Integrated Application of Risk Management Techniques in Developing an Analysis Method for Traditional Chinese Medicine: A Case Study of a Percolation Solution for Xiaochaihu Capsules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Sample Solution
2.3. Preparation of Standard Solution
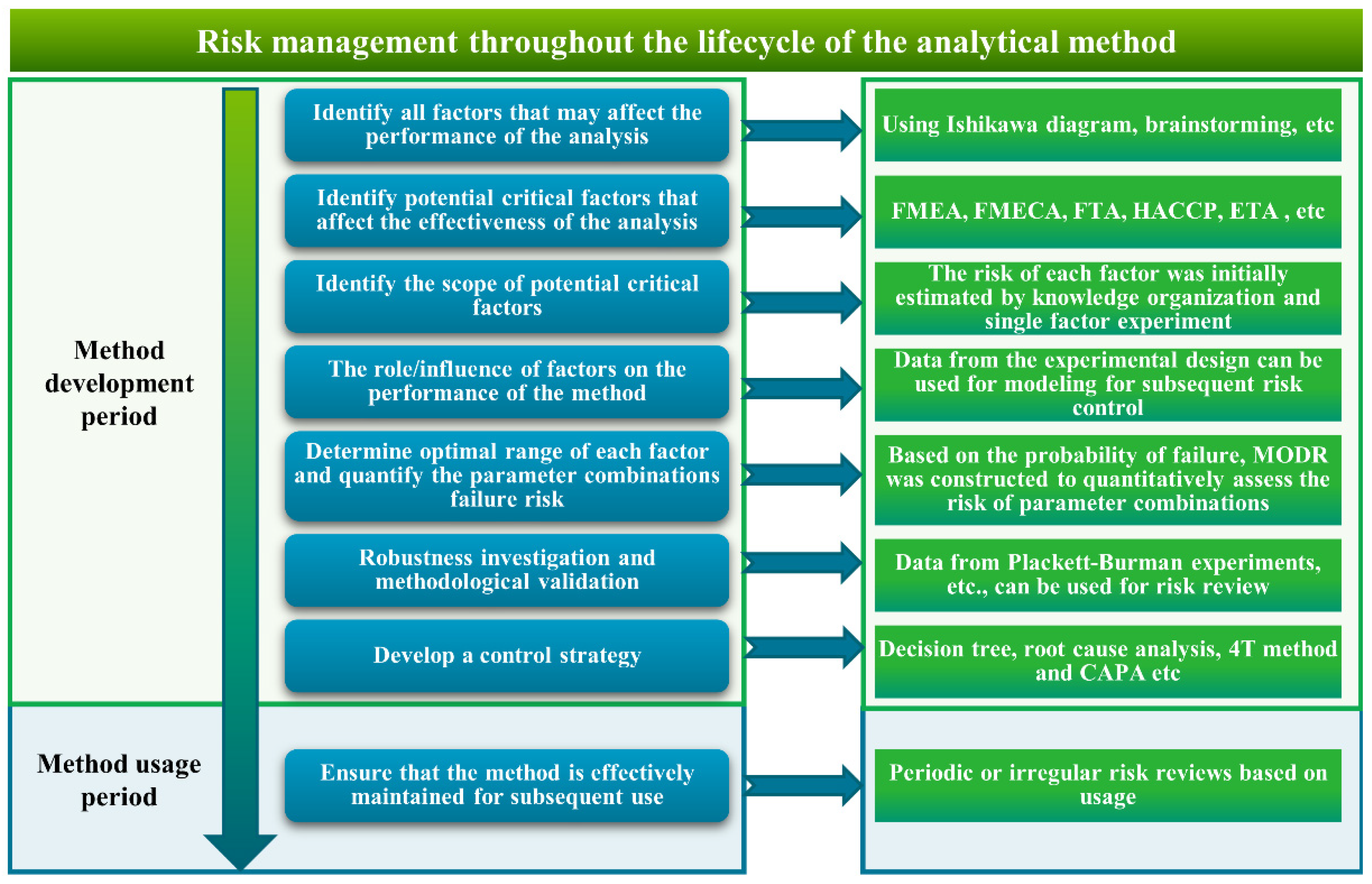
2.4. Risk Management Process throughout the Entire Lifecycle of the Analytical Method
2.5. Preliminary Screening of CMPs through Single-Factor Experiments
2.6. Experimental Design
2.7. Data Processing
2.8. Control Strategy Formulation
3. Results and Discussion
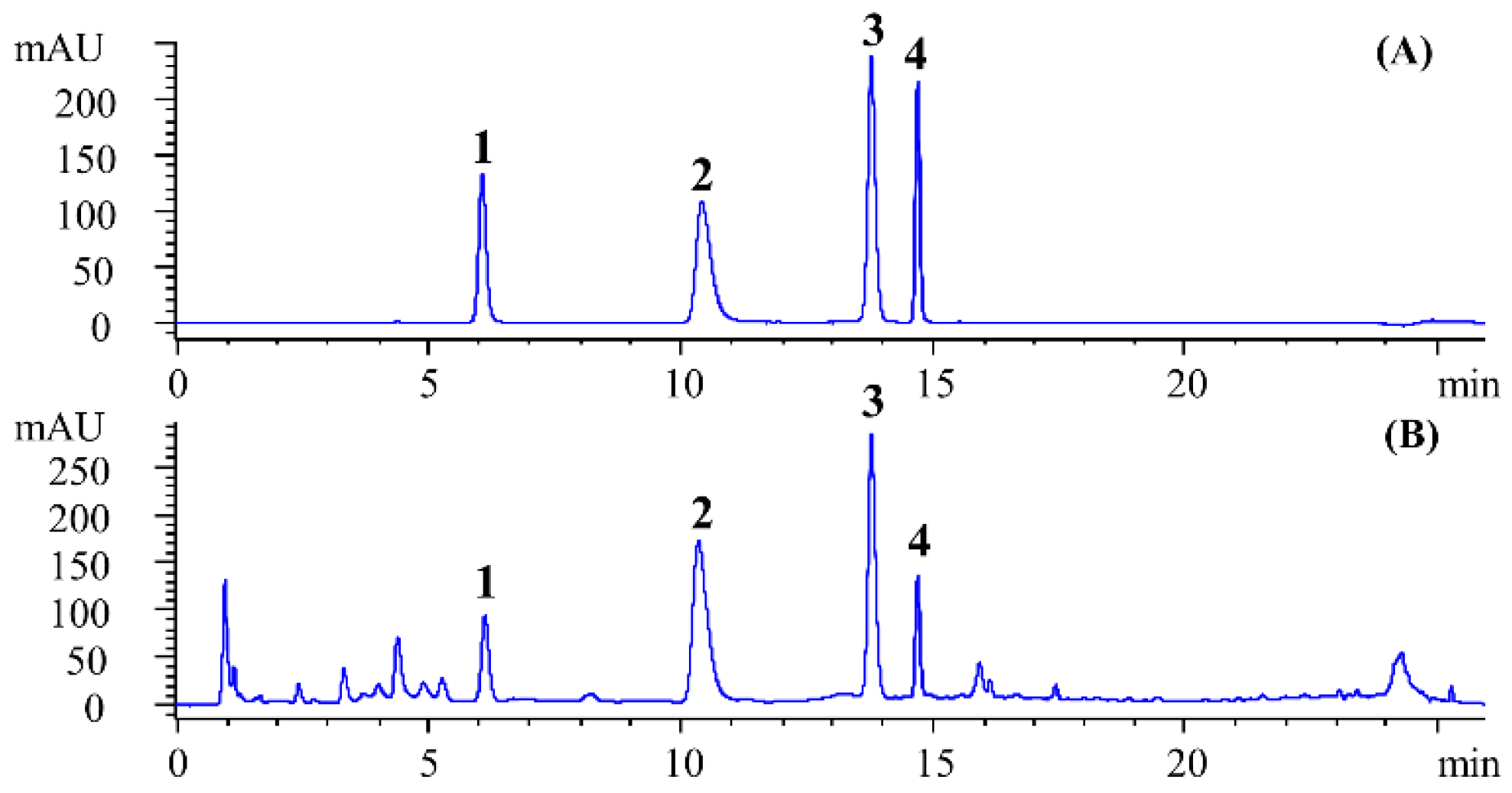
3.1. Determination of ATP
3.2. The Ishikawa Diagram Analysis
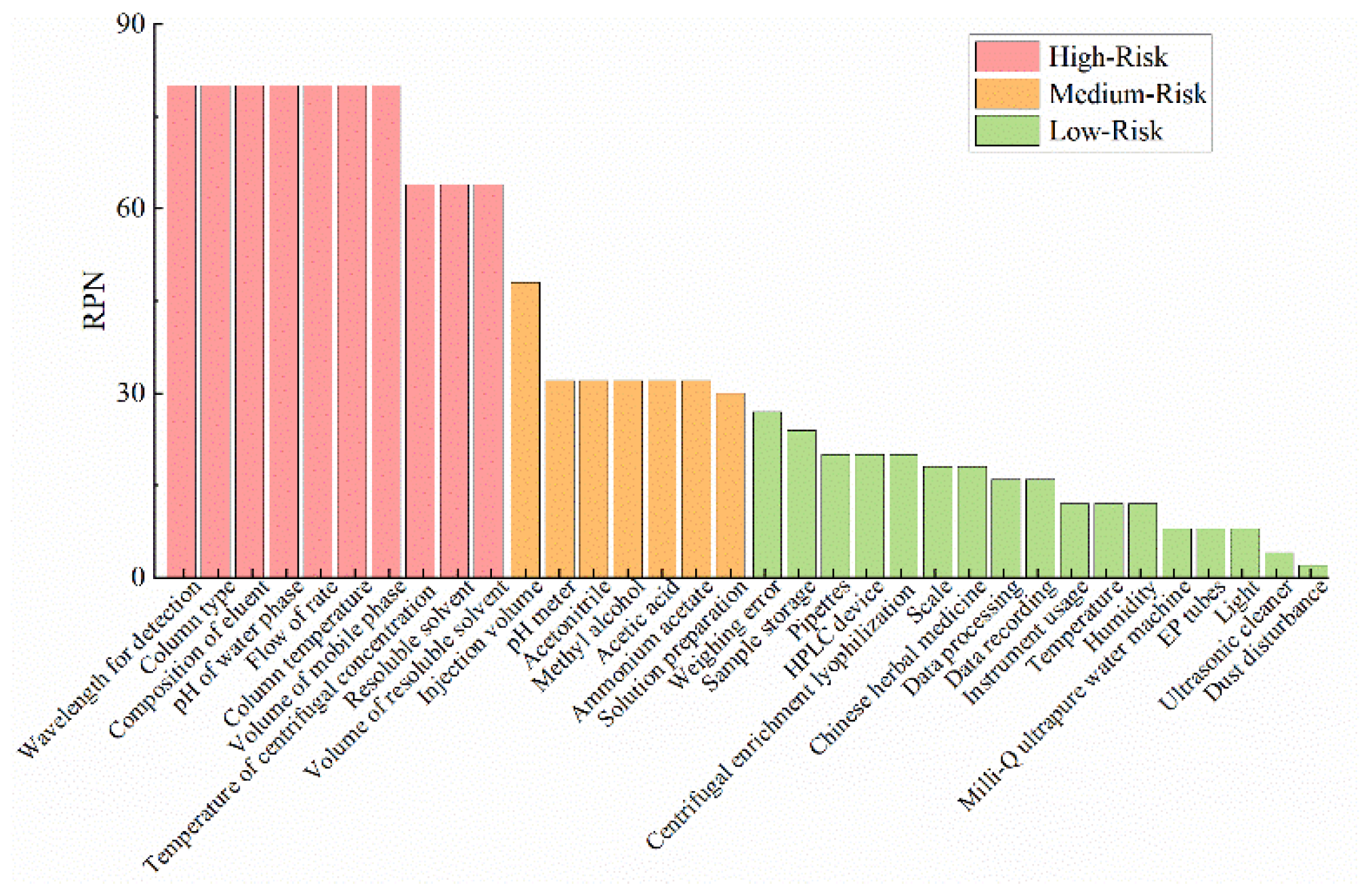
3.3. Preliminary FMEA Risk Assessment
3.4. Results of the Preliminary Experiments
3.4.1. Selection of the Detection Wavelength
3.4.2. Selection of the Chromatographic Column
3.4.3. Selection of the Mobile Phase System, Flow Rate, and Initial Gradient
3.4.4. Selection of Column Temperature
3.4.5. Selection of the Centrifugal Concentration Temperature
3.4.6. The Selection of Reconstitution Solvent and Volume
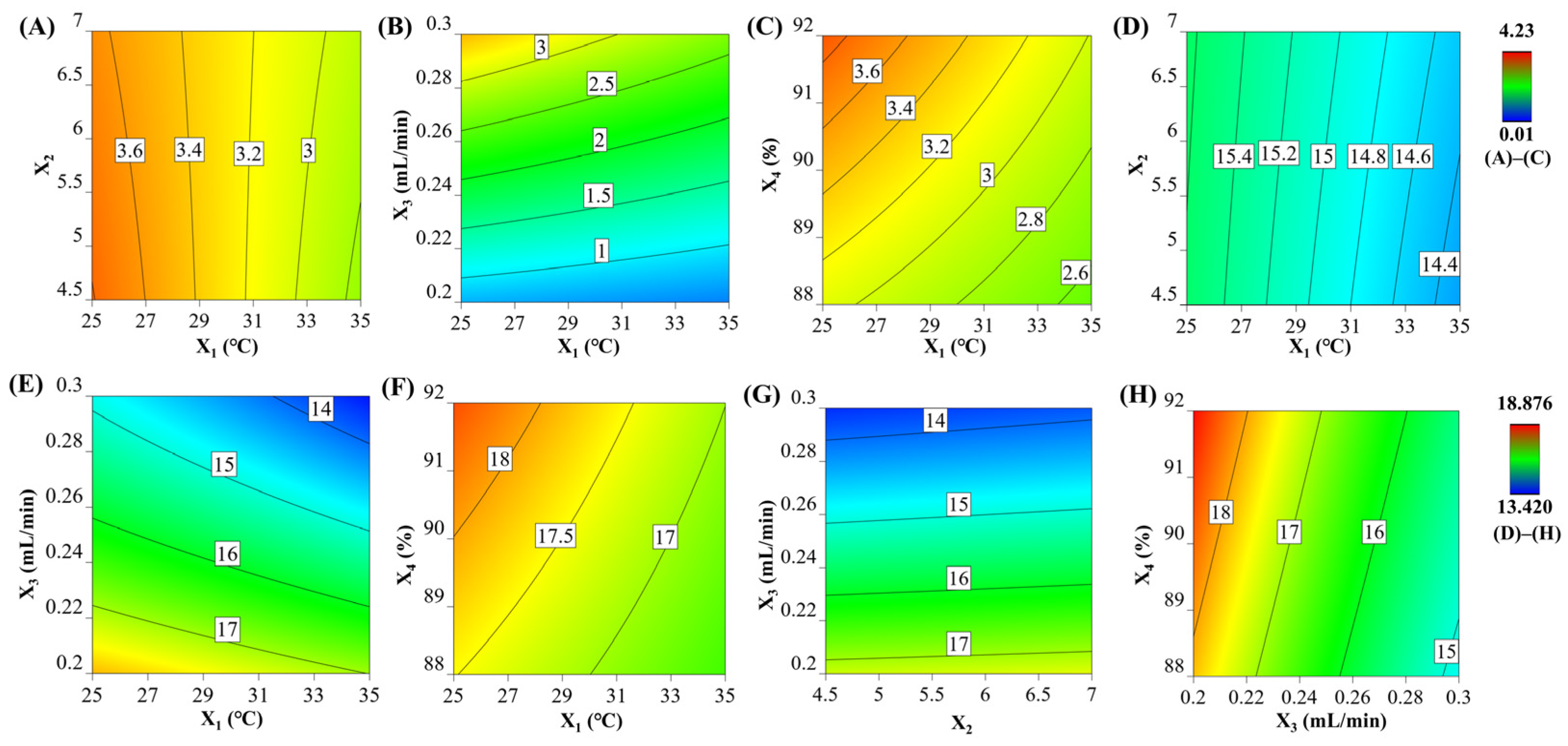
3.5. Results of the CCD
3.6. Data Modeling
3.7. Development and Verification of the MODRs
