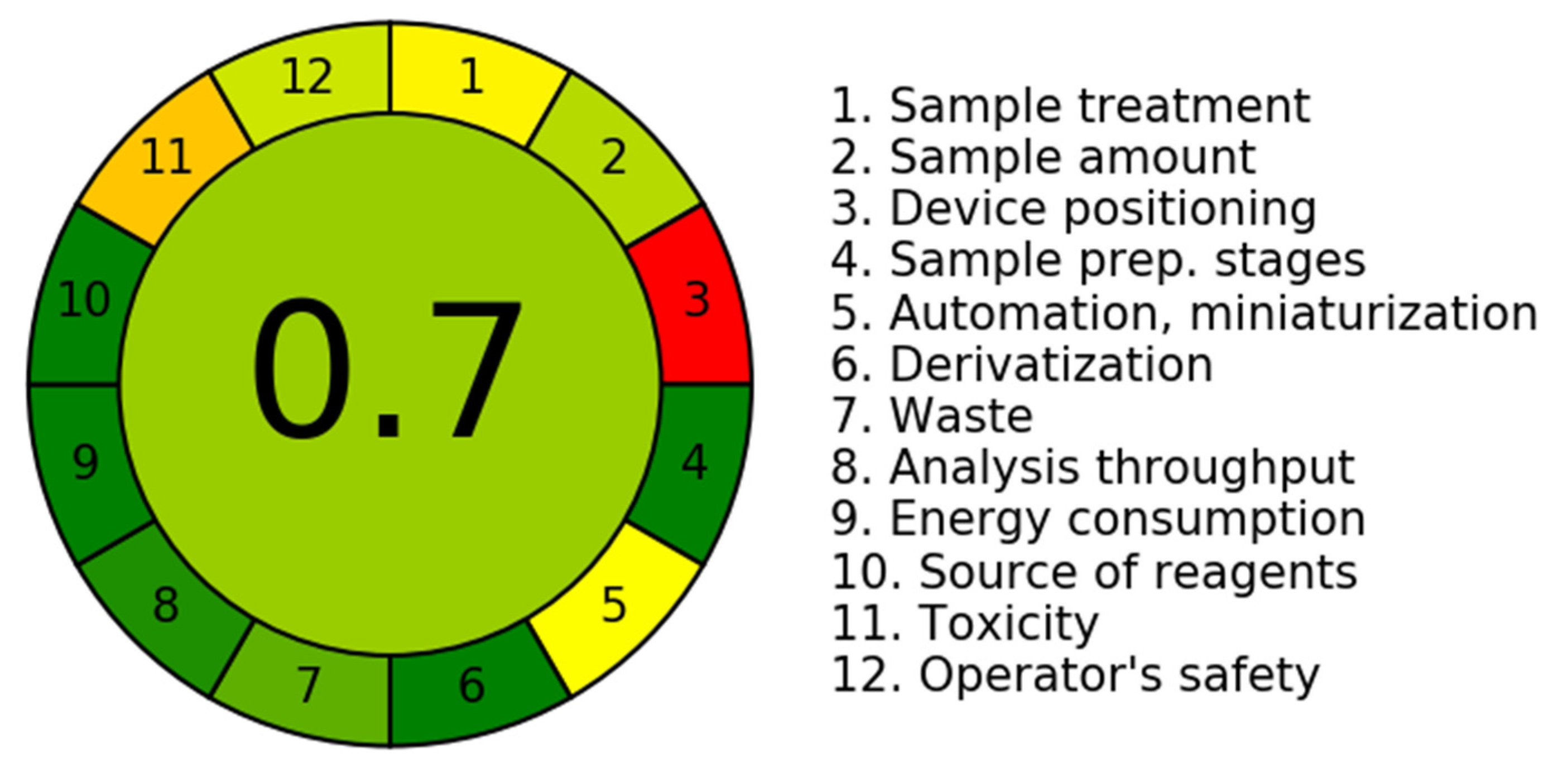
3.2. Assessment of Greenness Characteristics
- A.
- Greenness outline of the Novel Chromatographic Methods
- B.
- Greenness appraisal using the Analytical Greenness (AGREE) and Green Analytical Procedure Index (GAPI) approaches.
