Facile Fabrication of Bio-Nanohybrid Electrode with Guanine/Cytosine-Modified Electrochemically Reduced Graphene Oxide Electrode and Its Application in Doxorubicin Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. Electrochemical Measurements
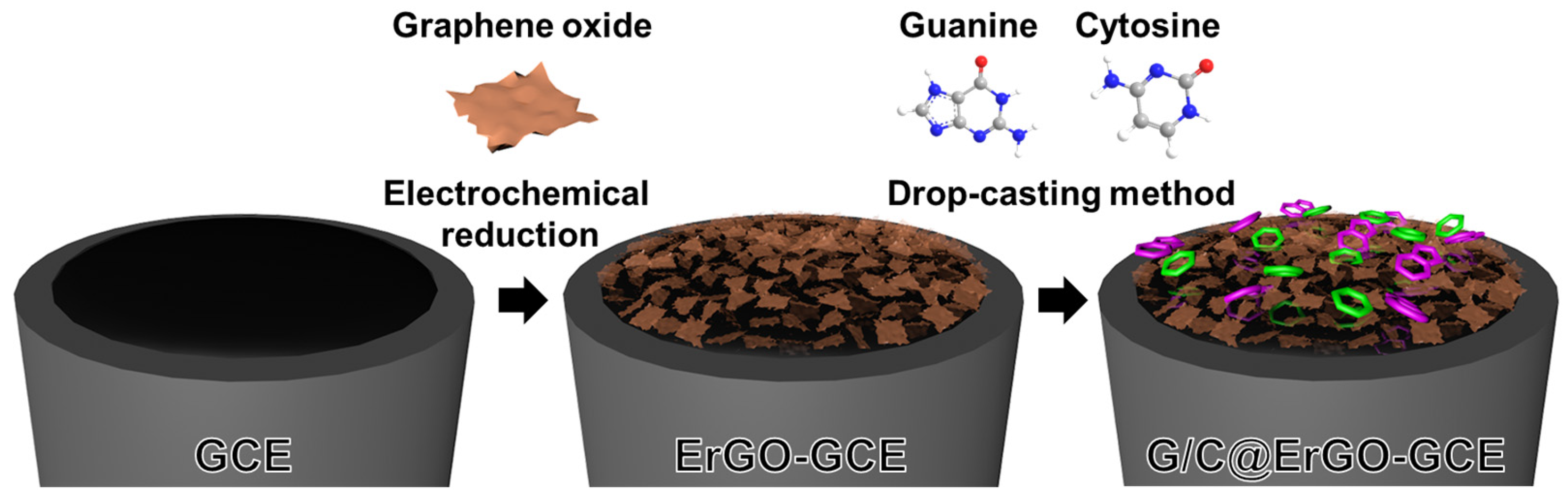
2.4. Preparation of G/C@ErGO-GCE Bio-Nanohybrid Electrode
3. Results and Discussion
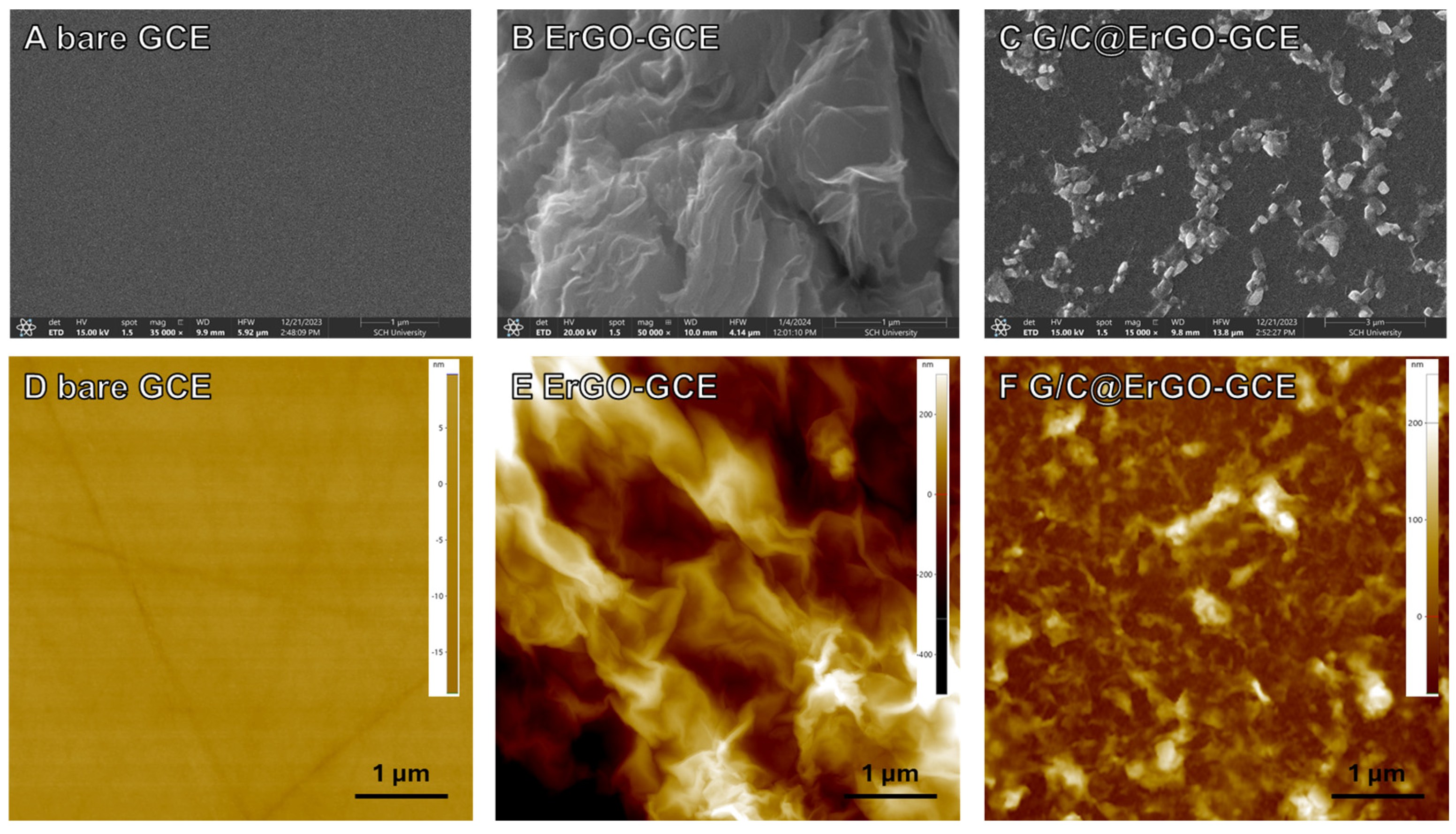
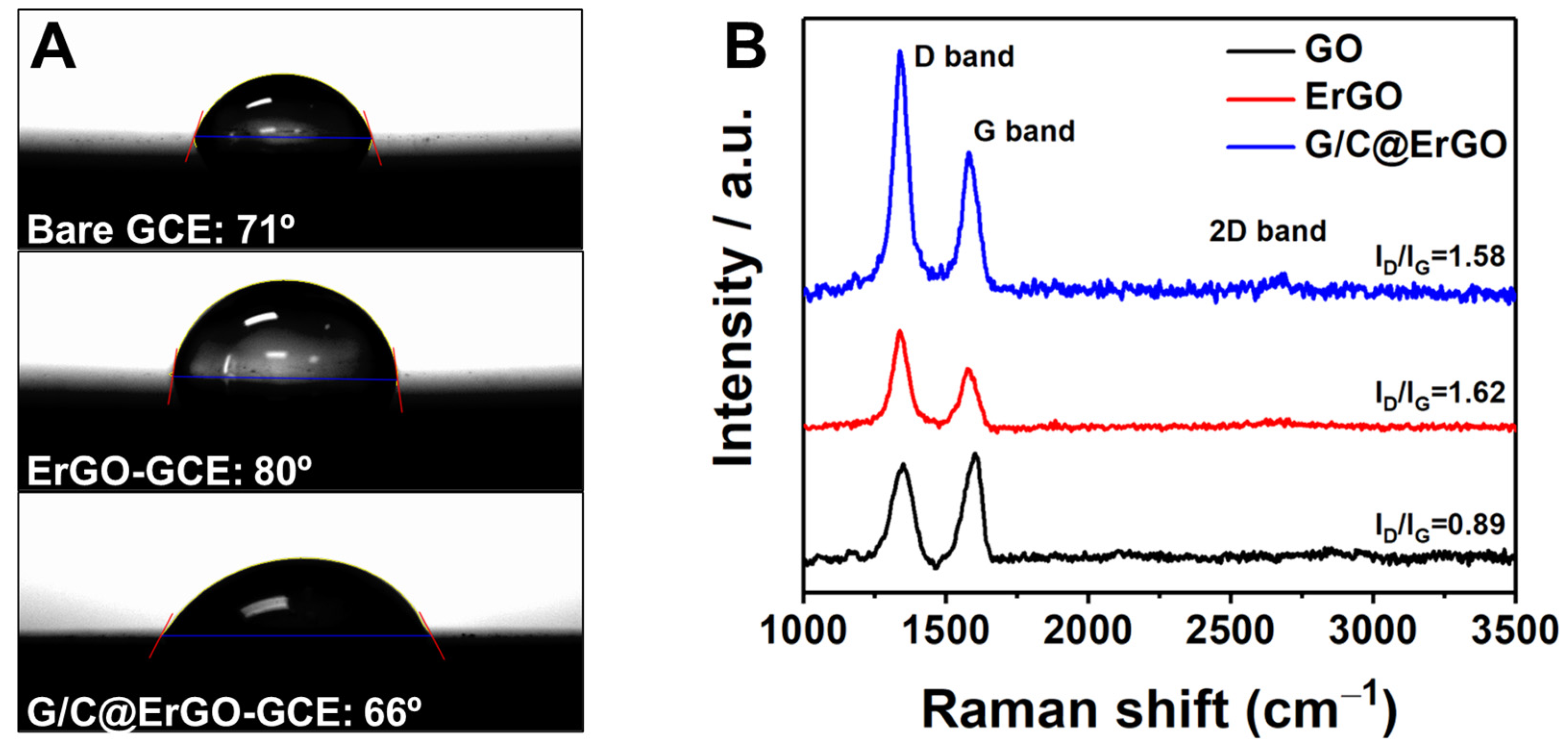
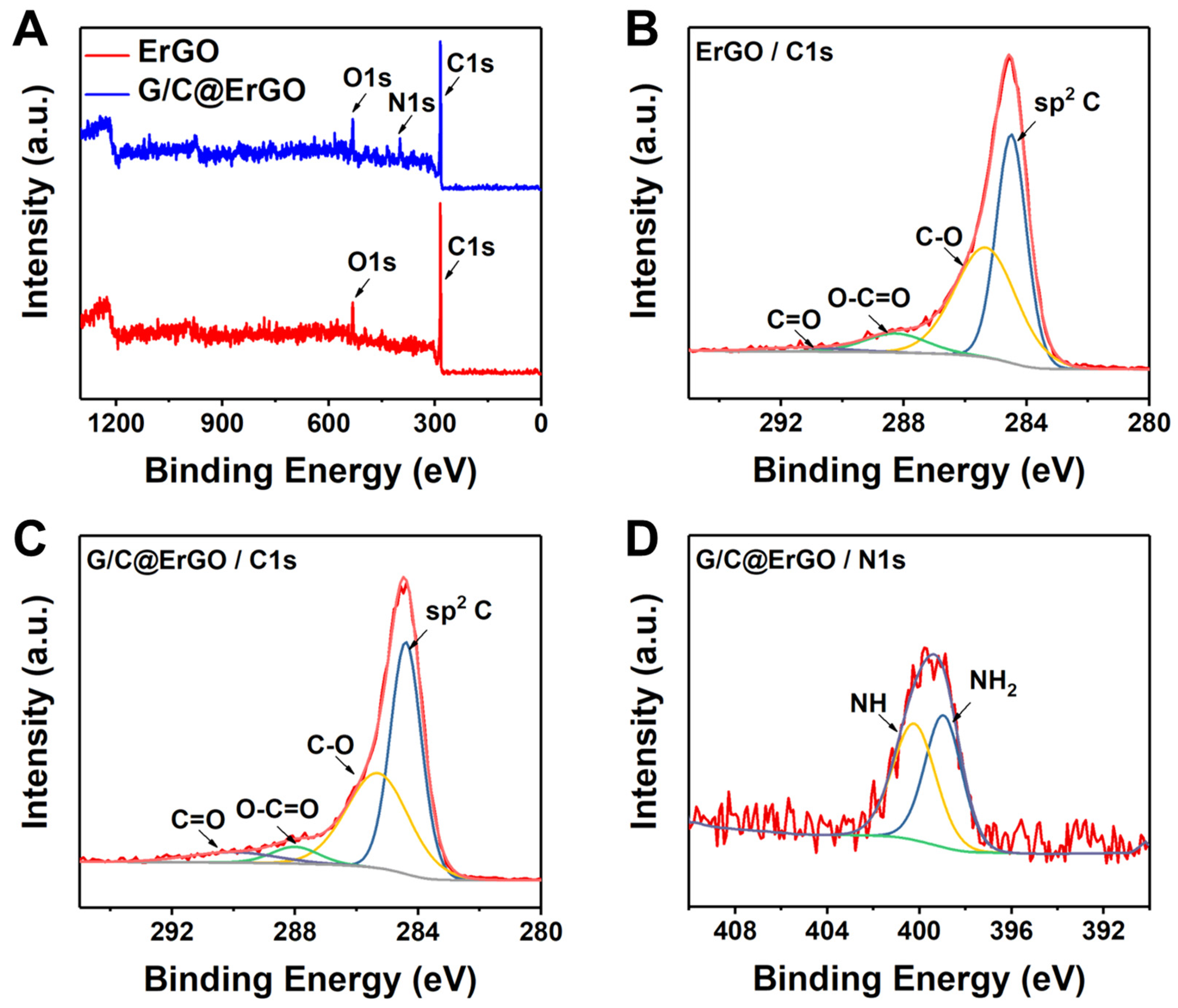
3.1. Preparation and Characterization
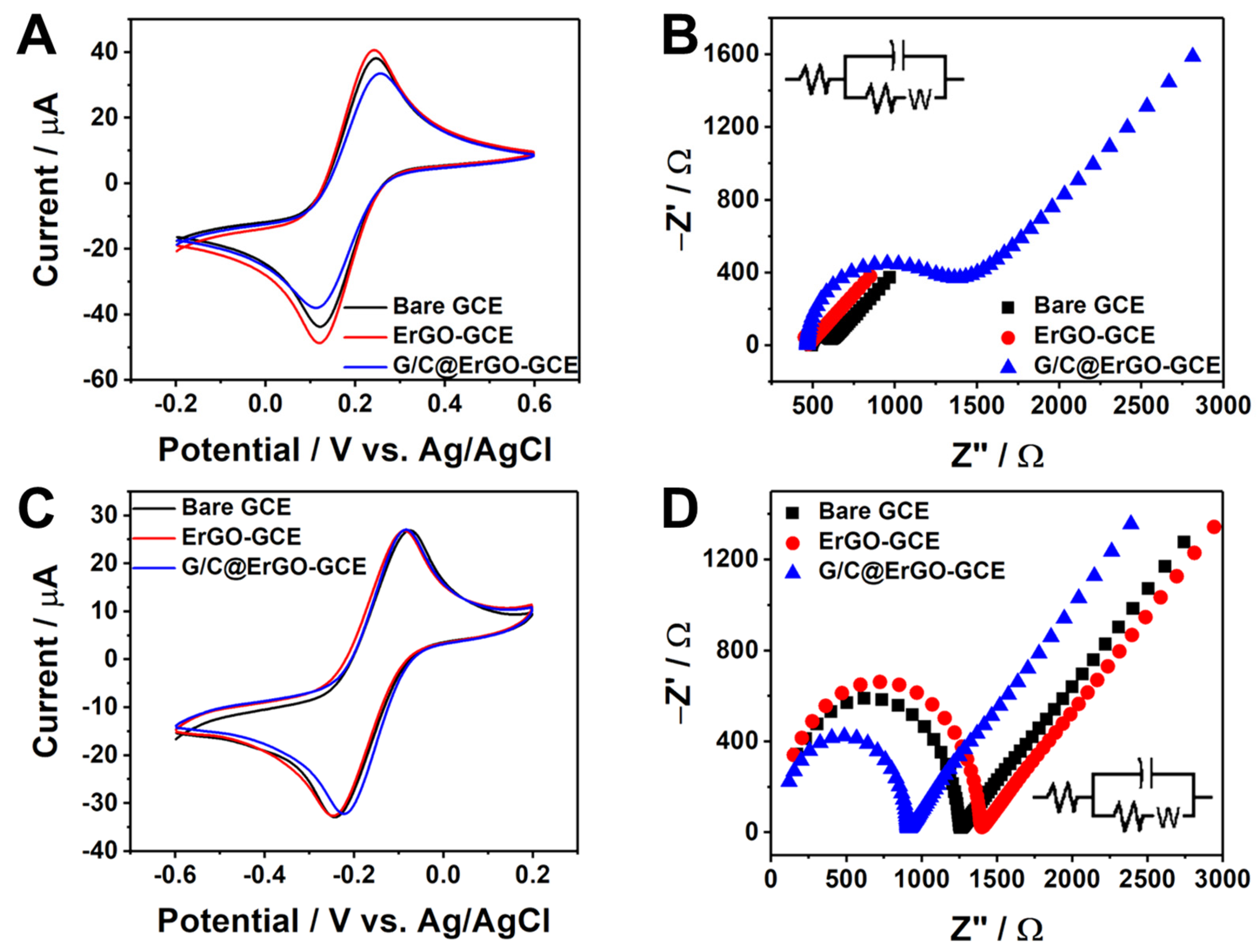
3.2. Electrochemical Property of G/C@ErGO-GCE
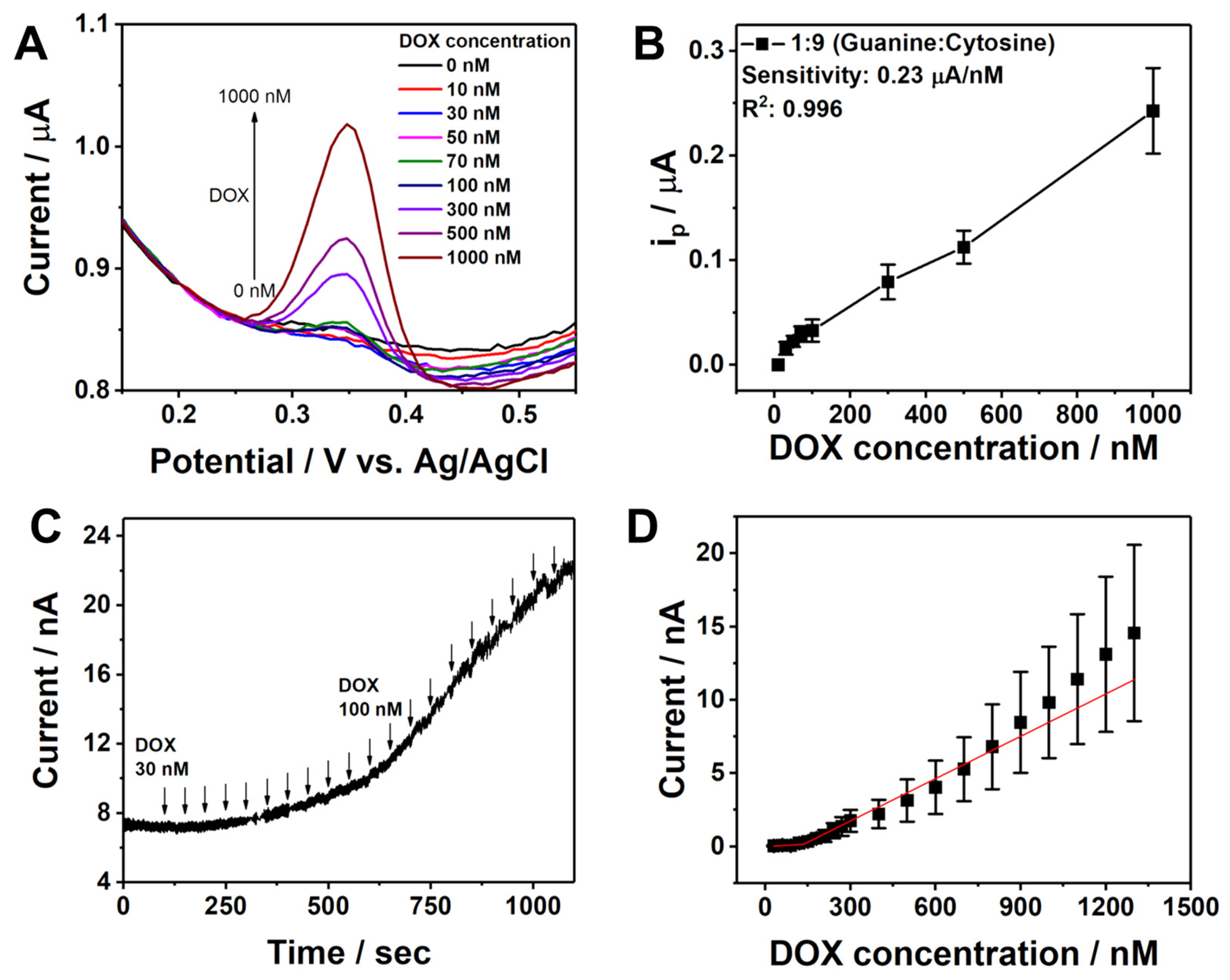
3.3. Electrochemical Detection of DOX
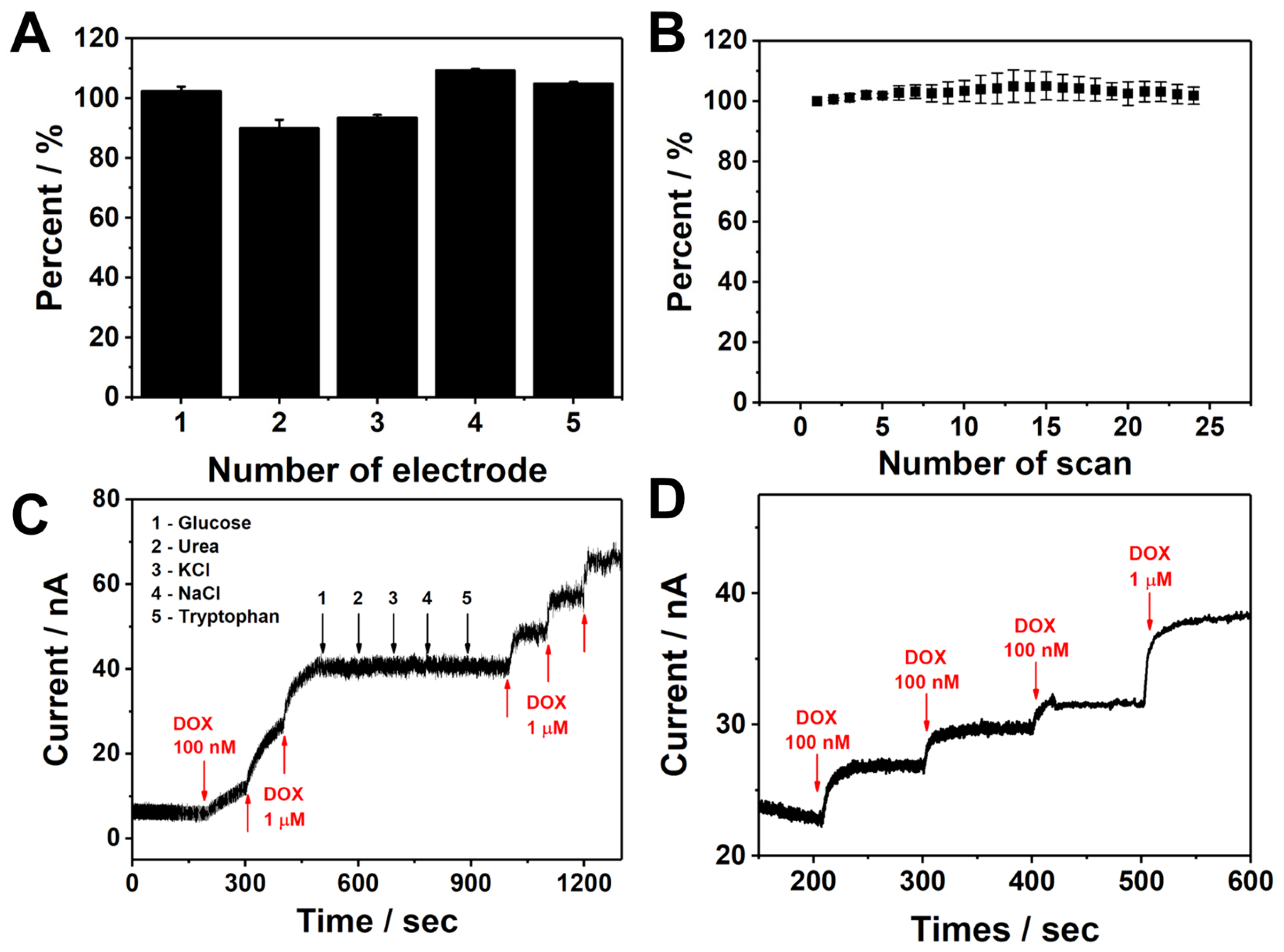