3.8. Risk Review Based on the Robustness and Verification Experiment Results
3.9. Results of Methodological Validation
3.10. Control Strategy
3.11. Application of the Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, S.; Wu, L.; Wang, X.; Gong, X.; Qu, H. Development of an HPLC-MS method for the determination of four terpene trilactones in Ginkgo biloba leaf extract via quality by design. Biomed. Chromatogr. 2021, 35, e5170. [Google Scholar] [CrossRef] [PubMed]
- Dispas, A.; Avohou, H.T.; Lebrun, P.; Hubert, P.; Hubert, C. ‘Quality by Design’ approach for the analysis of impurities in pharmaceutical drug products and drug substances. TRAC Trends Anal. Chem. 2018, 101, 24–33. [Google Scholar] [CrossRef]
- Prajapati, P.; Patel, M.; Kansara, Y.; Shah, P.; Pulusu, V.S.; Shah, S. Green LC-MS/MS method for in-vivo pharmacokinetics of mirabegron-encapsulated nanostructured lipid carriers in rat plasma: Integrating white analytical chemistry and analytical quality by design approach. Sustain. Chem. Pharm. 2024, 39, 101523. [Google Scholar] [CrossRef]
- Lan, J.; Wu, G.; Wu, L.; Qu, H.; Gong, P.; Xie, Y.; Zhou, P.; Gong, X. Development of a Quantitative Chromatographic Fingerprint Analysis Method for Sugar Components of Xiaochaihu Capsules Based on Quality by Design Concept. Separations 2022, 10, 13. [Google Scholar] [CrossRef]
