Influence of Synthesis Method and Electrode Geometry on GHG-Sensing Properties of 5%Gd-Doped SnO2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Powders Synthesis
2.2. Structural and Morphological Investigations
2.3. Textural Analysis
2.4. Layer Deposition and Sensing Investigations
3. Results and Discussions
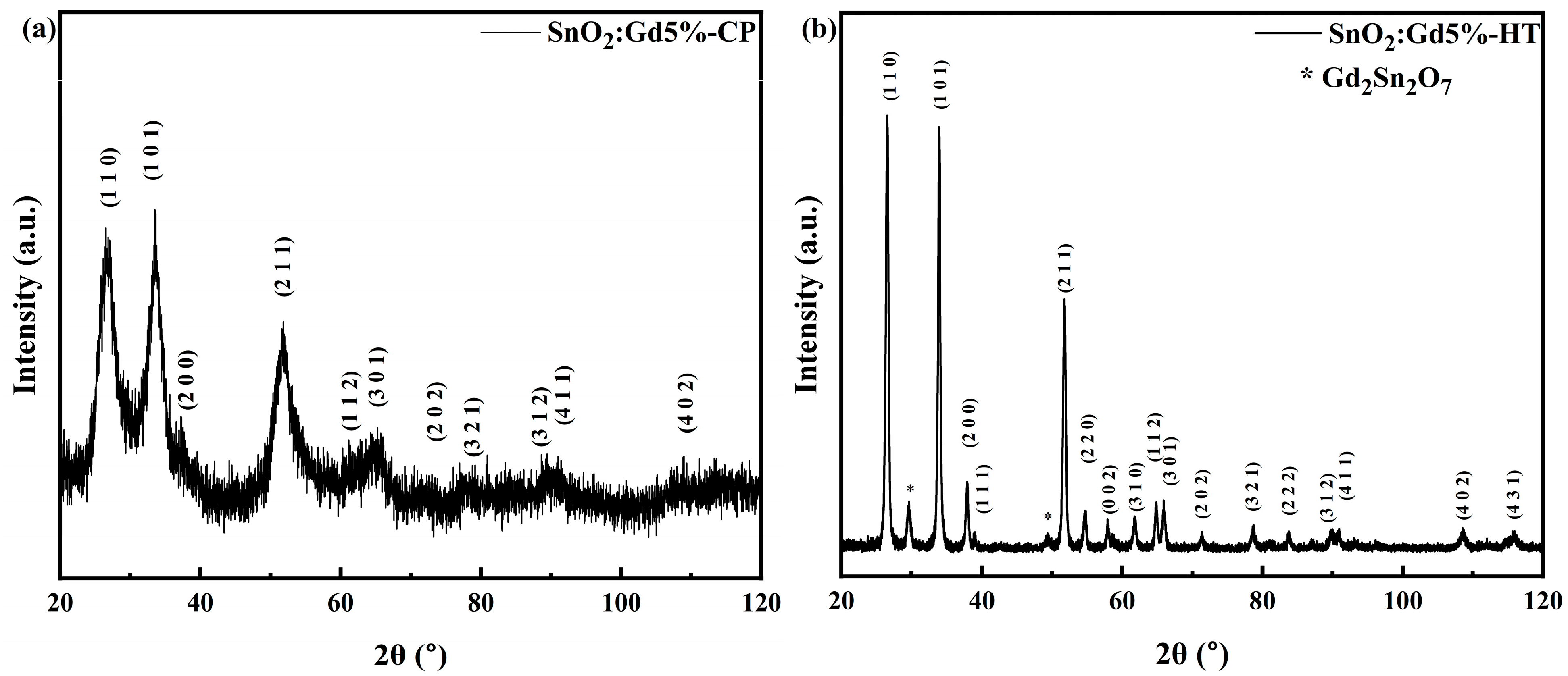
3.1. XRD Characterisation
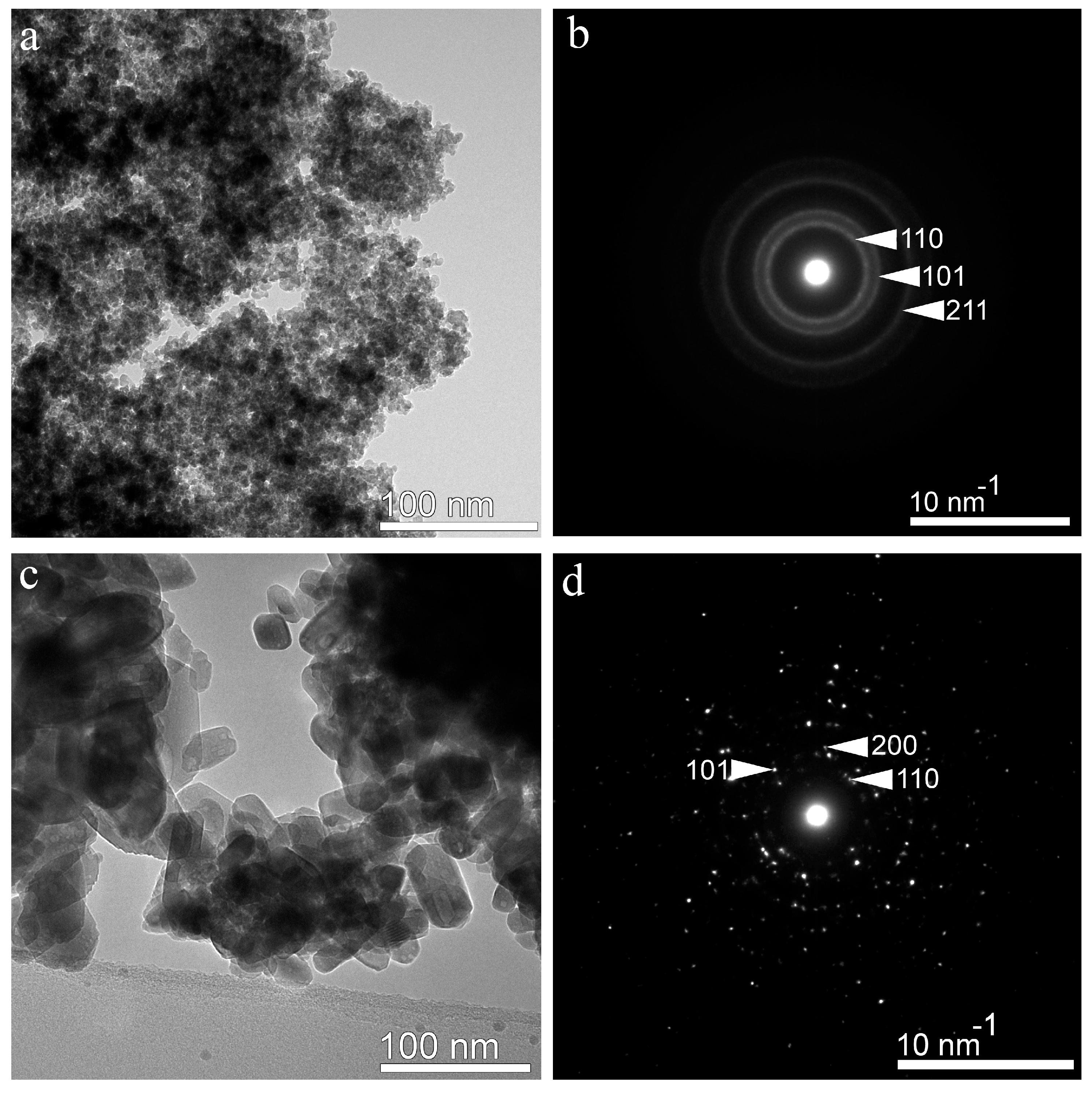
3.2. Analytical TEM Characterisation