3.4. Analytical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akinwande, D.; Huyghebaert, C.; Wang, C.-H.; Serna, M.I.; Goossens, S.; Li, L.-J.; Wong, H.-S.P.; Koppens, F.H.L. Graphene and Two-Dimensional Materials for Silicon Technology. Nature 2019, 573, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhu, J.; Huang, L. A Review of Three-Dimensional Graphene-Based Materials: Synthesis and Applications to Energy Conversion/Storage and Environment. Carbon 2019, 143, 610–640. [Google Scholar] [CrossRef]
- Han, S.; Sun, J.; He, S.; Tang, M.; Chai, R. The Application of Graphene-Based Biomaterials in Biomedicine. Am. J. Transl. Res. 2019, 11, 3246–3260. [Google Scholar] [PubMed]
- Song, S.; Shen, H.; Wang, Y.; Chu, X.; Xie, J.; Zhou, N.; Shen, J. Biomedical Application of Graphene: From Drug Delivery, Tumor Therapy, to Theranostics. Colloids Surf. B Biointerfaces 2020, 185, 110596. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical Properties of Graphene and Graphene-Based Nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Akinwande, D.; Brennan, C.J.; Bunch, J.S.; Egberts, P.; Felts, J.R.; Gao, H.; Huang, R.; Kim, J.-S.; Li, T.; Li, Y.; et al. A Review on Mechanics and Mechanical Properties of 2D Materials—Graphene and Beyond. Extrem. Mech. Lett. 2017, 13, 42–77. [Google Scholar] [CrossRef]
- Guo, B.; Fang, L.; Zhang, B.; Gong, J.R. Graphene Doping: A Review. Insci. J. 2011, 1, 80–89. [Google Scholar] [CrossRef]
- Yang, Z.; Yao, Z.; Li, G.; Fang, G.; Nie, H.; Liu, Z.; Zhou, X.; Chen, X.; Huang, S. Sulfur-Doped Graphene as an Efficient Metal-Free Cathode Catalyst for Oxygen Reduction. ACS Nano 2012, 6, 205–211. [Google Scholar] [CrossRef]
- Geng, D.; Yang, S.; Zhang, Y.; Yang, J.; Liu, J.; Li, R.; Sham, T.-K.; Sun, X.; Ye, S.; Knights, S. Nitrogen Doping Effects on the Structure of Graphene. Appl. Surf. Sci. 2011, 257, 9193–9198. [Google Scholar] [CrossRef]