- Shao, J.; Cao, W.; Qu, H.; Pan, J.; Gong, X. A novel quality by design approach for developing an HPLC method to analyze herbal extracts: A case study of sugar content analysis. PLoS ONE 2018, 13, e0198515. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Park, S.C.; Park, G.; Choi, E.; Ji, Y.; Jang, Y.P. Analytical quality by design methodology for botanical raw material analysis: A case study of flavonoids in Genkwa Flos. Sci. Rep. 2021, 11, 11936. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Liu, S.; Wu, G.; Qu, H.; Gong, P.; Xie, Y.; Zhou, P.; Gong, X. A novel process for developing a quantitative chromatographic fingerprint analysis method based on analytical quality by design: A case study of Xiaochaihu capsules. Microchem. J. 2023, 194, 109253. [Google Scholar] [CrossRef]
- Peraman, R.; Bhadraya, K.; Reddy, Y.P. Analytical Quality by Design: A Tool for Regulatory Flexibility and Robust Analytics. Int. J. Anal. Chem. 2015, 2015, 868727. [Google Scholar] [CrossRef]
- Santhanam, M.K.; Nagarajan, N.C.; Ponraj, P.B.; Hilurudeen, M.S.M. A Complete Roadmap of Analytical Quality by Design in Various Analytical Techniques. Curr. Pharm. Anal. 2023, 19, 184–215. [Google Scholar] [CrossRef]