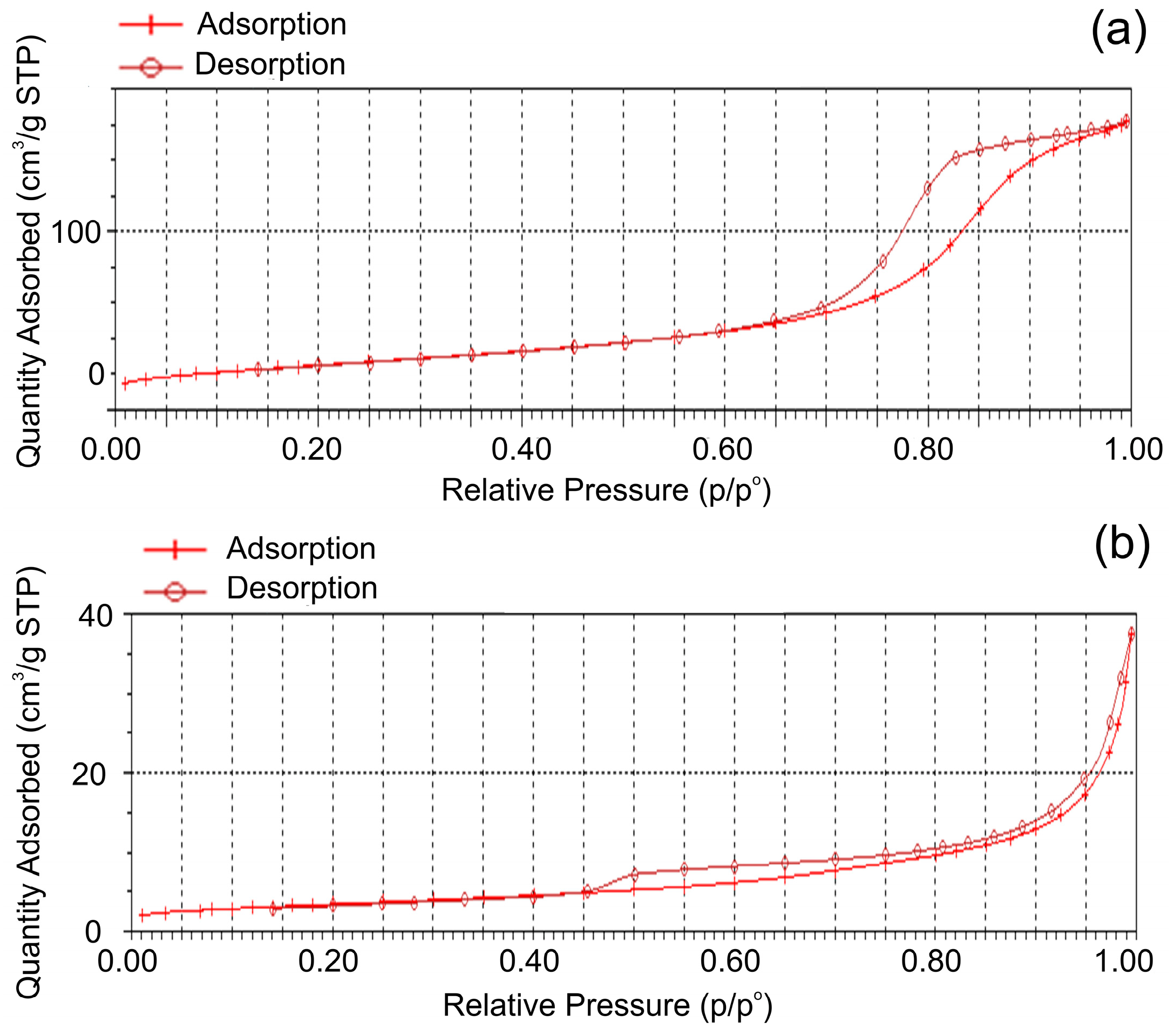
3.3. Textural Characterisation
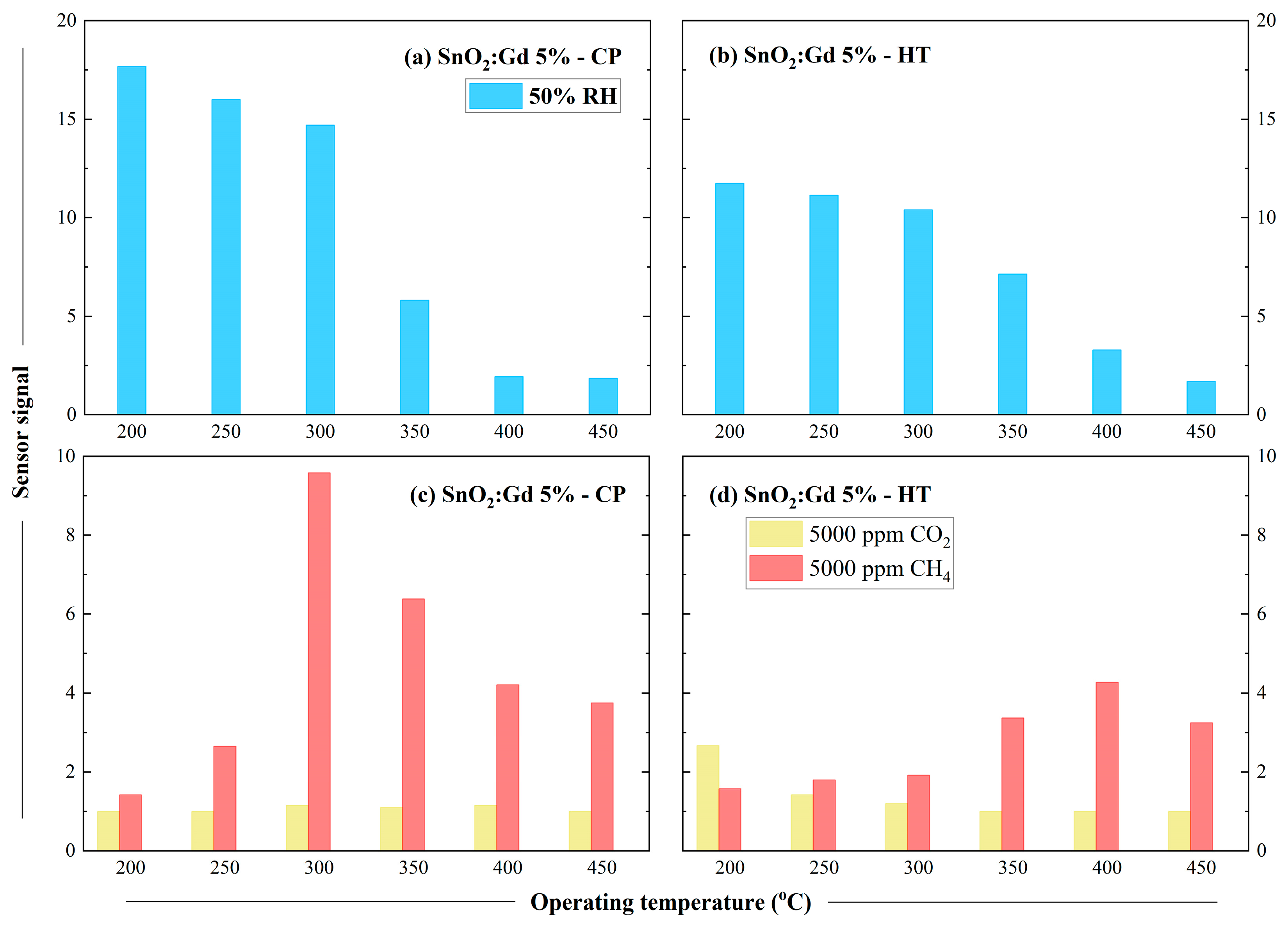
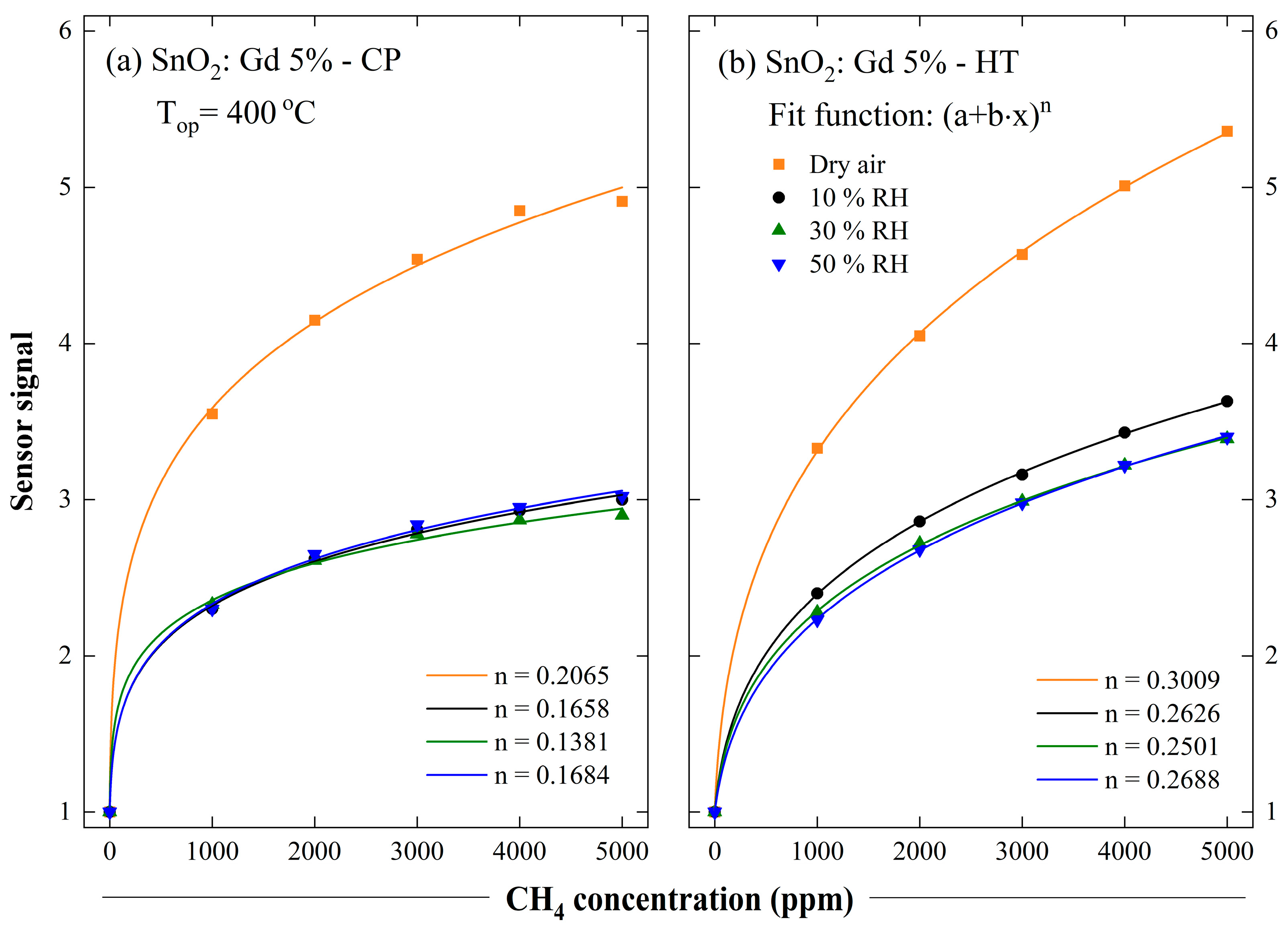
3.4. Sensing Characterisation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Meteorological Organization. Greenhouse Gas Concentrations Hit Record High. Again. Available online: https://wmo.int/news/media-centre/greenhouse-gas-concentrations-hit-record-high-again (accessed on 6 May 2024).
- National Oceanic and Atmospheric Administration—NOOA. Topping the Charts: September 2023 was Earth’s Warmest September in 174-Year Record. Available online: https://www.noaa.gov/news/topping-charts-september-2023-was-earths-warmest-september-in-174-year-record (accessed on 6 May 2024).