3.3. Investigation of the Methods Functionalities Using Blue Applicability Grade Index (BAGI) Software
3.4. Validation
3.4.1. Linearity
3.4.2. Accuracy Assessment and Application to Avil Ampoule Formulation
3.4.3. Repeatability and Intermediate Precision
- -
- Repeatability
- -
- Intermediate Precision
3.4.4. Selectivity
3.4.5. Robustness
3.4.6. Assessment of Chromatographic System Suitability
3.5. Comparisons between Novel UPLC and TLC Methods and Those of Previously Reported LC and TLC Methods
3.6. Merits of the Two Novel UPLC and TLC Methods
4. Conclusions
Study Limitations and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kelani, K.M.; Hegazy, M.A.; Hassan, A.M.; Tantawy, M.A. Determination of naphazoline HCl, pheniramine maleate and their official impurities in eye drops and biological fluid rabbit aqueous humor by a validated LC-DAD method. RSC Adv. 2021, 11, 7051–7058. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Chen, N.; Wang, D.; Lai, Y.; Cao, Z. A validated stability-indicating HPLC method for the simultaneous determination of pheniramine maleate and naphazoline hydrochloride in pharmaceutical formulations. Chem. Cent. J. 2014, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Sweetman, S.C. Martindale: The Complete Drug Reference; Pharmaceutical Press: London, UK, 2009; Volume 3709. [Google Scholar]
- British Pharmacopoeia. Pheniramine Maleate Monograph. 2013. Available online: https://search.worldcat.org/title/British-pharmacopoeia-2013/oclc/809536883 (accessed on 1 October 2024).
- Raghu, M.; Basavaiah, K. Rapid and Sensitive Extraction-Free Spectrophotometric Methods for the Determination of Pheniramine Maleate Using Three Sulphonthalein Dyes. Proc. Natl. Acad. Sci. India Sect. A Phys. Sci. 2012, 82, 187–196. [Google Scholar] [CrossRef]
- Raghu, M.; Basavaiah, K. Two charge-transfer complexation reactions for spectrophotometric determination of pheniramine maleate using π-acceptors. J. Sci. Ind. Res. 2011, 70, 851–858. [Google Scholar]
- Raghu, M.S.; Basavaiah, K.; Ramesh, P.J.; Abdulrahman, S.A.; Vinay, K.B. Development and validation of a uv-spectrophotometric method for the determination of pheniramine maleate and its stability studies. J. Appl. Spectrosc. 2012, 79, 131–138. [Google Scholar] [CrossRef]
- Mehmood, Y.; Saleem, N.; Mahmood, R.K.; Riaz, H.; Raza, S.A. Analytical Method Development and Validation of Pheniramine Maleate Injection. International J. Drug Dev. Res. 2016, 8, 044–048. [Google Scholar]