- Prajapati, P.; Shahi, A.; Acharya, A.; Shah, S. Chemometry and Green Chemistry-Based Chromatographic Analysis of Azilsartan Medoxomil, Cilnidipine and Chlorthalidone in Human Plasma Using Analytical Quality by Design Approach. J. Chromatogr. Sci. 2024, 62, 201–212. [Google Scholar] [CrossRef]
- Deidda, R.; Orlandini, S.; Hubert, P.; Hubert, C. Risk-based approach for method development in pharmaceutical quality control context: A critical review. J. Pharm. Biomed. Anal. 2018, 161, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, P.B.; Patel, P.R.; Shah, S.A. Chemometric and DoE-Based Analytical Quality Risk Management to HPTLC Method for Simultaneous Estimation of Metronidazole and Norfloxacin. J. Chromatogr. Sci. 2023, 61, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, P.; Patel, A.; Shah, S. DoE-Based Analytical Quality Risk Management for Enhanced AQbD Approach to Economical and Eco-Friendly RP-HPLC Method for Synchronous Estimation of Multiple FDC Products of Antihypertensive Drugs. J. Chromatogr. Sci. 2022, 60, 786–799. [Google Scholar] [CrossRef] [PubMed]
- International Conference on Harmonization (ICH). Quality Risk Management Q9. Available online: https://www.cde.org.cn/ichWeb/guideIch/toGuideIch/1/0 (accessed on 1 May 2024).