- Chataut, G.; Bhatta, B.; Joshi, D.; Subedi, K.; Kafle, K. Greenhouse gases emission from agricultural soil: A review. J. Agric. Food Res. 2023, 11, 100533. [Google Scholar] [CrossRef]
- Ahmed, R.; Liu, G.; Yousaf, B.; Abbas, Q.; Ullah, H.; Ali, M.U. Recent advances in carbon-based renewable adsorbent for selective carbon dioxide capture and separation―A review. J. Clean. Prod. 2020, 242, 118409. [Google Scholar] [CrossRef]
- Jatmiko, A.R.; Suryani, E.; Octabriyantiningtyas, D. The Analysis of Greenhouse Gas Emissions Mitigation: A System Thinking Approach (Case Study: East Java). Procedia Comput. Sci. 2019, 161, 951–958. [Google Scholar] [CrossRef]
- Dutta, T.; Noushin, T.; Tabassum, S.; Mishra, S.K. Road Map of Semiconductor Metal-Oxide-Based Sensors: A Review. Sensors 2023, 23, 6849. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, A.; Shakoor, S.; Rehman, A.; Ashraf, F.; Abdullah, M.; Shahzad, S.M.; Farooq, T.H.; Ashraf, M.; Manzoor, M.A.; Altaf, M.M.; et al. Effect of animal manure, crop type, climate zone, and soil attributes on greenhouse gas emissions from agricultural soils—A global meta-analysis. J. Clean. Prod. 2021, 278, 124019. [Google Scholar] [CrossRef]
- Paul, R.; Das, B.; Ghosh, R. Novel approaches towards design of metal oxide based hetero-structures for room temperature gas sensor and its sensing mechanism: A recent progress. J. Alloys Compd. 2023, 941, 168943. [Google Scholar] [CrossRef]
- Gautam, Y.K.; Sharma, K.; Tyagi, S.; Ambedkar, A.K.; Chaudhary, M.; Singh, B.P. Nanostructured metal oxide semiconductor-based sensors for greenhouse gas detection: Progress and challenges. R. Soc. Open Sci. 2021, 8, 201324. [Google Scholar] [CrossRef]
- Tyagi, S.; Chaudhary, M.; Ambedkar, A.K.; Sharma, K.; Gautam, Y.K.; Singh, B.P. Metal oxide nanomaterial-based sensors for monitoring environmental NO2 and its impact on the plant ecosystem: A review. Sens. Diagn. 2022, 1, 106. [Google Scholar] [CrossRef]
- Abdullah, A.N.; Kamarudin, K.; Kamarudin, L.M.; Adom, A.H.; Mamduh, S.M.; Juffry, Z.H.M.; Bennetts, V.H. Correction Model for Metal Oxide Sensor Drift Caused by Ambient Temperature and Humidity. Sensors 2022, 22, 3301. [Google Scholar] [CrossRef]
- Xue, D.; Wang, P.; Zhang, Z.; Wang, Y. Enhanced methane sensing property of flower-like SnO2 doped by Pt nanoparticles: A combined experimental and first-principle study. Sens. Actuators B Chem. 2019, 296, 126710. [Google Scholar] [CrossRef]
- Shi, J.; Liu, S.; Zhang, P.; Sui, N.; Cao, S.; Zhou, T.; Zhang, T. Sb/Pd co-doped SnO2 nanoparticles for methane detection: Resistance reduction and sensing performance studies. J. Nanotechnol. 2021, 32, 475506. [Google Scholar] [CrossRef] [PubMed]
- Hoefer, U.; Kühner, G.; Schweizer, W.; Sulz, G.; Steiner, K. CO and CO2 thin-film SnO2 gas sensors on Si substrates. Sens. Actuators B Chem. 1994, 22, 115–119. [Google Scholar] [CrossRef]
- Singh, A.; Yadav, B.C. Photo-responsive highly sensitive CO2 gas sensor based on SnO2@CdO heterostructures with DFT calculations. Surf. Interfaces 2022, 34, 102368. [Google Scholar] [CrossRef]
- Kuncser, A.C.; Vlaicu, I.D.; Dinu, I.V.; Simion, C.E.; Iacoban, A.C.; Florea, O.G.; Stanoiu, A. The impact of the synthesis temperature on SnO2 morphology and sensitivity to CO2 under in-field conditions. Mater. Lett. 2022, 325, 132855. [Google Scholar] [CrossRef]
