Precise Optical Fiber-Based Ammonia Sensor Using CdS Quantum Dots Decorated with ZnO at Heterointerface
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
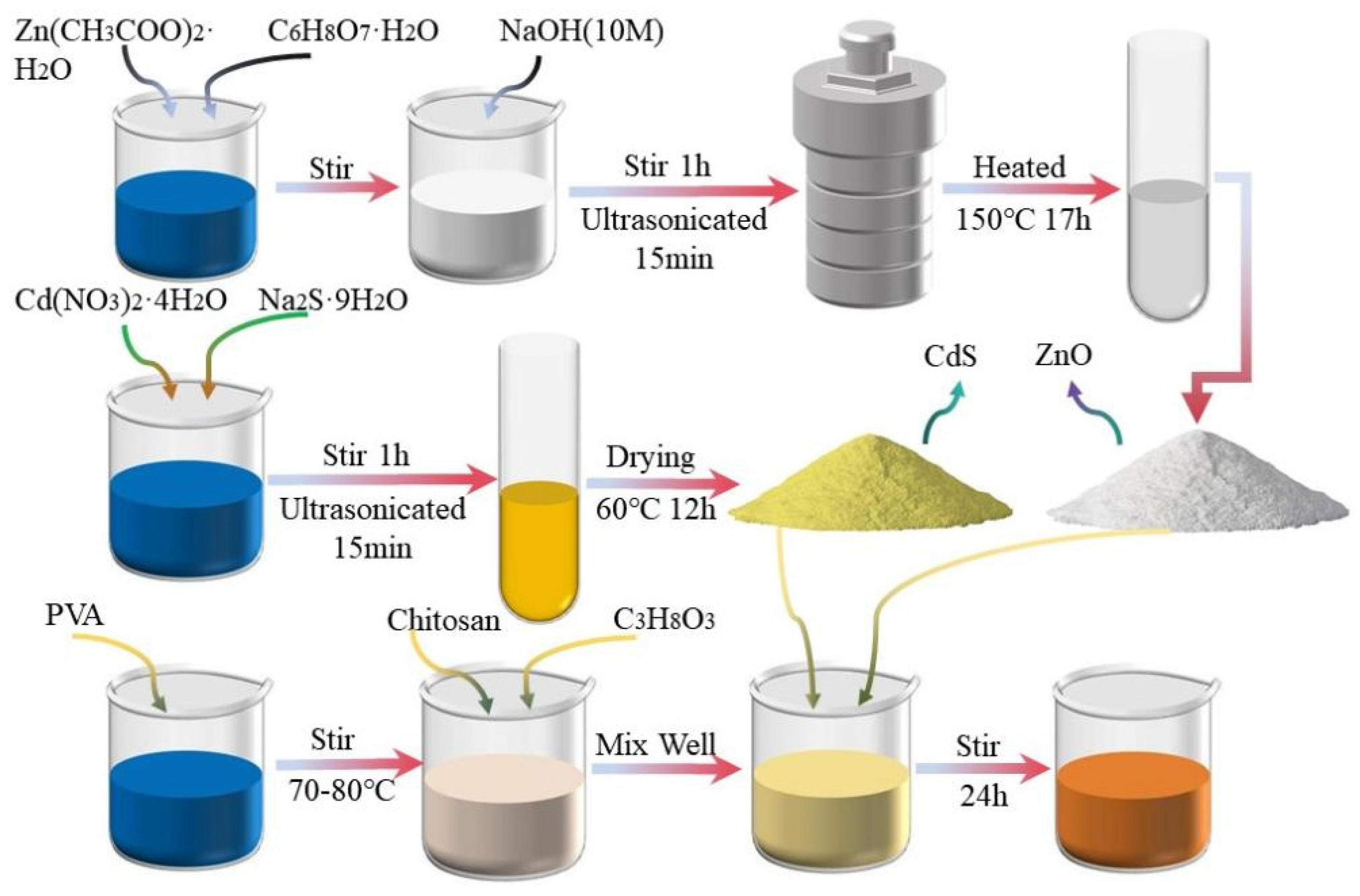
2.2. Preparation of Gas-Sensitive Materials
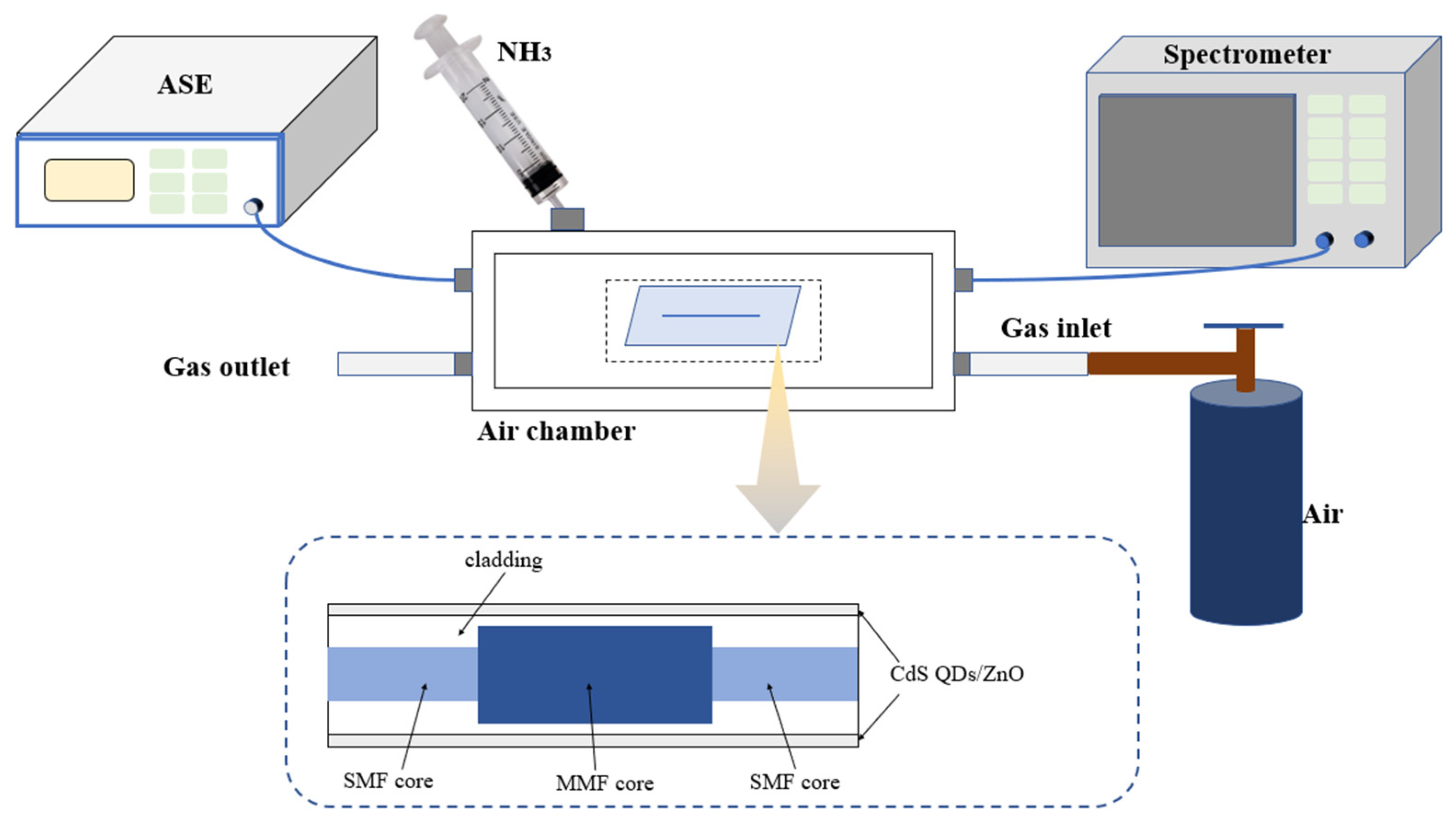
2.3. Construction of Experimental Equipment
3. Results and Discussion
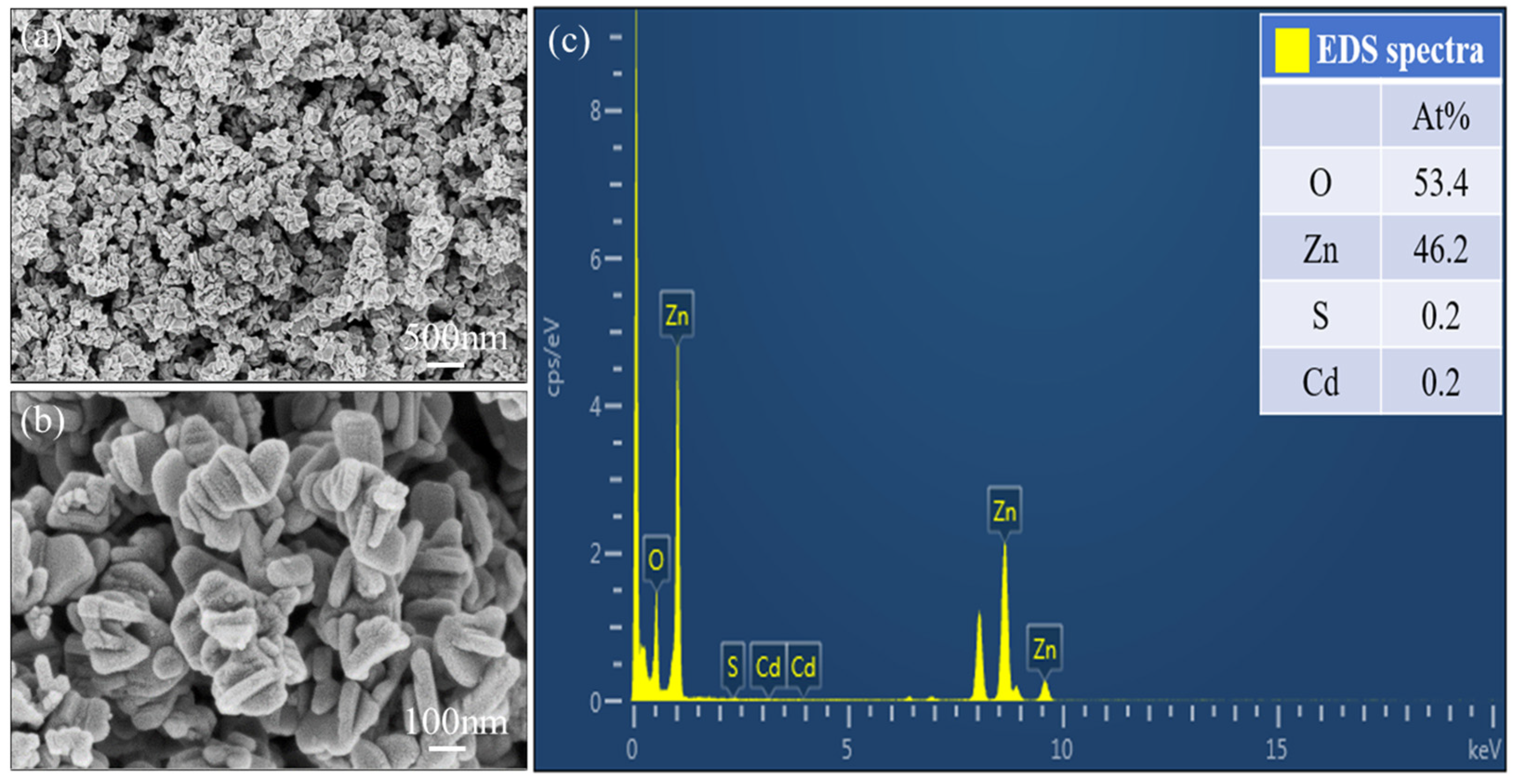
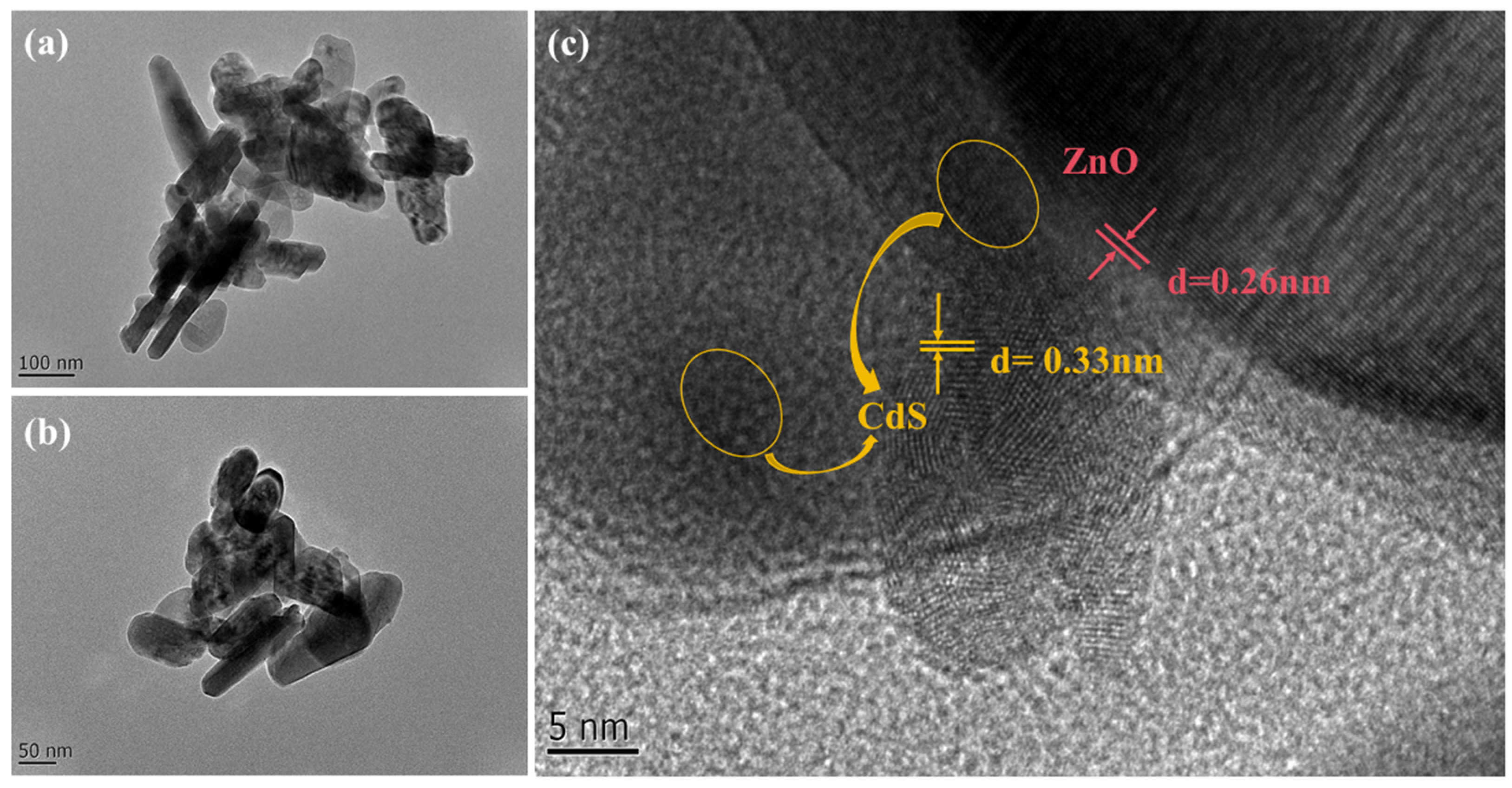
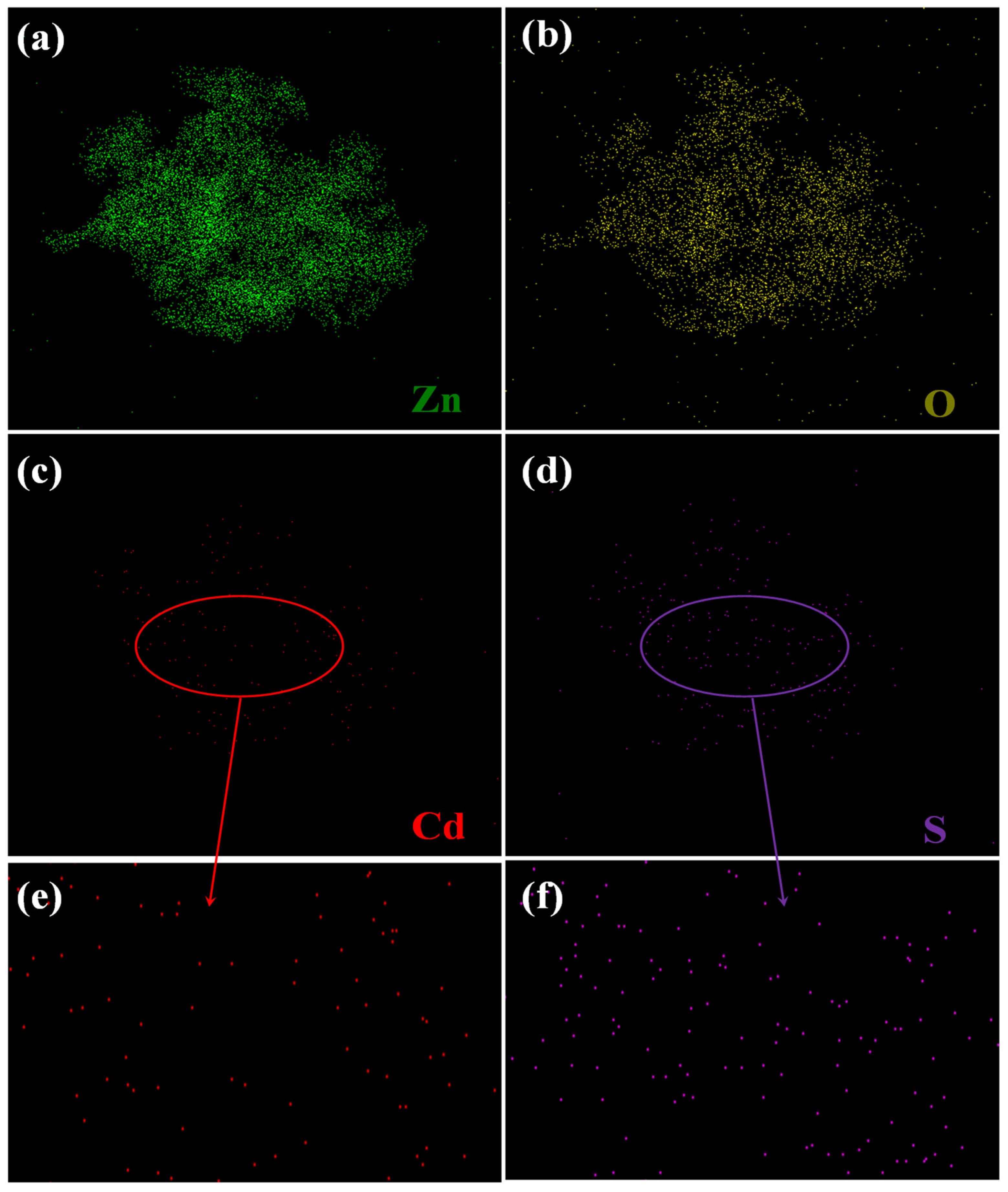
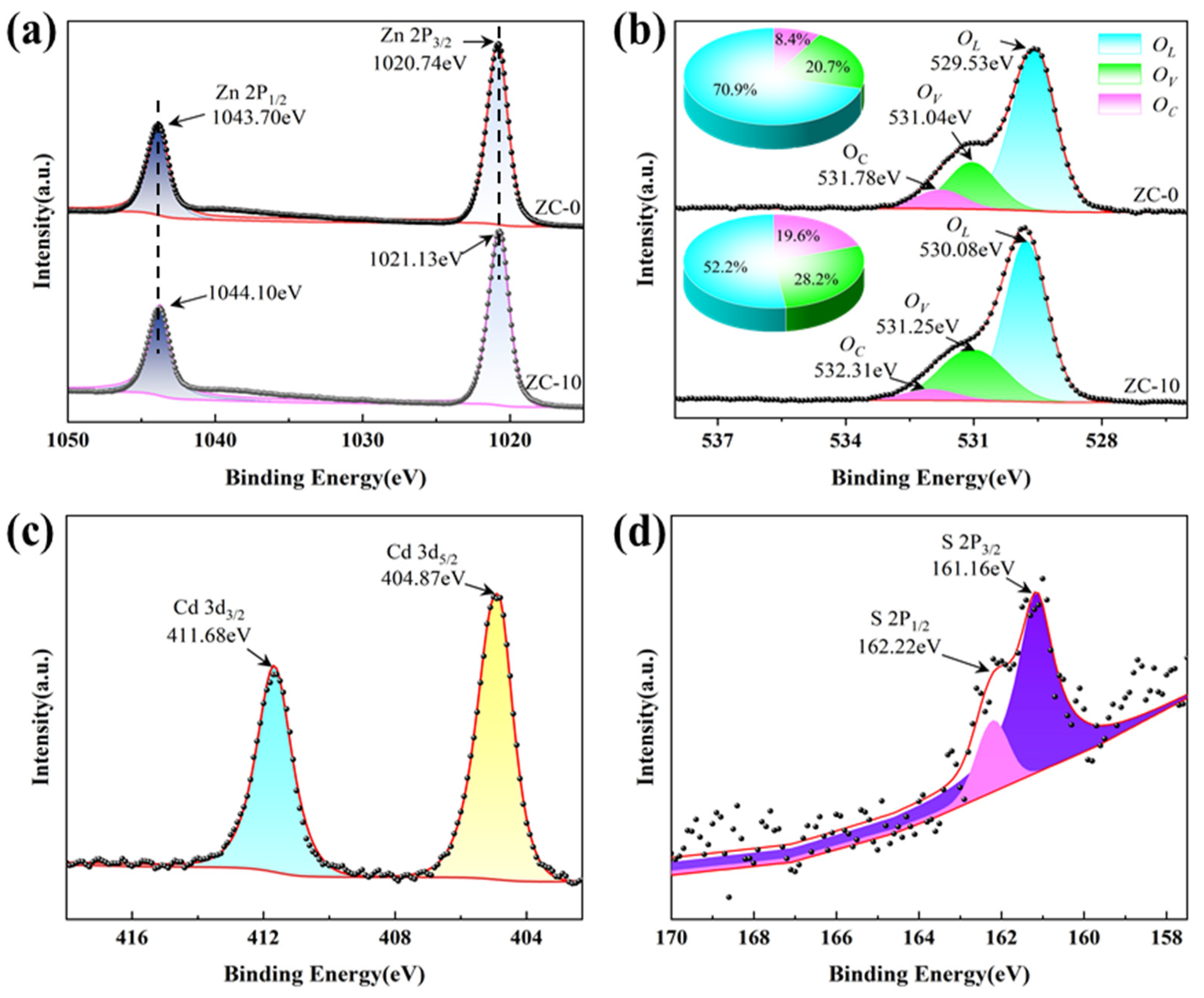
3.1. Materials Characterization
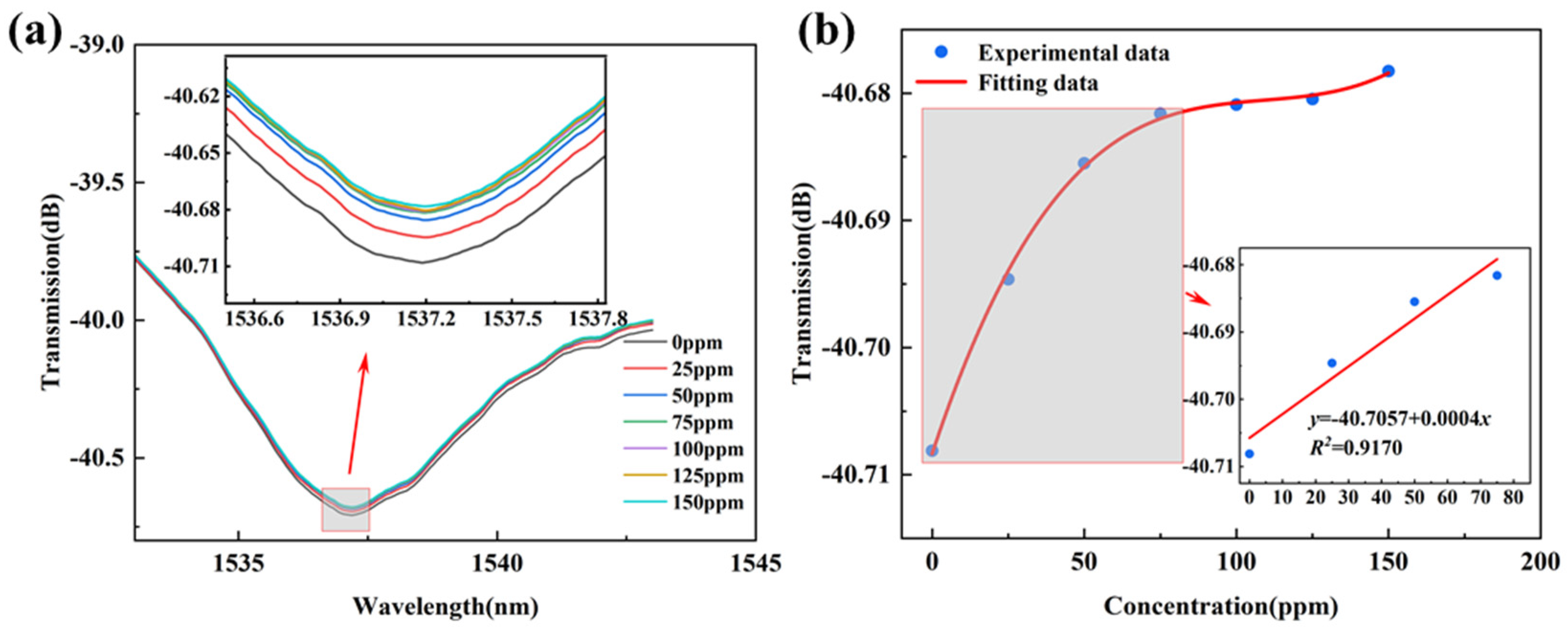
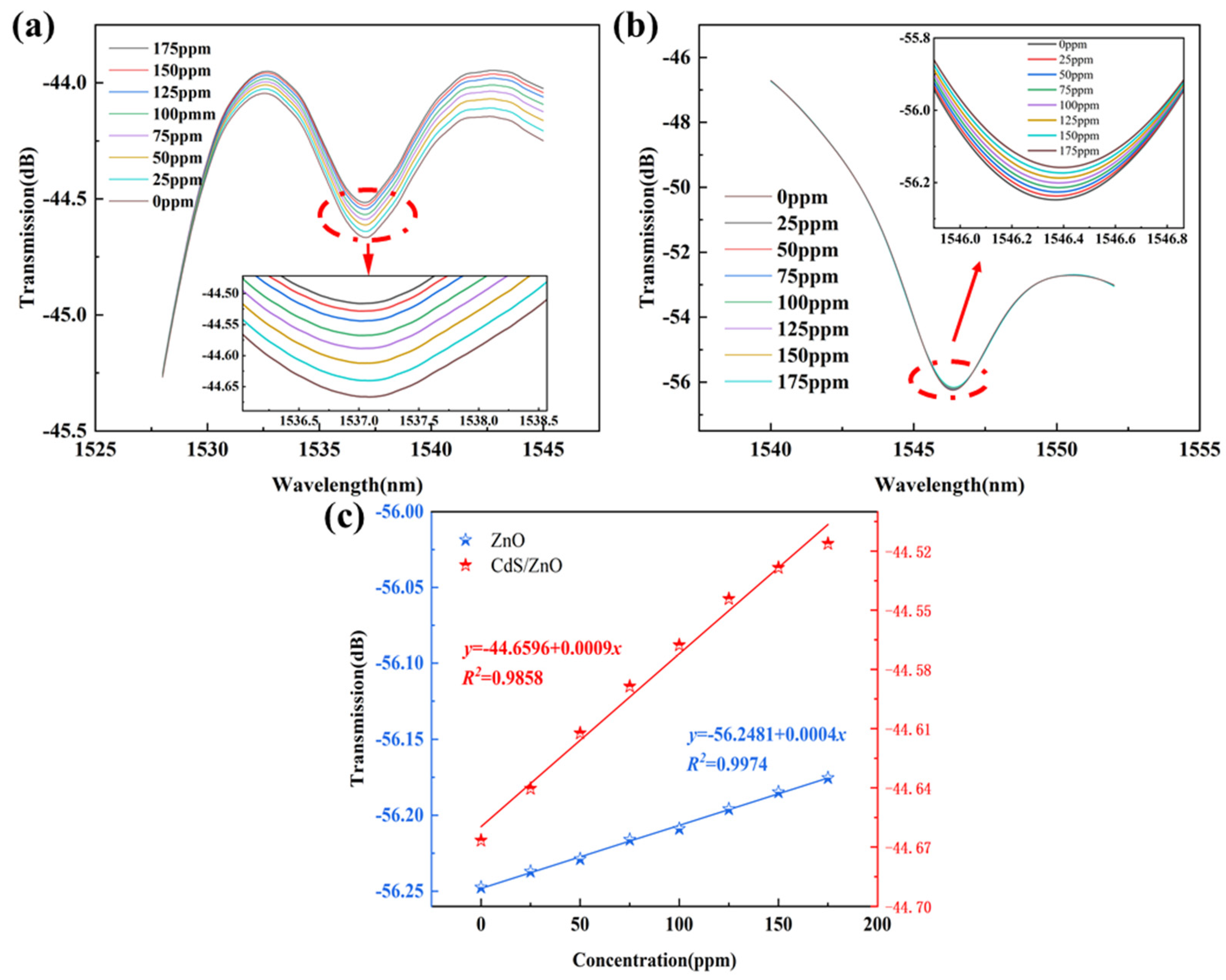
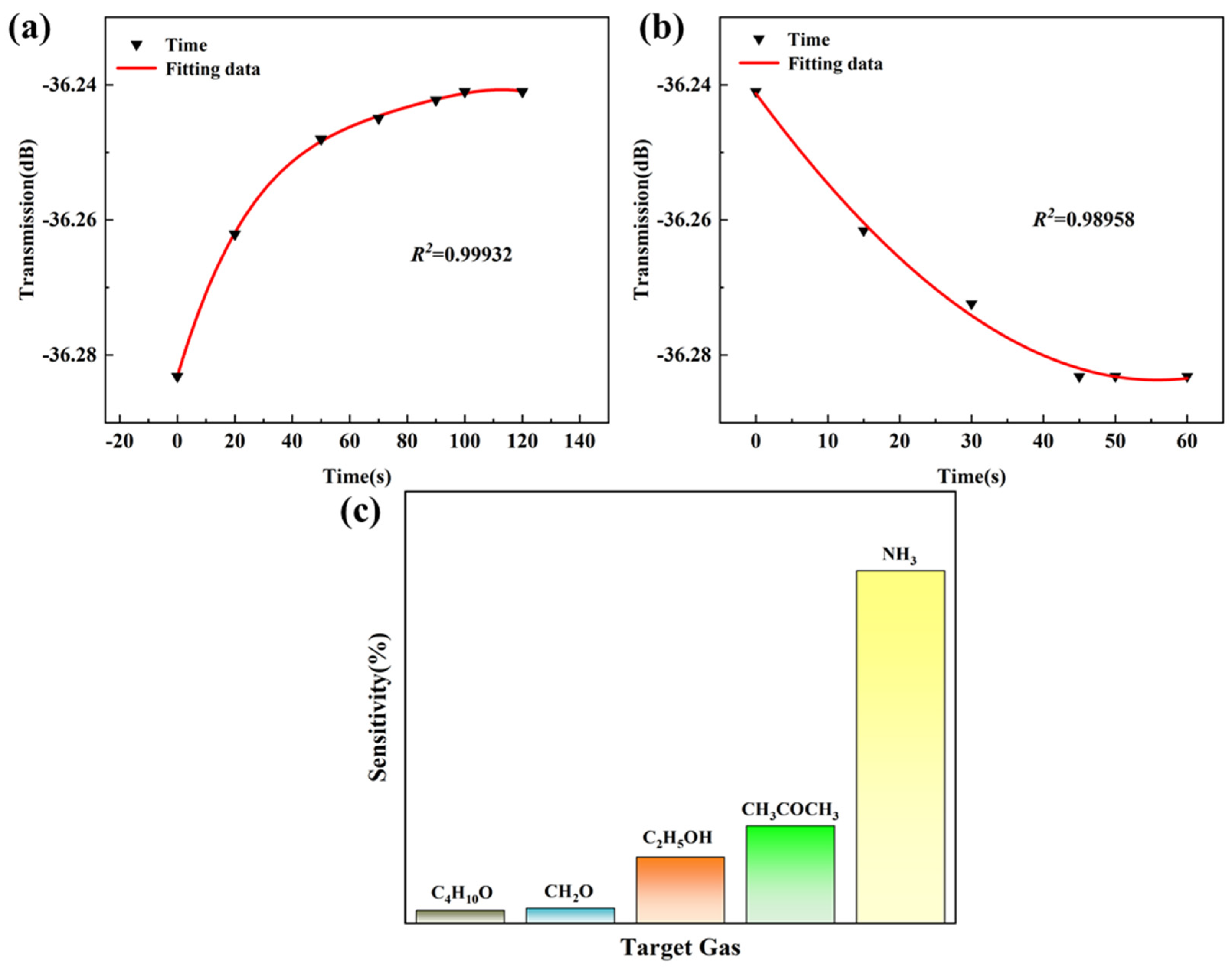
3.2. Gas-Sensing Properties
3.3. Gas-Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Li, Y.; Yuan, Z.; Lei, Y.; Li, X.; Meng, F. Enhanced Ammonia Sensing Performance Based on FeCo2O4/WO3/rGO Ternary Nanocomposites. IEEE Sens. J. 2023, 23, 25698–25707. [Google Scholar] [CrossRef]
- Kumar, N.; Srivastava, A.K.; Nath, R.; Gupta, B.K.; Varma, G.D. Probing the highly efficient room temperature ammonia gas sensing properties of a luminescent ZnO nanowire array prepared via an AAO-assisted template route. Dalton Trans. 2014, 43, 5713–5720. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Li, F.; Xie, Y.; Luo, J.; Fan, P.; Zhong, A. High performance ammonia gas sensor based on GaN honeycomb nanonetwork. Sens. Actuators A Phys. 2020, 312, 112172. [Google Scholar] [CrossRef]