- Chalikwar, S.S.; Surana, S.J.; Goyal, S.N.; Chaturvedi, K.K.; Dangre, P.V. Solid self-microemulsifying nutraceutical delivery system for hesperidin using quality by design: Assessment of biopharmaceutical attributes and shelf-life. J. Microencapsul. 2021, 38, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Chudiwal, V.S.; Shahi, S.; Chudiwal, S. Development of sustained release gastro-retentive tablet formulation of nicardipine hydrochloride using quality by design (QbD) approach. Drug Dev. Ind. Pharm. 2018, 44, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Chiarentin, L.; Cardoso, C.; Miranda, M.; Vitorino, C. Rheology of Complex Topical Formulations: An Analytical Quality by Design Approach to Method Optimization and Validation. Pharmaceutics 2023, 15, 1810. [Google Scholar] [CrossRef] [PubMed]
- Goswami, A.; Rahman, S.N.R.; Pawde, D.M.; Shunmugaperumal, T. Analytical Quality by Design-Driven RP-HPLC Method Conditions to Concomitantly Determine Cinnarizine and Morin Hydrate in Combined Drug Solution and Dual Drug-Loaded Formulations. J. AOAC Int. 2023, 106, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.S.; Bera, R.; Acharjya, A.K.; Sahoo, P.; Beg, S. Analytical lifecycle management approach: Application to development of a reliable LC method for estimation of lacidipine. Sep. Sci. Plus 2019, 2, 18–25. [Google Scholar] [CrossRef]
- Prajapati, P.; Salunkhe, M.; Pulusu, V.; Shah, S.L. Implementation of white analytical chemistry-driven analytical quality risk assessment and design of experiments to multipurpose chromatographic method for the synchronous estimation of multiple drugs co-formulated with paracetamol. JPC-J. Planar Chromatogr.-Mod. TLC 2023, 37, 69–86. [Google Scholar] [CrossRef]
- Rampini, D.; de Araújo, R.V.; Foitinho, R.E.; Lourenço, F.R.; Giarolla, J. Analytical Quality by Design as an Important Tool to Determine the Best Analytical Conditions for Isoniazid and Its Respective Succinylated Prodrug. J. AOAC Int. 2021, 104, 239–247. [Google Scholar] [CrossRef]
- Deidda, R.; Avohou, H.T.; Baronti, R.; Davolio, P.L.; Pasquini, B.; Del Bubba, M.; Hubert, C.; Hubert, P.; Orlandini, S.; Furlanetto, S. Analytical quality by design: Development and control strategy for a LC method to evaluate the cannabinoids content in cannabis olive oil extracts. J. Pharm. Biomed. Anal. 2019, 166, 326–335. [Google Scholar] [CrossRef]
- Liu, Q.; Bian, G.; Chen, X.; Han, J.; Chen, Y.; Wang, M.; Yang, F. Application of a six sigma model to evaluate the analytical performance of urinary biochemical analytes and design a risk-based statistical quality control strategy for these assays: A multicenter study. J. Clin. Lab. Anal. 2021, 35, e24059. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y. Interpretation of Febrile Disease; Shanghai Scientific and Technical Publishers: Shanghai, China, 2010. [Google Scholar]
- Xu, G.; Wang, H.; Deng, Y.; Xie, K.; Zhao, W.; Gong, X. Research Progress on Quality Control Methods for Xiaochaihu Preparations. Separations 2021, 8, 199. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, C.; Sun, L.; Xue, M. Combination of fingerprint and chemometrics for identification of four processed products of Pinelliae Rhizoma based on nucleosides and nucleobases. Chin. J. Pharm. Anal. 2021, 41, 919–928. [Google Scholar]
- Yu, L.; Zhao, J.; Zhu, Q.; Li, S.P. Macrophage biospecific extraction and high performance liquid chromatography for hypothesis of immunological active components in Cordyceps sinensis. J. Pharm. Biomed. Anal. 2007, 44, 439–443. [Google Scholar] [CrossRef]
- Evaldsson, C.; Rydén, I.; Uppugunduri, S. Anti-inflammatory effects of exogenous uridine in an animal model of lung inflammation. Int. Immunopharmacol. 2007, 7, 1025–1032. [Google Scholar] [CrossRef]
- Ding, X.; Xiong, L.; Zhou, Q.; Ye, Q.; Guo, L.; Liu, F. Advances in Studies on Chemical Structure and Pharmacological Activities of Natural Nucleosides. J. Chengdu Univ. Tradit. Chin. Med. 2018, 41, 102–108. [Google Scholar]
- Wang, M.; Wang, Y.; Wu, Y.; Li, N.; Feng, M.; Zhang, W.; Jing, R.; Hao, X.; Ni, Y. Research progress on content changes of 5-hydroxymethyl furfural during processing of Chinese medicine and it’s pharmacological effects. Drug Eval. Res. 2020, 43, 2319–2327. [Google Scholar]
- Li, J.; Duan, K.; Yang, J.; Li, P.; Xing, T.; Wang, W.; Li, Z.; Shao, X.; Ji, X. Synthesis of 5-hydroxymethyl furfural from dehydration of fructose catalyzed by heteropoly acid. Mod. Chem. Ind. 2021, 41, 269–273. [Google Scholar]
- Guan, L.; Li, X.; Li, J.; Yang, C.; Tan, X.; Cui, Y.; Gao, Y.; Huang, J. Effect of 5-HMF on hemoglobin-oxygen affinity, swimming endurance and spatial memory ability of rats in high altitude simulated environment. J. Army Med. Univ. 2015, 37, 1561–1565. [Google Scholar]
- Yalcin, O.; Cabrales, P. Increased hemoglobin O2 affinity protects during acute hypoxia. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H271–H281. [Google Scholar] [CrossRef] [PubMed]
- International Conference on Harmonization (ICH). Analytical Procedure Development Q14. Available online: https://database.ich.org/sites/default/files/ICH_Q14_Document_Step2_Guideline_2022_0324.pdf (accessed on 1 May 2024).