- Raghu, M.S.; Basavaiah, K.; Abdulrahaman, S.A.; Prashanth, K.N.; Vinay, K.B. Sensitive and selective spectrophotometric methods for the determination of pheniramine maleate in bulk drug and in its formulations using sodium hypochlorite. J. Anal. Chem. 2013, 68, 969–976. [Google Scholar] [CrossRef]
- Fattah, S.A.; Kelany, K.O.; El-Zeany, B.A.; El-Tarras, M.F. Analysis of Pheniramine Naleate and Cblorpheniramine Maleate Via Their Fe (III) Cohplexes. Anal. Lett. 1987, 20, 1667–1678. [Google Scholar] [CrossRef]
- Raghu, M.S.; Basavaiah, K.; Prashanth, K.N.; Vinay, K.B. Acid-base titrimetric assay of pheniramine maleate in pharmaceuticals in hydro-alcoholic medium. Der Pharm. Lett. 2012, 4, 1523–1529. [Google Scholar]
- Pandey, A.K.; Dwivedi, D. Titrimetric assay of some antihistamine drugs with pyridinium fluoro chromate (pfc) reagent. J. Drug Deliv. Ther. 2018, 8, 407–410. [Google Scholar] [CrossRef]
- Caglar, H.; Buyuktuncel, S. HPLC method development and validation: Simultaneous determination of active ingredients in cough and cold pharmaceuticals. Int. J. Pharm. Pharm. Sci. 2014, 6, 421–428. [Google Scholar]
- Dongala, T.; Katari, N.K.; Palakurthi, A.K.; Jonnalagadda, S.B. Development and validation of a generic RP-HPLC PDA method for the simultaneous separation and quantification of active ingredients in cold and cough medicines. Biomed. Chromatogr. 2019, 33, e4641. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, B.; Xue, M.; Jiang, Z.; Guo, X. Studies on the chiral separation of pheniramine and its enantioselective pharmacokinetics in rat plasma by HPLC-MS/MS. Microchem. J. 2020, 156, 104989. [Google Scholar] [CrossRef]
- Zheng, R.; Wu, Y.H.; Jiang, D.X.; Zhang, D. Determination of metabolite of nicergoline in human plasma by high-performance liquid chromatography and its application in pharmacokinetic studies. J. Pharm. Anal. 2012, 2, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Subramaniyan, S.P.; Das, S.K. Rapid identification and quantification of chlorpheniramine maleate or pheniramine maleate in pharmaceutical preparations by thin-layer chromatography-densitometry. J. AOAC Int. 2004, 87, 1319–1322. [Google Scholar] [CrossRef]
- Kelani, K.M.; Hegazy, M.A.; Hassan, A.M.; Tantawy, M.A. A green TLC densitometric method for the simultaneous detection and quantification of naphazoline HCl, pheniramine maleate along with three official impurities. BMC Chem. 2022, 16, 24. [Google Scholar] [CrossRef]