- Hibbard, T.; Killard, A.J. Breath Ammonia Analysis: Clinical Application and Measurement. Crit. Rev. Anal. Chem. 2011, 41, 21–35. [Google Scholar] [CrossRef]
- Reston, R.R.; Kolesar, E.S. Silicon-micromachined gas chromatography system used to separate and detect ammonia and nitrogen dioxide. I. Design, fabrication, and integration of the gas chromatography system. J. Microelectromechanical Syst. 1994, 3, 134–146. [Google Scholar] [CrossRef]
- Renganathan, B.; Sastikumar, D.; Gobi, G.; Rajeswari Yogamalar, N.; Chandra Bose, A. Nanocrystalline ZnO coated fiber optic sensor for ammonia gas detection. Opt. Laser Technol. 2011, 43, 1398–1404. [Google Scholar] [CrossRef]
- Cao, W.; Duan, Y. Optical fiber-based evanescent ammonia sensor. Sens. Actuators B Chem. 2005, 110, 252–259. [Google Scholar] [CrossRef]
- Devendiran, S.; Sastikumar, D. Gas sensing based on detection of light radiation from a region of modified cladding (nanocrystalline ZnO) of an optical fiber. Opt. Laser Technol. 2017, 89, 186–191. [Google Scholar] [CrossRef]
- Narasimman, S.; Balakrishnan, L.; Alex, Z.C. Fiber-Optic Ammonia Sensor Based on Amine Functionalized ZnO Nanoflakes. IEEE Sens. J. 2017, 18, 201–208. [Google Scholar] [CrossRef]