- Dai, J.; Yuan, J. Adsorption of Molecular Oxygen on Doped Graphene: Atomic, Electronic, and Magnetic Properties. Phys. Rev. B 2010, 81, 165414. [Google Scholar] [CrossRef]
- Jose, P.P.A.; Kala, M.S.; Kalarikkal, N.; Thomas, S. Reduced Graphene Oxide Produced by Chemical and Hydrothermal Methods. Mater. Today Proc. 2018, 5, 16306–16312. [Google Scholar] [CrossRef]
- Long, D.; Li, W.; Ling, L.; Miyawaki, J.; Mochida, I.; Yoon, S.-H. Preparation of Nitrogen-Doped Graphene Sheets by a Combined Chemical and Hydrothermal Reduction of Graphene Oxide. Langmuir 2010, 26, 16096–16102. [Google Scholar] [CrossRef]
- Lee, C.-S.; Yu, S.; Kim, T. One-Step Electrochemical Fabrication of Reduced Graphene Oxide/Gold Nanoparticles Nanocomposite-Modified Electrode for Simultaneous Detection of Dopamine, Ascorbic Acid, and Uric Acid. Nanomaterials 2017, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-S.; Oh, D.E.; Kim, T.H. Label-Free Assay of Protein Kinase A Activity and Inhibition in Cancer Cell Using Electrochemically-Prepared AuNP/rGO Nanohybrid Electrode Modified with C-Kemptide. Talanta 2020, 215, 120899. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.E.; Lee, C.-S.; Kim, T.H. Simultaneous and Individual Determination of Seven Biochemical Species Using a Glassy Carbon Electrode Modified with a Nanocomposite of Pt Nanoparticle and Graphene by a One-Step Electrochemical Process. Talanta 2022, 247, 123590. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Liu, L. Peptide-Based Electrochemical Biosensing. Sens. Actuators B Chem. 2021, 344, 130232. [Google Scholar] [CrossRef]
- Vanova, V.; Mitrevska, K.; Milosavljevic, V.; Hynek, D.; Richtera, L.; Adam, V. Peptide-Based Electrochemical Biosensors Utilized for Protein Detection. Biosens. Bioelectron. 2021, 180, 113087. [Google Scholar] [CrossRef]
- Jang, S.J.; Lee, C.-S.; Kim, T.H. α-Synuclein Oligomer Detection with Aptamer Switch on Reduced Graphene Oxide Electrode. Nanomaterials 2020, 10, 832. [Google Scholar] [CrossRef]
- Lee, C.-S.; Yu, S.H.; Kim, T.H. A “Turn-on” Electrochemical Aptasensor for Ultrasensitive Detection of Cd2+ Using Duplexed Aptamer Switch on Electrochemically Reduced Graphene Oxide Electrode. Microchem. J. 2020, 159, 105372. [Google Scholar] [CrossRef]
- Huizenga, D.E.; Szostak, J.W. A DNA Aptamer That Binds Adenosine and ATP. Available online: https://pubs.acs.org/doi/pdf/10.1021/bi00002a033 (accessed on 20 June 2024).