- Mikuš, P.; Valášková, I.; Havránek, E. Enantioselective determination of pheniramine in pharmaceuticals by capillary electrophoresis with charged cyclodextrin. J. Pharm. Biomed. Anal. 2005, 38, 442–448. [Google Scholar] [CrossRef]
- Phatthiyaphaibun, K.; Som-Aum, W.; Srisa-Ard, M.; Threeprom, J. Determination of pheniramine enantiomers in eye drop by capillary electrophoresis using hydroxypropyl-β-cyclodextrin as chiral selector. J. Anal. Chem. 2010, 65, 755–759. [Google Scholar] [CrossRef]
- Ribeiro, M.M.; Marra, M.C.; Costa, B.M.; Oliveira, T.C.; Batista, A.D.; Muñoz, R.A.; Richter, E.M. Sub-Minute Method for Determination of Naphazoline in the Presence of Diphenhydramine, Pheniramine or Chlorpheniramine by Capillary Electrophoresis. J. Braz. Chem. Soc. 2018, 29, 1959–1964. [Google Scholar] [CrossRef]
- Jain, R.; Sharma, S. Glassy carbon electrode modified with multi-walled carbon nanotubes sensor for the quantification of antihistamine drug pheniramine in solubilized systems. J. Pharm. Anal. 2012, 2, 56–61. [Google Scholar] [CrossRef]
- Kelani, K.M.; Hegazy, M.A.; Hassan, A.M.; Tantawy, M.A. Application of multivariate chemometrics tools for spectrophotometric determination of naphazoline HCl, pheniramine maleate and three official impurities in their eye drops. Sci. Rep. 2023, 13, 19678. [Google Scholar] [CrossRef] [PubMed]
- Elzanfaly, E.S.; Hegazy, M.A.; Saad, S.S.; Salem, M.Y.; Abd El Fattah, L.E. Validated green high-performance liquid chromatographic methods for the determination of coformulated pharmaceuticals: A comparison with reported conventional methods. J. Sep. Sci. 2015, 38, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Gamal, M.; Naguib, I.A.; Panda, D.S.; Abdallah, F.F. Comparative study of four greenness assessment tools for selection of greenest analytical method for assay of hyoscine N -butyl bromide. Anal. Methods 2021, 13, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE-Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef] [PubMed]
- Płotka-Wasylka, J. A new tool for the evaluation of the analytical procedure: Green Analytical Procedure Index. Talanta 2018, 181, 204–209. [Google Scholar] [CrossRef]
- Manousi, N.; Wojnowski, W.; Płotka-Wasylka, J.; Samanidou, V. Blue applicability grade index (BAGI) and software: A new tool for the evaluation of method practicality. Green Chem. 2023, 25, 7598–7604. [Google Scholar] [CrossRef]