- Renganathan, B.; Ganesan, A.R. Fiber optic gas sensor with nanocrystalline ZnO. Opt. Fiber Technol. 2014, 20, 48–52. [Google Scholar] [CrossRef]
- Zeng, Y.; Lou, Z.; Wang, L.; Zou, B.; Zhang, T.; Zheng, W.; Zou, G. Enhanced ammonia sensing performances of Pd-sensitized flowerlike ZnO nanostructure. Sens. Actuators B Chem. 2011, 156, 395–400. [Google Scholar] [CrossRef]
- Renganathan, B.; Sastikumar, D.; Gobi, G.; Yogamalar, N.R.; Bose, A.C. Gas sensing properties of a clad modified fiber optic sensor with Ce, Li and Al doped nanocrystalline zinc oxides. Sens. Actuators B Chem. 2011, 156, 263–270. [Google Scholar] [CrossRef]
- Lupan, O.; Postica, V.; Gröttrup, J.; Mishra, A.K.; de Leeuw, N.H.; Adelung, R. Enhanced UV and ethanol vapour sensing of a single 3-D ZnO tetrapod alloyed with Fe2O3 nanoparticles. Sens. Actuators B Chem. 2017, 245, 448–461. [Google Scholar] [CrossRef]
- Gavgani, J.N.; Hasani, A.; Nouri, M.; Mahyari, M.; Salehi, A. Highly sensitive and flexible ammonia sensor based on S and N co-doped graphene quantum dots/polyaniline hybrid at room temperature. Sens. Actuators B Chem. 2016, 229, 239–248. [Google Scholar] [CrossRef]
- Hu, S.; Yan, G.; Wu, C.; He, S. An Ethanol Vapor Sensor Based on a Microfiber with a Quantum-Dot Gel Coating. Sensors 2019, 19, 300. [Google Scholar] [CrossRef]