- Arebo, M.A.; Feyisa, J.D.; Tafa, K.D.; Satheesh, N. Optimization of spray-drying parameter for production of better quality orange fleshed sweet potato (Ipomoea batatas L.) powder: Selected physiochemical, morphological, and structural properties. Heliyon 2023, 9, e13078. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, K.K.; Jiang, M.Y.; Wan, L. Interactions between mesocellular foam silica carriers and model drugs constructed by central composite design. Colloids Surf. B-Biointerfaces 2019, 180, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wan, L.; Zhang, Q.; Cui, B.; Li, C.; Jiang, Y.; Jiang, M.; Wang, K. Constructing a drug release model by central composite design to investigate the interaction between drugs and temperature-sensitive controlled release nanoparticles. Eur. J. Pharm. Biopharm. 2023, 183, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.; Qu, H.; Gong, X. Design space calculation and continuous improvement considering a noise parameter: A case study of ethanol precipitation process optimization for the Carthami Flos extract. Separations 2021, 8, 74. [Google Scholar] [CrossRef]
- Shao, J.; Qu, H.; Gong, X. Comparison of two algorithms for development of design space-overlapping method and probability-based method. China J. Chin. Mater. Medica 2018, 43, 2074–2080. [Google Scholar]
- Jayagopal, B.; Murugesh, S. QbD-mediated RP-UPLC method development invoking an FMEA-based risk assessment to estimate nintedanib degradation products and their pathways. Arab. J. Chem. 2020, 13, 7087–7103. [Google Scholar] [CrossRef]
- International Electrotechnical Commission (IEC). Failure Modes and Effects Analysis (FMEA and FMECA) IEC60812 Edition 3.0; IEC: Geneva, Switzerland, 2018. [Google Scholar]
- Szoleczky, R.; Budai-Szűcs, M.; Csányi, E.; Berkó, S.; Tonka-Nagy, P.; Csóka, I.; Kovács, A. Analytical Quality by Design (AQbD) Approach to the Development of In Vitro Release Test for Topical Hydrogel. Pharmaceutics 2022, 14, 707. [Google Scholar] [CrossRef]
- Kothari, B.H.; Fahmy, R.; Claycamp, H.G.; Moore, C.M.V.; Chatterjee, S.; Hoag, S.W. A Systematic Approach of Employing Quality by Design Principles: Risk Assessment and Design of Experiments to Demonstrate Process Understanding and Identify the Critical Process Parameters for Coating of the Ethylcellulose Pseudolatex Dispersion Using Non-Conventional Fluid Bed Process. AAPS PharmSciTech 2017, 18, 1135–1157. [Google Scholar]
- Tai, Y.; Zhao, M.; Ding, F.; Wu, G.; Qu, H.; Gong, P.; Xie, Y.; Zhou, P.; Gong, X. Analytical Quality by Design based on knowledge organization: A case study of developing a UHPLC method for the detection of phenolic compounds. Phytochem. Anal. 2024, 35, 1088–1099. [Google Scholar] [CrossRef]



| Element | Targets |
|---|---|
| Intended purpose | Determination of four target compounds in GGPP |
| Analysis object | GGPP |
| Main characteristic of the analysis object | There are multiple types of components, and the chemical structures of most components are unclear. |
| Quantitatively detected compounds | Uridine, adenine, guanosine, and 5-hydroxymethylfurfural |
| Analytical method performance requirements |
|
| Attribute | Failure Mode | Potential Risk | O | Resulting Influence | S | Detection Method | D | RPN |
|---|---|---|---|---|---|---|---|---|
| Human | data recording | misregistration | 2 | analysis result error | 4 | manual examination | 2 | 16 |
| data processing | miscalculation | 2 | analysis result error | 4 | manual examination | 2 | 16 | |
| instrument using | disoperation | 2 | analysis result error | 3 | instrument history | 2 | 12 | |
| weigh | weighing error | 3 | influence in solvents, mobile phases, and percolations | 3 | visual check | 3 | 27 | |
| solution preparation | solution formulation error | 2 | influence in solvents, mobile phases, and percolations | 3 | visual check | 5 | 30 | |
| Instrument | pipette | error of scale | 2 | solution formulation error | 2 | visual check | 5 | 20 |
| HPLC | instrument failure | 2 | unable to analyze | 5 | service | 2 | 20 | |
| pH meter | instrument failure | 2 | influence in mobile phase pH | 4 | pH test paper | 4 | 32 | |
| balance | instrument uncalibrated | 2 | weighing error | 3 | visual check | 3 | 18 | |
| Milli-Q ultrapure water machine | fault filter | 1 | influences solution preparation | 2 | service | 4 | 8 | |
| ultrasonic cleaning machine | instrument failure | 2 | influences solution preparation | 1 | visual check | 2 | 4 | |
| centrifugal enrichment freeze-drying system | instrument failure | 2 | influences solution preparation | 5 | visual check | 2 | 20 | |