- Breaker, R.R. DNA Aptamers and DNA Enzymes. Curr. Opin. Chem. Biol. 1997, 1, 26–31. [Google Scholar] [CrossRef]
- Sharma, T.K.; Bruno, J.G.; Dhiman, A. ABCs of DNA Aptamer and Related Assay Development. Biotechnol. Adv. 2017, 35, 275–301. [Google Scholar] [CrossRef] [PubMed]
- Hynek, D.; Krejcova, L.; Zitka, O.; Adam, V.; Trnkova, L.; Sochor, J.; Stiborova, M.; Eckschlager, T.; Hubalek, J.; Kizek, R. Electrochemical Study of Doxorubicin Interaction with Different Sequences of Single Stranded Oligonucleotides, Part I. Int. J. Electrochem. Sci. 2012, 7, 13–33. [Google Scholar] [CrossRef]
- Jawad, B.; Poudel, L.; Podgornik, R.; Ching, W.-Y. Thermodynamic Dissection of the Intercalation Binding Process of Doxorubicin to dsDNA with Implications of Ionic and Solvent Effects. J. Phys. Chem. B 2020, 124, 7803–7818. [Google Scholar] [CrossRef]
- Gnapareddy, B.; Reddy Dugasani, S.; Ha, T.; Paulson, B.; Hwang, T.; Kim, T.; Hoon Kim, J.; Oh, K.; Park, S.H. Chemical and Physical Characteristics of Doxorubicin Hydrochloride Drug-Doped Salmon DNA Thin Films. Sci. Rep. 2015, 5, 12722. [Google Scholar] [CrossRef]
- Shandilya, M.; Sharma, S.; Das, P.P.; Charak, S.; Shandilya, M.; Sharma, S.; Das, P.P.; Charak, S. Molecular-Level Understanding of the Anticancer Action Mechanism of Anthracyclines. In Advances in Precision Medicine Oncology; IntechOpen: London, UK, 2020; ISBN 978-1-83968-868-3. [Google Scholar]
- Vail, D.M.; Amantea, M.A.; Colbern, G.T.; Martin, F.J.; Hilger, R.A.; Working, P.K. Pegylated Liposomal Doxorubicin: Proof of Principle Using Preclinical Animal Models and Pharmacokinetic Studies. Semin. Oncol. 2004, 31, 16–35. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, M.G.; Poppi, R.J. Determination of Doxorubicin in Human Plasma by Excitation–Emission Matrix Fluorescence and Multi-Way Analysis. Anal. Chim. Acta 2003, 493, 69–81. [Google Scholar] [CrossRef]
- Sakai-Kato, K.; Saito, E.; Ishikura, K.; Kawanishi, T. Analysis of Intracellular Doxorubicin and Its Metabolites by Ultra-High-Performance Liquid Chromatography. J. Chromatogr. B 2010, 878, 1466–1470. [Google Scholar] [CrossRef]
- Arnold, R.D.; Slack, J.E.; Straubinger, R.M. Quantification of Doxorubicin and Metabolites in Rat Plasma and Small Volume Tissue Samples by Liquid Chromatography/Electrospray Tandem Mass Spectroscopy. J. Chromatogr. B 2004, 808, 141–152. [Google Scholar] [CrossRef]
- Lee, C.-S.; Shim, S.J.; Kim, T.H. Scalable Preparation of Low-Defect Graphene by Urea-Assisted Liquid-Phase Shear Exfoliation of Graphite and Its Application in Doxorubicin Analysis. Nanomaterials 2020, 10, 267. [Google Scholar] [CrossRef]
- Frederick, C.A.; Williams, L.D.; Ughetto, G.; Van der Marel, G.A.; Van Boom, J.H.; Rich, A.; Wang, A.H.J. Structural comparison of anticancer drug-DNA complexes: Adriamycin and daunomycin. Biochemistry 1990, 29, 2538–2549. [Google Scholar] [CrossRef]
- Trnkova, L.; Huska, D.; Adam, V.; Kizek, R.; Eckschlager, T.; Stiborova, M.; Hubalek, J. Electrochemical biosensor for investigation of anticancer drugs interactions (doxorubicin and ellipticine) with DNA. IEEE Sens. 2009. [CrossRef]
- Hajiana, R.; Tayebib, Z.; Shams, N. Fabrication of an electrochemical sensor for determination of doxorubicin in human plasma and its interaction with DNA. J. Pharm. Anal. 2017, 7, 27–33. [Google Scholar] [CrossRef]
- Zaaba, N.I.; Foo, K.L.; Hashim, U.; Tan, S.J.; Liu, W.-W.; Voon, C.H. Synthesis of Graphene Oxide Using Modified Hummers Method: Solvent Influence. Procedia Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]
- Shahriary, L.; Athawale, A.A. Graphene Oxide Synthesized by Using Modified Hummers Approach. Int. J. Energy Environ. Eng. 2014, 2, 58–63. [Google Scholar]
- Breazu, C.; Socol, M.; Preda, N.; Rasoga, O.; Costas, A.; Socol, G.; Petre, G.; Stanculescu, A. Nucleobases Thin Films Deposited on Nanostructured Transparent Conductive Electrodes for Optoelectronic Applications. Sci. Rep. 2021, 11, 7551. [Google Scholar] [CrossRef]