- Gao, W.; Wang, X.; He, Y.; Yu, H.; Zheng, Y.; Yin, R.; Jiang, X. Sub-ppm NO2 gas sensing in CdTe quantum dots functionalized hollow-core anti-resonant fiber. Sens. Actuators B Chem. 2024, 405, 135350. [Google Scholar] [CrossRef]
- Ding, L.; Ruan, Y.; Li, T.; Huang, J.; Warren-Smith, S.C.; Ebendorff-Heidepriem, H.; Monro, T.M. Nitric oxide optical fiber sensor based on exposed core fibers and CdTe/CdS quantum dots. Sens. Actuators B Chem. 2018, 273, 9–17. [Google Scholar] [CrossRef]
- Sung, T.-W.; Lo, Y.-L. Ammonia vapor sensor based on CdSe/SiO2 core–shell nanoparticles embedded in sol–gel matrix. Sens. Actuators B Chem. 2013, 188, 702–708. [Google Scholar] [CrossRef]
- Brahma, S.; Huang, P.C.; Mwakikunga, B.W.; Saasa, V.; Akande, A.A.; Huang, J.-L.; Liu, C.-P. Cd doped ZnO nanorods for efficient room temperature NH3 sensing. Mater. Chem. Phys. 2023, 294, 127053. [Google Scholar] [CrossRef]
- Li, G.; Zhu, X.; Tang, X.; Song, W.; Yang, Z.; Dai, J.; Sun, Y.; Pan, X.; Dai, S. Doping and annealing effects on ZnO:Cd thin films by sol–gel method. J. Alloys Compd. 2011, 509, 4816–4823. [Google Scholar] [CrossRef]
- Jule, L.T.; Dejene, F.B.; Ali, A.G.; Roro, K.T.; Hegazy, A.; Allam, N.K.; El Shenawy, E. Wide visible emission and narrowing band gap in Cd-doped ZnO nanopowders synthesized via sol-gel route. J. Alloys Compd. 2016, 687, 920–926. [Google Scholar] [CrossRef]