| EP tube | error of scale | 2 | influences solution preparation and sample preparation | 1 | visual check | 4 | 8 | |
| Material | TCM herbal slices | quality difference in herbal slices | 2 | difference in active ingredient content | 3 | HPLC | 3 | 18 |
| acetonitrile | contaminated | 2 | influences mobile phase | 4 | HPLC | 4 | 32 | |
| methanol | contaminated | 2 | influences mobile phase | 4 | HPLC | 4 | 32 | |
| acetic acid | contaminated | 2 | influences mobile phase pH | 4 | HPLC | 4 | 32 | |
| ammonium acetate | contaminated | 2 | influences mobile phase pH | 4 | HPLC | 4 | 32 | |
| Sample | volume of redissolved solvent | unreasonable volume of redissolved solvent | 4 | low chromatographic peak response value | 4 | HPLC | 4 | 64 |
| resoluble solvent | effect on sample dissolution | 4 | influences chromatographic peak response and peak shape | 4 | HPLC | 4 | 64 | |
| sample bomb | contaminated/sample volatilization | 2 | influences chromatographic peak response | 3 | HPLC | 4 | 24 | |
| centrifugal concentration temperature | if the temperature is too low, the preparation time is long, and if the temperature is too high, the components are volatilized. | 4 | influences chromatographic peak response | 4 | HPLC | 4 | 64 | |
| HPLC mothod | chromatographic column | unsuitable for target component separation | 4 | influences component separation and analysis time | 5 | HPLC | 4 | 80 |
| column temperature | unsuitable for target component separation | 4 | influences component separation and analysis time | 5 | HPLC | 4 | 80 | |
| composition of the mobile phase system | unsuitable for target component separation | 4 | influences component separation and analysis time | 5 | HPLC | 4 | 80 | |
| flow rate | unsuitable for target component separation | 4 | influences component separation and analysis time | 5 | HPLC | 4 | 80 | |
| pH | unsuitable for target component separation | 4 | influences component separation and peak shape | 5 | HPLC | 4 | 80 | |
| injection volume | unsuitable for target component separation | 3 | low chromatographic peak response | 4 | HPLC | 4 | 48 | |
| detection wavelength | unsuitable for target component separation | 4 | low absorption and large interference | 5 | HPLC | 4 | 80 | |
| volume ratio of mobile phase | unsuitable for target component separation | 4 | influences component separation and analysis time | 5 | HPLC | 5 | 80 | |
| Environment | temperature | influence on column gentle sample | 3 | influences component separation and analysis time | 2 | thermometer | 2 | 12 |
| humidity | effect on instruments, weighing, etc. | 3 | influences component separation and analysis time | 2 | thermometer | 2 | 12 | |
| illumination | changes in the chemical composition of the reagent or side reactions occur | 2 | influences component separation and analysis time | 1 | HPLC | 4 | 8 | |
| dust disturbance | effect on some precision instruments | 1 | influences component separation and analysis time | 1 | HPLC | 2 | 2 |
| Run | Analytical Method Parameter | Chromatographic Response | |||||
|---|---|---|---|---|---|---|---|
| X1 (°C) | X2 | X3 (mL/min) | X4 (%) | Y1 | Y2 | Y3 (min) | |
| 1 | 35.0 | 4.50 | 0.200 | 88.0 | 1.68 | 0.0100 | 16.9 |
| 2 | 35.0 | 7.00 | 0.200 | 92.0 | 0.860 | 0.840 | 17.6 |
| 3 | 30.0 | 5.75 | 0.200 | 90.0 | 0.530 | 1.02 | 17.8 |
| 4 | 35.0 | 4.50 | 0.200 | 92.0 | 1.29 | 0.910 | 17.5 |
| 5 | 25.0 | 7.00 | 0.200 | 88.0 | 3.00 | 0.500 | 17.9 |
| 6 | 35.0 | 5.75 | 0.250 | 90.0 | 1.08 | 1.57 | 15.2 |
| 7 | 25.0 | 4.50 | 0.200 | 92.0 | 2.79 | 1.09 | 18.9 |
| 8 | 25.0 | 5.75 | 0.250 | 90.0 | 3.16 | 1.90 | 16.5 |
| 9 | 25.0 | 7.00 | 0.300 | 88.0 | 2.83 | 2.88 | 14.9 |
| 10 | 35.0 | 7.00 | 0.300 | 88.0 | 1.32 | 2.63 | 13.7 |
| 11 | 30.0 | 5.75 | 0.250 | 90.0 | 1.29 | 1.87 | 15.9 |
| 12 | 30.0 | 5.75 | 0.250 | 88.0 | 1.39 | 1.43 | 15.7 |
| 13 | 30.0 | 7.00 | 0.250 | 90.0 | 3.44 | 2.02 | 16.0 |
| 14 | 35.0 | 7.00 | 0.300 | 92.0 | 1.84 | 2.90 | 13.8 |
| 15 | 35.0 | 4.50 | 0.300 | 92.0 | 2.07 | 2.77 | 13.6 |
| 16 | 25.0 | 7.00 | 0.300 | 92.0 | 2.16 | 3.96 | 15.6 |
| 17 | 30.0 | 5.75 | 0.250 | 90.0 | 1.33 | 1.84 | 15.9 |
| 18 | 30.0 | 4.50 | 0.250 | 90.0 | 2.88 | 2.11 | 15.8 |
| 19 | 30.0 | 5.75 | 0.300 | 90.0 | 1.25 | 3.05 | 14.5 |
| 20 | 25.0 | 7.00 | 0.200 | 92.0 | 2.50 | 0.980 | 18.9 |
| 21 | 30.0 | 5.75 | 0.250 | 90.0 | 1.30 | 1.96 | 15.9 |
| 22 | 35.0 | 4.50 | 0.300 | 88.0 | 2.02 | 2.58 | 13.4 |
| 23 | 25.0 | 4.50 | 0.300 | 88.0 | 3.24 | 3.17 | 14.8 |
| 24 | 35.0 | 7.00 | 0.200 | 88.0 | 1.17 | 0.480 | 17.0 |