- Elbordiny, H.S.; Elonsy, S.M.; Daabees, H.G.; Belal, T.S. Design of Trio-colored Validated HPLC Method for Synchronized Multianalyte quantitation of Four Top Selling Antihyperlipidemic Drugs in Different Fixed-Dose Combined Tablets. Green Anal. Chem. 2024, 8, 100100. [Google Scholar] [CrossRef]
- Yenduri, S.; Varalakshm, H.N.I. Assessment and comparison of sustainability aspects of UV-spectroscopy methods for simultaneous determination of anti-hypertensive combination. Green Anal. Chem. 2024, 9, 100108. [Google Scholar] [CrossRef]
- Habib, N.M.; Tony, R.M.; AlSalem, H.S.; Algethami, F.K.; Gamal, M. Evaluation of the Greenness, Whiteness, and blueness profiles of stability indicating HPLC method for determination of Denaverine hydrochloride and benzyl alcohol in pharmaceuticals. Microchem. J. 2024, 201, 110733. [Google Scholar] [CrossRef]
- Abdelfatah, R.M.; Abd Elhalim, L.M.; Darwish, H.W.; Ayoub, B.M.; Tony, R.M.; Gamal, M. A stability-indicating HPLC assay of ten different vitamins in a food supplement: Appraisal of the method’s greenness, whiteness, and blueness. Talanta 2024, 277, 126324. [Google Scholar] [CrossRef]
- Joshi, D.R.; Adhikari, N. An overview on common organic solvents and their toxicity. J. Pharm. Res. Int. 2019, 28, 1–18. [Google Scholar] [CrossRef]
- Swetha, S.R.; Sri, K.B.; Mounika, C. A Review on Comparative study of HPLC and UPLC. Res. J. Pharm. Technol. 2020, 13, 1570–1574. [Google Scholar]
- Kumar, A.; Saini, G.; Nair, A.; Sharma, R. UPLC: A preeminent technique in pharmaceutical analysis. Acta Pol. Pharm. 2012, 69, 371–380. [Google Scholar] [PubMed]
- Faraji, H.; Mirzaie, A.; Waqif-Husain, S. Liquid phase microextraction-ion exchange-high performance thin layer chromatography for the preconcentration, separation, and determination of plasticizers in aqueous samples. J. Sep. Sci. 2013, 36, 1486–1492. [Google Scholar] [CrossRef]
- Faraji, H.; Saber-Tehrani, M.; Mirzaie, A.; Waqif-Husain, S. Application of liquid-liquid microextraction-high-performance thin-layer chromatography for preconcentration and determination of phenolic compounds in aqueous samples. JPC–J. Planar Chromatogr. –Mod. TLC 2011, 24, 214–217. [Google Scholar] [CrossRef]

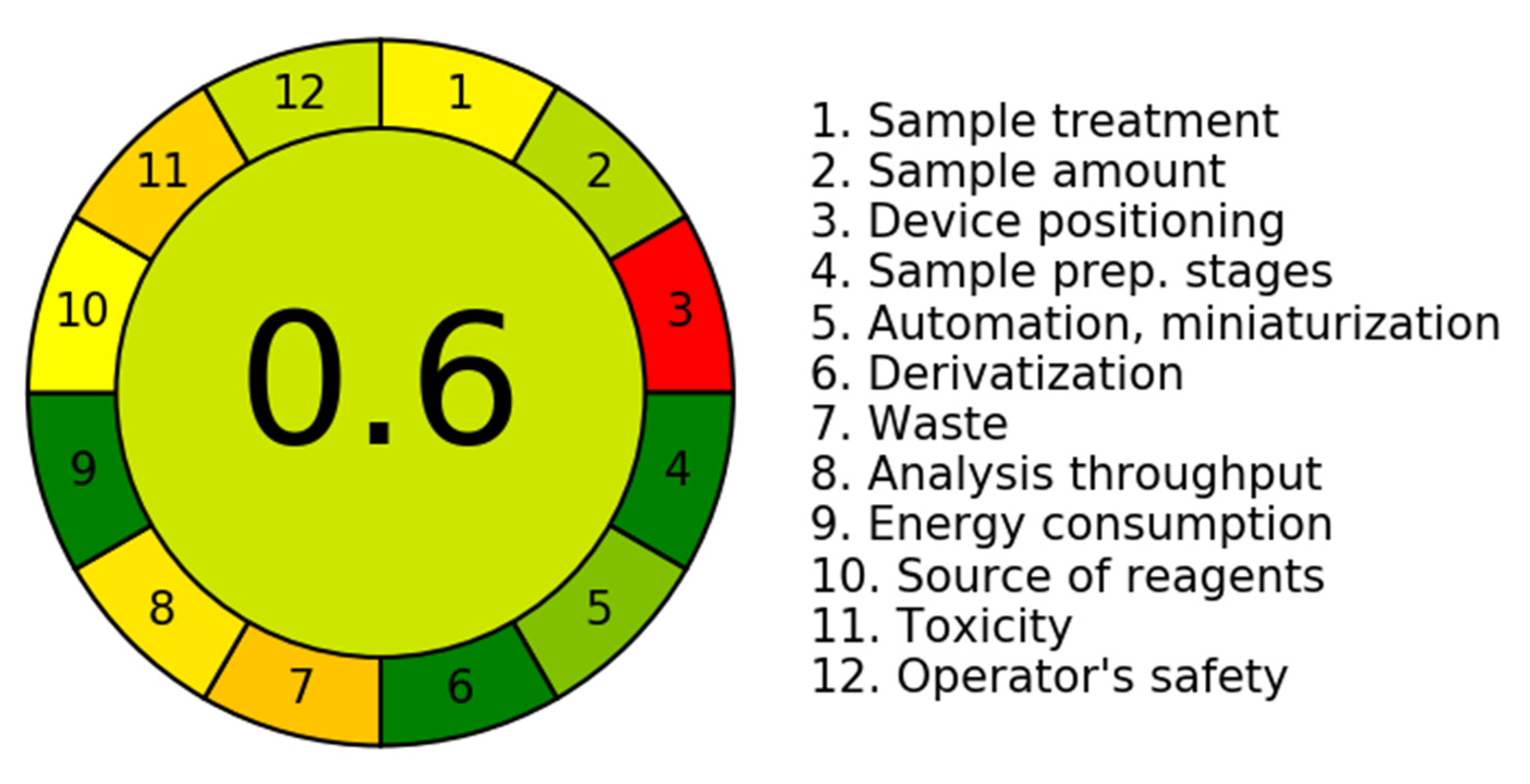
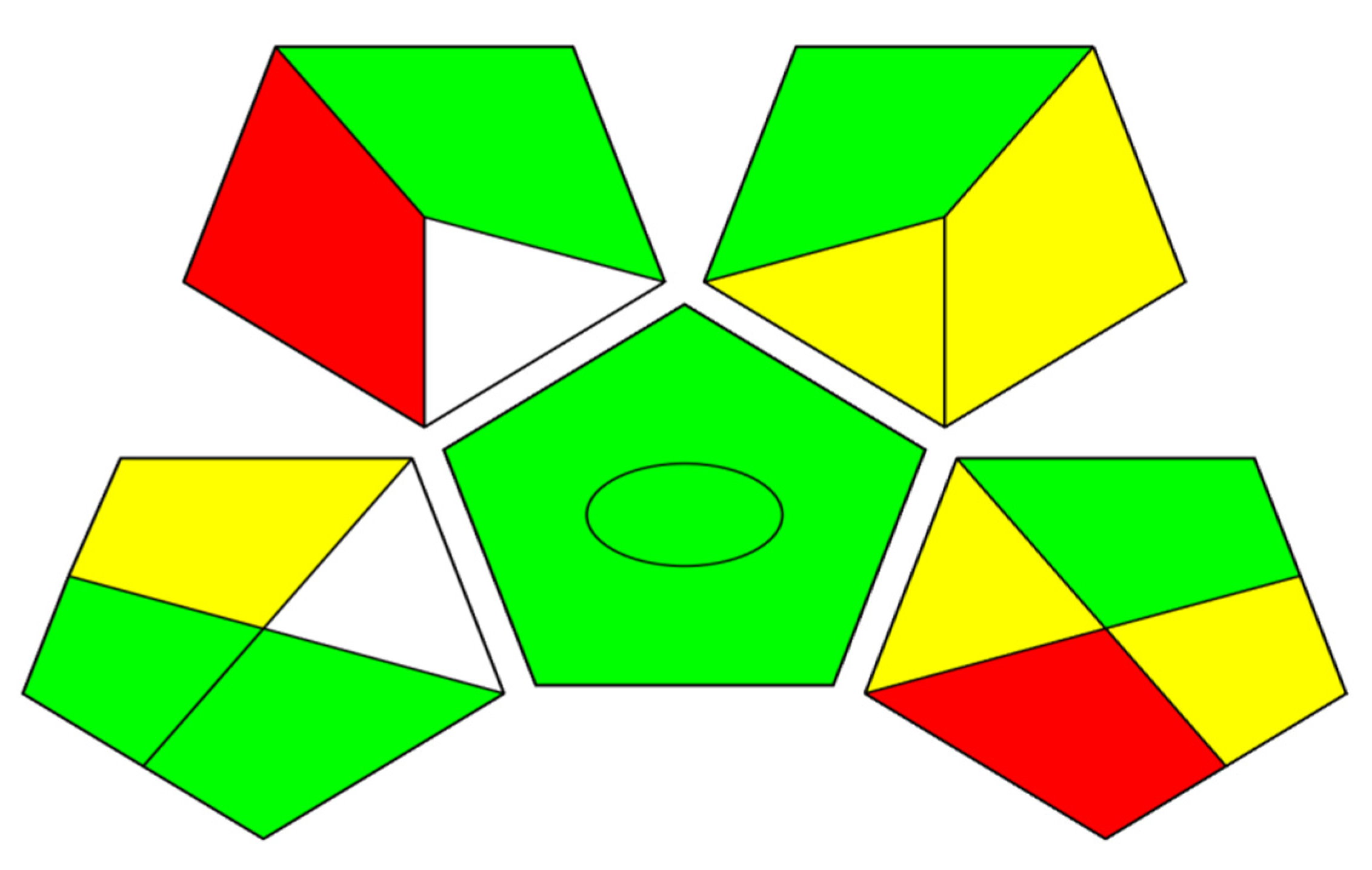


| Methods | Mobile Phase | Run Time (min) | Flow Rate (mL/min) | Waste * (g/run) | Greenness Profile ** |
|---|---|---|---|---|---|
| Developed UHPLC | Methanol/water (60:40 v/v) | 3.90 | 0.1 | 0.39 |  |