- Zhang, H.; Jin, Z.; Xu, M.-D.; Zhang, Y.; Huang, J.; Cheng, H.; Wang, X.-F.; Zheng, Z.-L.; Ding, Y. Enhanced Isopropanol Sensing Performance of the CdS Nanoparticle Decorated ZnO Porous Nanosheets-Based Gas Sensors. IEEE Sens. J. 2021, 21, 13041–13047. [Google Scholar] [CrossRef]
- Wang, Y.-n.; Qin, L.; Yuan, Z.; Li, J.; Meng, F. UV photosensitized N-CQDs@In2O3 ordered porous film elaborated optical fiber acetone gas sensor with ppb-level at room temperature. Sens. Actuators B Chem. 2024, 418, 136283. [Google Scholar] [CrossRef]
- Li, H.; Shih, W.Y.; Shih, W.-H. Synthesis and Characterization of Aqueous Carboxyl-Capped CdS Quantum Dots for Bioapplications. Ind. Eng. Chem. Res. 2007, 46, 2013–2019. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Wu, Z.; Wang, M.; Luo, J.; Torun, H.; Hu, P.; Yang, C.; Grundmann, M.; Liu, X.; et al. Advances in designs and mechanisms of semiconducting metal oxide nanostructures for high-precision gas sensors operated at room temperature. Mater. Horiz. 2019, 6, 470–506. [Google Scholar] [CrossRef]
- Marikutsa, A.; Rumyantseva, M.; Konstantinova, E.A.; Gaskov, A. The Key Role of Active Sites in the Development of Selective Metal Oxide Sensor Materials. Sensors 2021, 21, 2554. [Google Scholar] [CrossRef] [PubMed]