- Melucci, M.; Treossi, E.; Ortolani, L.; Giambastiani, G.; Morandi, V.; Klar, P.; Casiraghi, C.; Samorì, P.; Palermo, V. Facile Covalent Functionalization of Graphene Oxide Using Microwaves: Bottom-up Development of Functional Graphitic Materials. J. Mater. Chem. 2010, 20, 9052–9060. [Google Scholar] [CrossRef]
- He, D.; Peng, Z.; Gong, W.; Luo, Y.; Zhao, P.; Kong, L. Mechanism of a Green Graphene Oxide Reduction with Reusable Potassium Carbonate. RSC Adv. 2015, 5, 11966–11972. [Google Scholar] [CrossRef]
- Claramunt, S.; Varea, A.; López-Díaz, D.; Velázquez, M.M.; Cornet, A.; Cirera, A. The Importance of Interbands on the Interpretation of the Raman Spectrum of Graphene Oxide. J. Phys. Chem. C 2015, 119, 10123–10129. [Google Scholar] [CrossRef]
- Abhishek Singh, T.; Sharma, V.; Thakur, N.; Tejwan, N.; Sharma, A.; Das, J. Selective and Sensitive Electrochemical Detection of Doxorubicin via a Novel Magnesium Oxide/Carbon Dot Nanocomposite Based Sensor. Inorg. Chem. Commun. 2023, 150, 110527. [Google Scholar] [CrossRef]
- Ehsani, M.; Soleymani, J.; Mohammadalizadeh, P.; Hasanzadeh, M.; Jouyban, A.; Khoubnasabjafari, M.; Vaez-Gharamaleki, Y. Low Potential Detection of Doxorubicin Using a Sensitive Electrochemical Sensor Based on Glassy Carbon Electrode Modified with Silver Nanoparticles-Supported Poly(Chitosan): A New Platform in Pharmaceutical Analysis. Microchem. J. 2021, 165, 106101. [Google Scholar] [CrossRef]
- Rong, S.; Zou, L.; Meng, L.; Yang, X.; Dai, J.; Wu, M.; Qiu, R.; Tian, Y.; Feng, X.; Ren, X.; et al. Dual Function Metal-Organic Frameworks Based Ratiometric Electrochemical Sensor for Detection of Doxorubicin. Anal. Chim. Acta 2022, 1196, 339545. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Luo, J.; Jing, L.; Wang, Y.; Shen, H.; Yu, R.; Sun, S.; Xing, Y.; Ming, T.; Liu, M.; et al. Reduced Graphene Oxide and Gold Nanoparticles-Modified Electrochemical Aptasensor for Highly Sensitive Detection of Doxorubicin. Nanomaterials 2023, 13, 1223. [Google Scholar] [CrossRef] [PubMed]
- Skalová, Š.; Langmaier, J.; Barek, J.; Vyskočil, V.; Navrátil, T. Doxorubicin Determination Using Two Novel Voltammetric Approaches: A Comparative Study. Electrochim. Acta 2020, 330, 135180. [Google Scholar] [CrossRef]
| Electrode Materials | Method | Linear Range (µM) | Sensitivity (µA/µM) | Limit of Detection (µM) | Ref. |
|---|---|---|---|---|---|
| Carbon dots/magnesium oxide | 1 CV | 0.1~1.0 | - | 0.09 | [41] |
| AgNP/poly(chitosan) | 2 SWV | 0.103~5.17 | 0.8028 | 0.103 | [42] |
| UiO-66-NH2/MWCNTs | 3 DPV | 0.1~75 | 0.01829 | 0.051 | [43] |
| rGO/AuNPs | SWV | 0.3~6.0 | 0.385 | 0.1 | [44] |
| p-AgSAE | DPV | 1.0~40 | 0.024 | 0.84 | [45] |
| LDG | DPV | 0.3~2.7 | 0.723 | 0.039 | [31] |
| 4 CA | 0.3~2.7 | - | 0.653 | ||
| G/C@ErGO | DPV | 0.01~1.0 0.03~1.3 | 2.17 6.79 | 0.084 (DPV) 0.034 (CA) | Present work |
| CA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, Y.; Oh, D.E.; Kim, M.; Lim, A.; Lee, C.-S.; Kim, T.H. Facile Fabrication of Bio-Nanohybrid Electrode with Guanine/Cytosine-Modified Electrochemically Reduced Graphene Oxide Electrode and Its Application in Doxorubicin Analysis. Chemosensors 2024, 12, 163. https://doi.org/10.3390/chemosensors12080163
Cho Y, Oh DE, Kim M, Lim A, Lee C-S, Kim TH. Facile Fabrication of Bio-Nanohybrid Electrode with Guanine/Cytosine-Modified Electrochemically Reduced Graphene Oxide Electrode and Its Application in Doxorubicin Analysis. Chemosensors. 2024; 12(8):163. https://doi.org/10.3390/chemosensors12080163
Chicago/Turabian StyleCho, Yoojin, Da Eun Oh, Myungeun Kim, Ahran Lim, Chang-Seuk Lee, and Tae Hyun Kim. 2024. "Facile Fabrication of Bio-Nanohybrid Electrode with Guanine/Cytosine-Modified Electrochemically Reduced Graphene Oxide Electrode and Its Application in Doxorubicin Analysis" Chemosensors 12, no. 8: 163. https://doi.org/10.3390/chemosensors12080163