| Developed TLC | Ethanol/ethyl acetate/ammonia (8:2:0.1, by volume) | 0.9 g/sample |  | ||
| Published HPLC for pheniramine maleate determination [1] | Phosphate buffer pH 6.0/acetonitrile (70:30, v/v) | 30 | 1.00 | 30 |  |
| Published TLC for pheniramine maleate determination [2] | Methanol/ethyl acetate/33.0% ammonia (2.0: 8.0: 1.0, by volume) |  |
| Parameter | UPLC | TLC | ||
|---|---|---|---|---|
| PAM | BNZ | PAM | BNZ | |
| Linearity range (μg/mL) | 5.00–70.00 | 0.05–10.00 | 0.5–8 | 0.1–3 |
| Slope | 0.0566 | 2.8495 | 0.8203 | 0.7319 |
| Intercept | 0.8645 | 0.4062 | 1.0418 | 1.9246 |
| Correlation coefficient | 0.9998 | 0.9999 | 0.9999 | 0.9999 |
| Accuracy | 99.28 | 98.07 | 99.54 | 99.12 |
| Precision (RSD%) * | ||||
| repeatability | 0.63 | 0.34 | 0.63 | 0.49 |
| Intermediate precision | 0.73 | 0.41 | 0.73 | 0.65 |
| LOD (µg/mL) ** | 1.59 | 0.007 | 0.13 | 0.02 |
| LOQ (µg/mL) ** | 4.77 | 0.02 | 0.41 | 0.08 |
| Method | Avil® Ampoule, Labeled to Contain 45.5 mg/2 mL, Batch No. 9EG060 | |||||
|---|---|---|---|---|---|---|
| Dosage form | Standard Addition | |||||
| Taken (µg/mL) | Found (µg/mL) | %Recovery * | Added (µg/mL) | Found (µg/mL) | %Recovery ** | |
| RP-UPLC | 45.00 | 45.71 | 101.57 ± 0.73 | 15.00 | 15.00 | 100.00 |
| 20.00 | 20.30 | 101.50 | ||||
| 25.00 | 25.25 | 101.00 | ||||
| Mean ± SD | 100.83 ± 0.52 | |||||
| TLC | 5.00 | 5.00 | 100.00 ± 0.57 | 0.50 | 0.50 | 100.00 |
| 1.00 | 1.01 | 101.00 | ||||
| 2.00 | 2.02 | 101.00 | ||||
| Mean ± SD | 100.67 ± 0.64 | |||||
| UPLC | Drug | Ratio of methanol 60 ± 0.1 mL | Wavelength 215 ± 2 nm | Flow rate 0.1 ± 0.01 mL/min |
| PAM | 0.01 * | 1.01 | 0.01 | |
| BNZ | 0.02 * | 0.01 | 0.00 | |
| TLC | Drug | Ratio of ethyl acetate 2.00 ± 0.03 mL | Wavelength 265 ± 2 nm | Saturation time 20 ± 5 min |
| PAM | 0.01 | 0.00 | 0.00 | |
| BNZ | 0.02 | 0.04 | 0.02 |
| UPLC Method | TLC Method | |||||
|---|---|---|---|---|---|---|
| Parameters | PAM | BNZ | Reference Value [3] | PAM | BNZ | Reference Value [3] |
| (T) Tailing factor | 1.00 | 1.10 | <1.50 | 0.98 | 1.00 | <1.50 |
| (K′) Capacity factor | 1.40 | 4.70 | 1.00–10.00 | |||
| (N) Column efficiency | 3249.00 | 5760.00 | Increase the efficiency of separation | |||
| (H) HETP * | 0.001 | 0.0008 | The smaller the value the higher the efficiency column | |||
| (α) Selectivity | 3.35 | α > 1.00 | 13.00 | α > 1.00 | ||
| (R) Resolution | 11.00 | R > 1.50 | 6.00 | R > 1.50 | ||