- Samavati, Z.; Samavati, A.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A. Comprehensive investigation of evanescent wave optical fiber refractive index sensor coated with ZnO nanoparticles. Opt. Fiber Technol. 2019, 52, 101976. [Google Scholar] [CrossRef]
- Meng, D.; Liu, D.; Wang, G.; Shen, Y.; San, X.; Li, M.; Meng, F. Low-temperature formaldehyde gas sensors based on NiO-SnO2 heterojunction microflowers assembled by thin porous nanosheets. Sens. Actuators B Chem. 2018, 273, 418–428. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Men, Y.; Bian, Z. TiO2 mesocrystal with exposed (001) facets and CdS quantum dots as an active visible photocatalyst for selective oxidation reactions. Appl. Catal. B Environ. 2016, 187, 115–121. [Google Scholar] [CrossRef]
- Wu, H.; Li, Y.; Feng, W.; Zhong, X.; Li, J.; Liu, S.; Liu, H.; Ma, G.; Xie, R. Three-dimensional zinc oxide decorated with cadmium sulfide nanoparticles heterogenous nanoarchitectures with expedited charge separation toward efficient photocatalytic degradation of organic pollutants. Mater. Sci. Eng. B 2023, 292, 116459. [Google Scholar] [CrossRef]
- Rajeswari Yogamalar, N.; Sadhanandham, K.; Chandra Bose, A.; Jayavel, R. Band alignment and depletion zone at ZnO/CdS and ZnO/CdSe hetero-structures for temperature independent ammonia vapor sensing. Phys. Chem. Chem. Phys. 2016, 18, 32057–32071. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, G.; Geng, X.; Wu, K.; Debliquy, M. Metal oxide semiconductors with highly concentrated oxygen vacancies for gas sensing materials: A review. Sens. Actuators A Phys. 2020, 309, 112026. [Google Scholar] [CrossRef]
- Subramanian, M.; Dhayabaran, V.V.; Sastikumar, D.; Shanmugavadivel, M. Development of room temperature fiber optic gas sensor using clad modified Zn3(VO4)2. J. Alloys Compd. 2018, 750, 153–163. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Han, B.; Wang, M.; Wang, Q.; Zhang, Y.-N. Fiber optic volatile organic compound gas sensors: A review. Coord. Chem. Rev. 2023, 493, 215297. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, B.; Liu, M.; Zhang, L.; Yu, J.; Zhou, M. Direct Z-scheme ZnO/CdS hierarchical photocatalyst for enhanced photocatalytic H2-production activity. Appl. Catal. B Environ. 2019, 243, 19–26. [Google Scholar] [CrossRef]
- Nie, Q.; Yang, L.; Cao, C.; Zeng, Y.; Wang, G.; Wang, C.; Lin, S. Interface optimization of ZnO nanorod/CdS quantum dots heterostructure by a facile two-step low-temperature thermal treatment for improved photoelectrochemical water splitting. Chem. Eng. J. 2017, 325, 151–159. [Google Scholar] [CrossRef]