- Suzuki, T.; Sackmann, A.; Lauxmann, F.; Berthold, C.; Weimar, U.; Bȃrsan, N. CO2 sensing with gas sensors based on rare-earth compounds: Material exploration. Sens. Actuators B Chem. 2020, 317, 128128. [Google Scholar] [CrossRef]
- Ghica, C.; Mihalcea, C.G.; Simion, C.E.; Vlaicu, I.D.; Ghica, D.; Dinu, I.V.; Florea, O.G.; Stanoiu, A. Influence of relative humidity on CO2 interaction mechanism for Gd-doped SnO2 with respect to pure SnO2 and Gd2O3. Sens. Actuators B Chem. 2022, 368, 132130. [Google Scholar] [CrossRef]
- Lee, S.P. Electrodes for semiconductor gas sensors. Sensors 2017, 17, 683. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.E.; Pratt, K.F.E. Theory of self-diagnostic sensor array devices using gas-sensitive resistors. J. Chem. Soc. Faraday Trans. 1995, 91, 1961–1966. [Google Scholar] [CrossRef]
- Shaalan, N.M.; Yamazaki, T.; Kikuta, T. Effect of micro-electrode geometry on NO2 gas-sensing characteristics of one-dimensional tin dioxide nanostructure microsensors. Sens. Actuators B Chem. 2011, 156, 784–790. [Google Scholar] [CrossRef]
- Gardner, J.W. Intelligent gas sensing using an integrated sensor pair. Sens. Actuators B Chem. 1995, 27, 261–266. [Google Scholar] [CrossRef]
- Tamaki, J.; Miyaji, A.; Makinodan, J.; Ogura, S.; Konishi, S. Effect of micro-gap electrode on detection of dilute NO2 using WO3 thin film microsensors. Sens. Actuators B Chem. 2005, 108, 202–206. [Google Scholar] [CrossRef]
- Meng, Z.; Kitagawa, C.; Takahashu, A.; Okochi, Y.; Tamaki, J. WO3 Crystals and Their NO2-Sensing Properties. Sens. Mater. 2009, 21, 259–264. [Google Scholar] [CrossRef]
- Cui, H.J.; Shi, J.W.; Yuan, B.; Fu, M.L. Synthesis of porous magnetic ferrite nanowires containing Mn and their application in water treatment. J. Mater. Chem. A 2013, 1, 5902–5907. [Google Scholar] [CrossRef]
- Shaikh, S.F.; Mane, R.S.; Min, B.K.; Hwang, Y.J.; Joo, O. D-sorbitol-induced phase control of TiO2 nanoparticles and its application for dye-sensitized solar cells. Sci. Rep. 2016, 6, 20103. [Google Scholar] [CrossRef] [PubMed]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Cai, Z.; Kim, K.-K.; Park, S. Room Temperature Detection of NO2 Gas under UV Irradiation Based on Au Nanoparticle-Decorated Porous ZnO Nanowires. J. Mater. Res. Technol. 2020, 9, 16289–16302. [Google Scholar] [CrossRef]
- Pandey, M.; Singh, M.; Wasnik, K.; Gupta, S.; Patra, S.; Gupta, P.S.; Pareek, D.; Chaitanya, N.S.N.; Maity, S.; Reddy, A.B.M.; et al. Targeted and Enhanced Antimicrobial Inhibition of Mesoporous ZnO−Ag2O/Ag, ZnO−CuO, and ZnO−SnO2 Composite Nanoparticles. ACS Omega 2021, 6, 31615–31631. [Google Scholar] [CrossRef]
- Barsan, N.; Tomescu, A. The temperature dependence of the response of SnO2-based gas sensing layers to O2, CH4 and CO. Sens. Actuators B Chem. 1995, 26, 45–48. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Conduction Model of Metal Oxide Gas Sensors. J. Electroceram. 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Jo, J.Y.; Kwon, Y.S.; Lee, J.W.; Park, J.S.; Rho, B.H.; Choi, W.I. Acute respiratory distress due to methane inhalation. Tuberc. Respir. Dis. 2013, 74, 120–123. [Google Scholar] [CrossRef]
- Mar, K.A.; Unger, C.; Walderdorff, L.; Butle, T. Beyond CO2 equivalence: The impacts of methane on climate, ecosystems, and health. Environ. Sci. Policy 2022, 134, 127–136. [Google Scholar] [CrossRef]