| 25 | 30.0 | 5.75 | 0.250 | 92.0 | 1.37 | 2.27 | 16.3 |
| 26 | 25.0 | 4.50 | 0.200 | 88.0 | 2.73 | 0.550 | 17.9 |
| 27 | 30.0 | 5.75 | 0.250 | 90.0 | 1.33 | 1.86 | 15.9 |
| 28 | 25.0 | 4.50 | 0.300 | 92.0 | 3.27 | 4.23 | 15.5 |
| Y1 | Y2 | Y3 (min) | ||||
|---|---|---|---|---|---|---|
| Model Term | Coefficient | p Value | Coefficient | p Value | Coefficient | p Value |
| Constant | 1.60 | 0 | 1.89 | 0 | 15.9 | 0 |
| X1 | −0.729 | <0.0001 | −0.264 | <0.0001 | −0.674 | <0.0001 |
| X2 | - | - | - | - | 0.0758 | <0.0001 |
| X3 | 0.267 | 0.0043 | 1.22 | <0.0001 | −1.71 | <0.0001 |
| X4 | - | - | 0.326 | <0.0001 | 0.296 | <0.0001 |
| X12 | 0.475 | 0.0264 | −0.191 | 0.0064 | ||
| X22 | 0.705 | 0.0015 | 0.221 | 0.0015 | - | - |
| X32 | −0.562 | 0.0097 | - | - | 0.225 | <0.0001 |
| X1X2 | - | - | 0.0622 | 0.0737 | - | - |
| X1X3 | - | - | −0.170 | <0.0001 | −0.107 | <0.0001 |
| X1X4 | - | - | −0.0983 | 0.0079 | −0.108 | <0.0001 |
| X2X3 | - | - | - | - | 0.0330 | 0.0495 |
| X3X4 | - | - | - | - | −0.0891 | <0.0001 |
| R2 | 0.7754 | 0.9856 | 0.9979 | |||
| Model p value | <0.0001 | <0.0001 | <0.0001 | |||
| Collection Time (min) | Uridine (μg/mL) | Adenine (μg/mL) | 5-Hydroxymethylfurfural (μg/mL) | Guanosine (μg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | The Lower Limit of the 95% Confidence Interval | The Upper Limit of the 95% Confidence Interval | Mean ± SD | The Lower Limit of the 95% Confidence Interval | The Upper Limit of the 95% Confidence Interval | Mean ± SD | The Lower Limit of the 95% Confidence Interval | The Upper Limit of the 95% Confidence Interval | Mean ± SD | The Lower Limit of the 95% Confidence Interval | The Upper Limit of the 95% Confidence Interval | |
| 10 | 26.8 ± 0.761 | 24.9 | 28.6 | 13.8 ± 0.185 | 13.3 | 14.3 | 21.3 ± 0.352 | 20.4 | 22.2 | 22.6 ± 0.642 | 21.0 | 24.2 |
| 20 | 31.4 ± 0.398 | 30.5 | 32.4 | 16.7 ± 0.170 | 16.3 | 17.1 | 24.9 ± 0.319 | 24.1 | 25.7 | 26.9 ± 0.964 | 24.5 | 29.3 |
| 30 | 32.1 ± 0.298 | 31.3 | 32.8 | 17.5 ± 0.0173 | 17.5 | 17.6 | 25.4 ± 0.0185 | 25.3 | 25.4 | 28.5 ± 0.564 | 27.1 | 29.9 |
| 50 | 27.9 ± 0.237 | 27.3 | 28.5 | 16.2 ± 0.185 | 15.8 | 16.7 | 21.6 ± 0.0555 | 21.4 | 21.7 | 25.3 ± 0.454 | 24.1 | 26.4 |
| 70 | 25.7 ± 0.635 | 24.1 | 27.3 | 15.1 ± 0.635 | 13.5 | 16.6 | 19.8 ± 0.0647 | 19.6 | 20.0 | 24.0 ± 0.285 | 23.3 | 24.8 |
| 100 | 21.5 ± 0.170 | 21.1 | 22.0 | 13.6 ± 0.0927 | 13.3 | 13.8 | 16.6 ± 0.0940 | 16.3 | 16.8 | 20.2 ± 0.377 | 19.3 | 21.1 |
| 130 | 19.5 ± 0.645 | 17.9 | 21.1 | 12.5 ± 0.170 | 12.0 | 12.9 | 15.7 ± 0.204 | 15.2 | 16.3 | 18.8 ± 0.282 | 18.1 | 19.5 |
| 160 | 18.6 ± 0.581 | 17.2 | 20.0 | 11.4 ± 0.0173 | 11.3 | 11.4 | 14.9 ± 0.138 | 14.5 | 15.2 | 17.5 ± 0.252 | 16.8 | 18.1 |
| 200 | 17.4 ± 0.102 | 17.1 | 17.6 | 10.1 ± 0.133 | 9.75 | 10.4 | 13.9 ± 0.0236 | 13.8 | 13.9 | 15.9 ± 0.152 | 15.5 | 16.2 |
| 250 | 14.8 ± 0.0657 | 14.6 | 14.9 | 8.55 ± 0.0378 | 8.45 | 8.64 | 12.2 ± 0.0415 | 12.1 | 12.3 | 13.6 ± 0.103 | 13.4 | 13.9 |
| 310 | 5.62 ± 0.180 | 5.17 | 6.06 | 4.49 ± 0.0351 | 4.41 | 4.58 | 3.80 ± 0.0558 | 3.66 | 3.94 | 6.39 ± 0.103 | 6.13 | 6.64 |
| 370 | 2.83 ± 0.0313 | 2.75 | 2.91 | 2.49 ± 0.0329 | 2.46 | 2.52 | 1.39 ± 0.0238 | 1.34 | 1.45 | 3.56 ± 0.0630 | 3.40 | 3.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Tai, Y.; Wu, G.; Ding, F.; Qu, H.; Gong, X. Integrated Application of Risk Management Techniques in Developing an Analysis Method for Traditional Chinese Medicine: A Case Study of a Percolation Solution for Xiaochaihu Capsules. Chemosensors 2024, 12, 161. https://doi.org/10.3390/chemosensors12080161
Zhao M, Tai Y, Wu G, Ding F, Qu H, Gong X. Integrated Application of Risk Management Techniques in Developing an Analysis Method for Traditional Chinese Medicine: A Case Study of a Percolation Solution for Xiaochaihu Capsules. Chemosensors. 2024; 12(8):161. https://doi.org/10.3390/chemosensors12080161
Chicago/Turabian StyleZhao, Mintong, Yanni Tai, Gelin Wu, Feng Ding, Haibin Qu, and Xingchu Gong. 2024. "Integrated Application of Risk Management Techniques in Developing an Analysis Method for Traditional Chinese Medicine: A Case Study of a Percolation Solution for Xiaochaihu Capsules" Chemosensors 12, no. 8: 161. https://doi.org/10.3390/chemosensors12080161
APA StyleZhao, M., Tai, Y., Wu, G., Ding, F., Qu, H., & Gong, X. (2024). Integrated Application of Risk Management Techniques in Developing an Analysis Method for Traditional Chinese Medicine: A Case Study of a Percolation Solution for Xiaochaihu Capsules. Chemosensors, 12(8), 161. https://doi.org/10.3390/chemosensors12080161