| Parameters | UPLC | TLC | Reported Method [1] |
|---|---|---|---|
| Mean | 101.57 | 100.00 | 99.52 |
| SD | 0.73 | 0.57 | 1.06 |
| Variance | 0.35 | 0.33 | 1.12 |
| n | 6 | 6 | 6 |
| t-test (2.220) * | 1.85 | 1.891 | - |
| F-test (5.050) * | 2.11 | 3.39 | - |
| Method | Mobile Phase/Total Run Time | PAM | BNZ | Reference |
|---|---|---|---|---|
| Novel UPLC | Methanol/water (60:40, in volume)/less than 5 min | 5–70 μg/mL | 0.05–10 μg/mL | |
| Novel TLC | Ethanol/ethyl acetate/liquid ammonia (8: 2: 0.1, in volume)/5 min | 0.5–8 μg/band | 0.1–3 μg/band | |
| Reported LC | Phosphate buffer pH 6.0/acetonitrile (70:30, in volume)/30 min | 10–110 μg/mL | 10–70 μg/mL | [1] |
| Reported TLC | Methanol/ethyl acetate/33.0% ammonia (2.0: 8.0: 1.0, in volume)/6 min | 10–110 μg/band | 2–5 μg/band | [18] |
| Reported direct spectrophotometry at 265 nm | Water | 5–25 μg/mL | ------------ | [8] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelhamid, N.S.; AlSalem, H.S.; K. Algethami, F.; Abdelaleem, E.A.; Mahmoud, A.M.; Ella, D.A.A.E.; Gamal, M. Green and Sensitive Analysis of the Antihistaminic Drug Pheniramine Maleate and Its Main Toxic Impurity Using UPLC and TLC Methods, Blueness Assessment, and Greenness Assessments. Chemosensors 2024, 12, 206. https://doi.org/10.3390/chemosensors12100206
Abdelhamid NS, AlSalem HS, K. Algethami F, Abdelaleem EA, Mahmoud AM, Ella DAAE, Gamal M. Green and Sensitive Analysis of the Antihistaminic Drug Pheniramine Maleate and Its Main Toxic Impurity Using UPLC and TLC Methods, Blueness Assessment, and Greenness Assessments. Chemosensors. 2024; 12(10):206. https://doi.org/10.3390/chemosensors12100206
Chicago/Turabian StyleAbdelhamid, Nessreen S., Huda Salem AlSalem, Faisal K. Algethami, Eglal A. Abdelaleem, Alaa M. Mahmoud, Dalal A. Abou El Ella, and Mohammed Gamal. 2024. "Green and Sensitive Analysis of the Antihistaminic Drug Pheniramine Maleate and Its Main Toxic Impurity Using UPLC and TLC Methods, Blueness Assessment, and Greenness Assessments" Chemosensors 12, no. 10: 206. https://doi.org/10.3390/chemosensors12100206
APA StyleAbdelhamid, N. S., AlSalem, H. S., K. Algethami, F., Abdelaleem, E. A., Mahmoud, A. M., Ella, D. A. A. E., & Gamal, M. (2024). Green and Sensitive Analysis of the Antihistaminic Drug Pheniramine Maleate and Its Main Toxic Impurity Using UPLC and TLC Methods, Blueness Assessment, and Greenness Assessments. Chemosensors, 12(10), 206. https://doi.org/10.3390/chemosensors12100206