- Sobanaa, M.; Prathiviraj, R.; Selvin, J.; Prathaban, M. A comprehensive review on methane’s dual role: Effects in climate change and potential as a carbon–neutral energy source. Environ. Sci. Pollut. Res. 2024, 31, 10379–10394. [Google Scholar] [CrossRef] [PubMed]
- Weisz, P.B. Effects of Electronic Charge Transfer between Adsorbate and Solid on Chemisorption and Catalysis. J. Chem. Phys. 1953, 21, 1531–1538. [Google Scholar] [CrossRef]
- Geistlinger, H. Electron theory of thin-film gas sensors. Sens. Actuators B Chem. 1993, 17, 47–60. [Google Scholar] [CrossRef]
- Gopel, W. Chemisorption and charge transfer at ionic semiconductor surfaces: Implications in designing gas sensors. Prog. Surf. Sci. 1985, 20, 9–103. [Google Scholar] [CrossRef]
- Alcantara, G.P.; Andrade, C.G.M. A short review of gas sensors based on interdigital electrode. In Proceedings of the 12th IEEE International Conference on Electronic Measurement & Instruments, Qingdao, China, 16–18 July 2015. [Google Scholar] [CrossRef]
- Barsan, N.; Huebner, M.; Weimar, U. Conduction mechanism in semiconducting metal oxide sensing films: Impact on transduction. Semiconductor Gas Sensors, 2nd ed.; Woodhead Publishing: Sawston, UK, 2020; Chapter 2, pp. 39–69; ISBN 9780081025598. [Google Scholar] [CrossRef]






| Sensing Material | Sensor Signal | Target Gas | Relative Humidity | Temperature °C | Reference |
|---|---|---|---|---|---|
| Pt/SnO2 | 3.6 | CH4 | Absent | 100 | [12] |
| Sb/Pd/SnO2 | 5/1.2 | CH4/CO2 | Absent | 280 | [13] |
| Ca/Pt/SnO2 | 1.02 | CO2 | Present | 270 | [14] |
| SnO2@CdO | 10.67 | CO2 | Absent | 30 | [15] |
| SnO2 | 2.48 | CO2 | Present | 200 | [16] |
| Crystallographic Parameters/Sample | a = b | c | dhkl (nm) |
|---|---|---|---|
| SnO2: Gd 5%-CP | 4.7304 ± 0.0018 | 3.2338 ± 0.0020 | 3.63 ± 0.03 |
| SnO2: Gd 5%-HT | 4.7457 ± 0.0001 | 3.1813 ± 0.0001 | 41.38 ± 0.34 |
| Sample | BET Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (nm) | BJH Pore Diameter (nm) |
|---|---|---|---|---|
| SnO2: Gd 5%-CP | 91.32 | 0.25 | 10.93 | 8.35 |
| SnO2: Gd 5%-HT | 12.48 | 0.058 | 18.53 | 13.25 |
| Sensing Material/Electrode Gap | Sensor Signal | Target Gas | Relative Humidity | Temperature |
|---|---|---|---|---|
| SnO2: Gd 5%-CP/200 μm | 3.01/1.14 | CH4/CO2 | 50% | 400 °C |
| SnO2: Gd 5%-CP/100 μm | 2.37/1.05 | |||
| SnO2: Gd 5%-HT/200 μm | 3.39/1 | |||
| SnO2: Gd 5%-HT/100 μm | 4.75/1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simion, C.E.; Mihalcea, C.G.; Iacoban, A.C.; Dinu, I.V.; Predoi, D.; Vlaicu, I.D.; Florea, O.G.; Stanoiu, A. Influence of Synthesis Method and Electrode Geometry on GHG-Sensing Properties of 5%Gd-Doped SnO2. Chemosensors 2024, 12, 148. https://doi.org/10.3390/chemosensors12080148
Simion CE, Mihalcea CG, Iacoban AC, Dinu IV, Predoi D, Vlaicu ID, Florea OG, Stanoiu A. Influence of Synthesis Method and Electrode Geometry on GHG-Sensing Properties of 5%Gd-Doped SnO2. Chemosensors. 2024; 12(8):148. https://doi.org/10.3390/chemosensors12080148
Chicago/Turabian StyleSimion, Cristian Eugen, Catalina Gabriela Mihalcea, Alexandra Corina Iacoban, Ion Viorel Dinu, Daniela Predoi, Ioana Dorina Vlaicu, Ovidiu Gabriel Florea, and Adelina Stanoiu. 2024. "Influence of Synthesis Method and Electrode Geometry on GHG-Sensing Properties of 5%Gd-Doped SnO2" Chemosensors 12, no. 8: 148. https://doi.org/10.3390/chemosensors12080148