- Saravanakumar, S.; Usha, K.S.; Prasath, G.V. Ammonia gas sensing performance of Co/Ni co-doped CdS thin films by chemical bath deposition. J. Mater. Sci. Mater. Electron. 2023, 34, 3. [Google Scholar] [CrossRef]
- Fu, T. Sensing behavior of CdS nanoparticles to SO2, H2S and NH3 at room temperature. Mater. Res. Bull. 2013, 48, 1784–1790. [Google Scholar] [CrossRef]


| Gas | A | B | C |
|---|---|---|---|
| Ammonia | 7.55466 | 1002.711 | 247.885 |
| Butanol | 6.78574 | 994.195 | 210.2 |
| Formaldehyde | 18.5875 | 3626.550 | −34.29 |
| Ethanol | 8.04494 | 1554.300 | 222.650 |
| Acetone | 7.0244 | 1161.000 | 224.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhao, C.; Wang, Y.; Yuan, Z. Precise Optical Fiber-Based Ammonia Sensor Using CdS Quantum Dots Decorated with ZnO at Heterointerface. Chemosensors 2024, 12, 169. https://doi.org/10.3390/chemosensors12080169
Li X, Zhao C, Wang Y, Yuan Z. Precise Optical Fiber-Based Ammonia Sensor Using CdS Quantum Dots Decorated with ZnO at Heterointerface. Chemosensors. 2024; 12(8):169. https://doi.org/10.3390/chemosensors12080169
Chicago/Turabian StyleLi, Xinxin, Chenxi Zhao, Yannan Wang, and Zhenyu Yuan. 2024. "Precise Optical Fiber-Based Ammonia Sensor Using CdS Quantum Dots Decorated with ZnO at Heterointerface" Chemosensors 12, no. 8: 169. https://doi.org/10.3390/chemosensors12080169





