A Novel Colorimetric Biosensor for the Detection of Catalase-Positive Staphylococcus aureus Based on an Onion-like Carbon Nanozyme
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Preparation of Onion-like Carbon Nanozyme
2.3. Detection of the POD-like Catalytic Activity of Onion-like Carbon Nanozyme
2.4. Catalytic Kinetic Determination of OLC’s POD-like Activity
2.5. Bacterial Culture
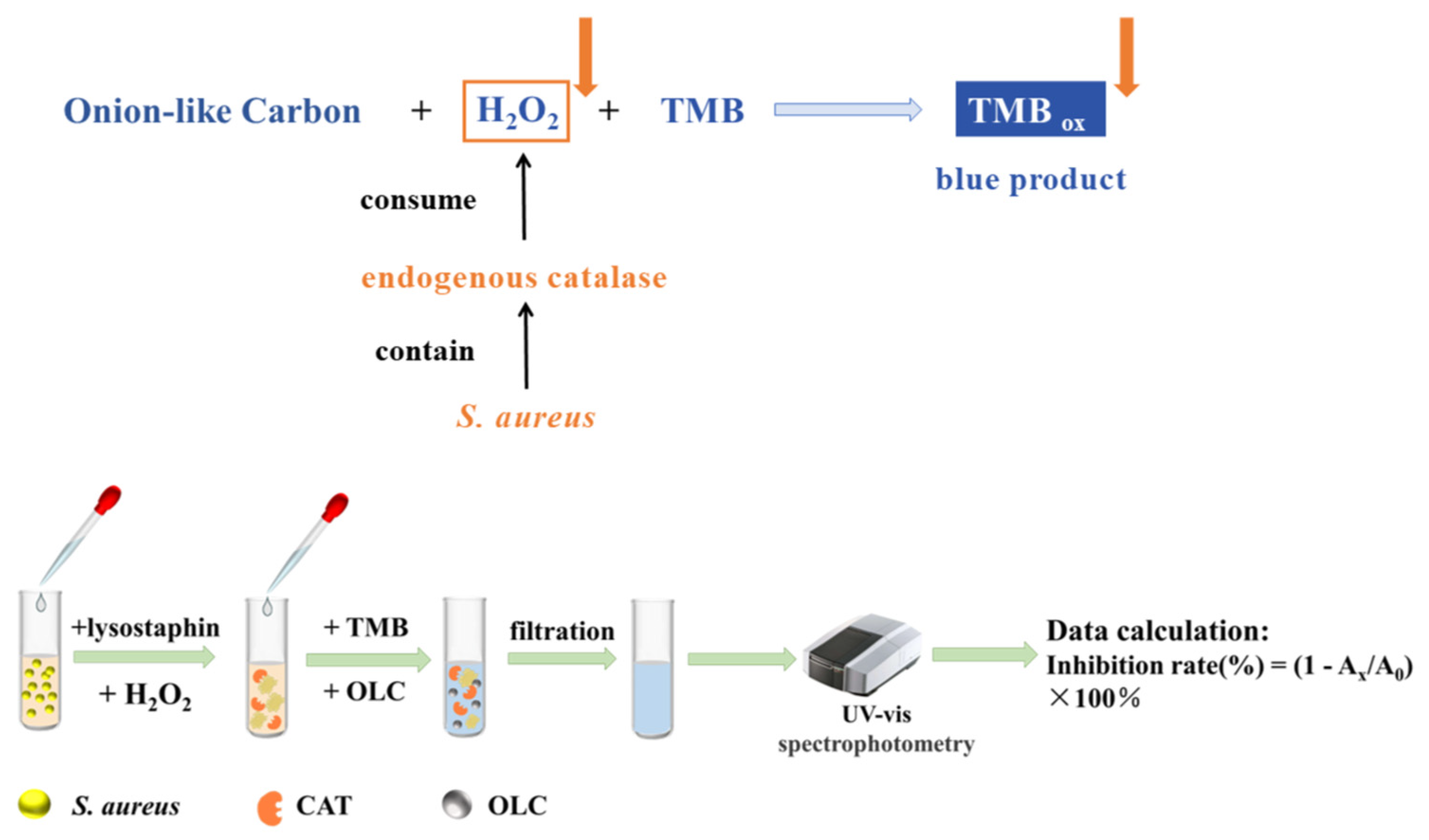
2.6. Colorimetric Detection of S. aureus
3. Results and Discussion
3.1. Structural and Catalytic Activity Characterization of OLC Nanozyme
3.2. Feasibility Verification of the As-Prepared Biosensor for S. aureus Detection
3.3. Optimization of Detection Conditions
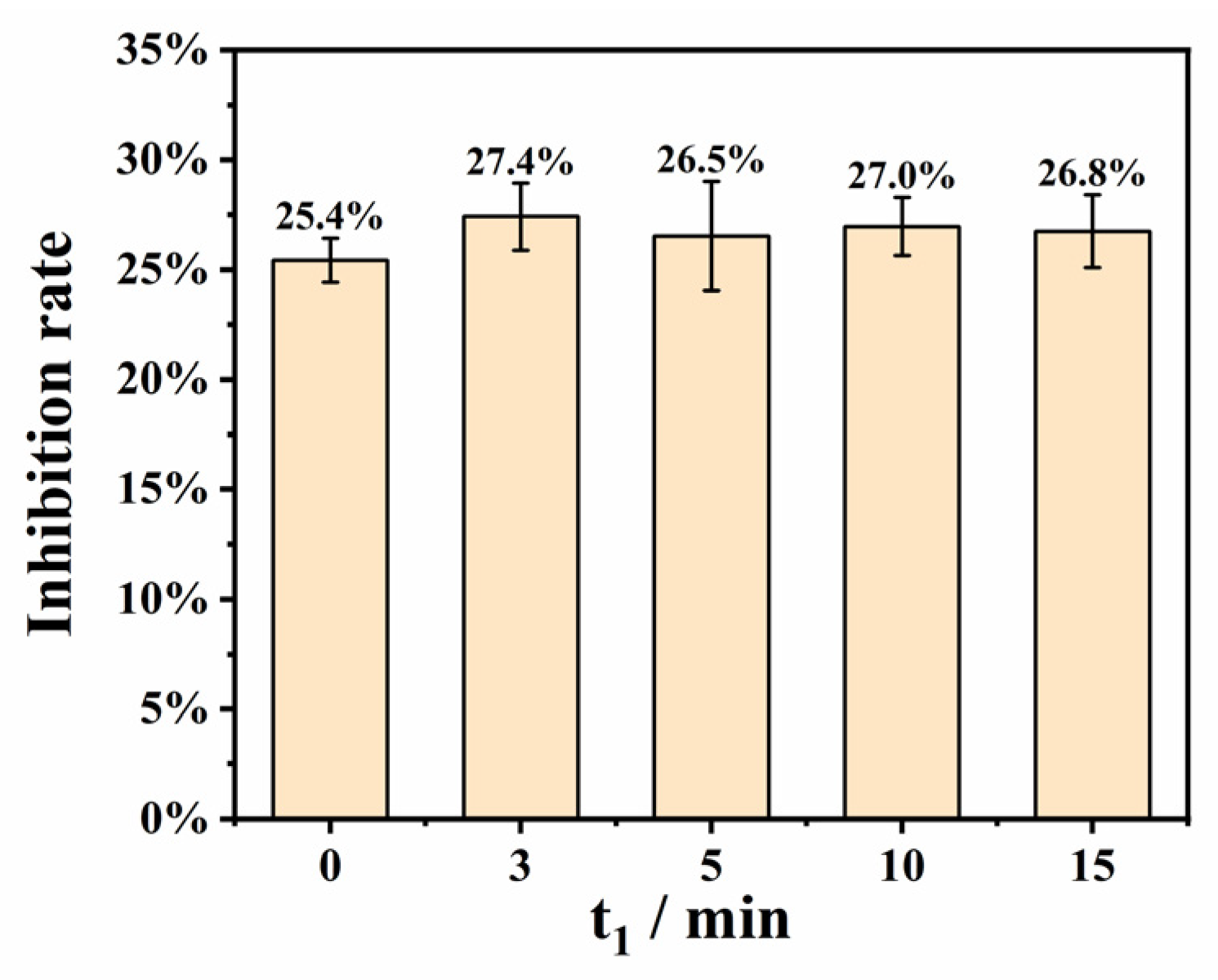
3.3.1. Optimization of the Incubation Time of Lysostaphin and S. aureus (t1)
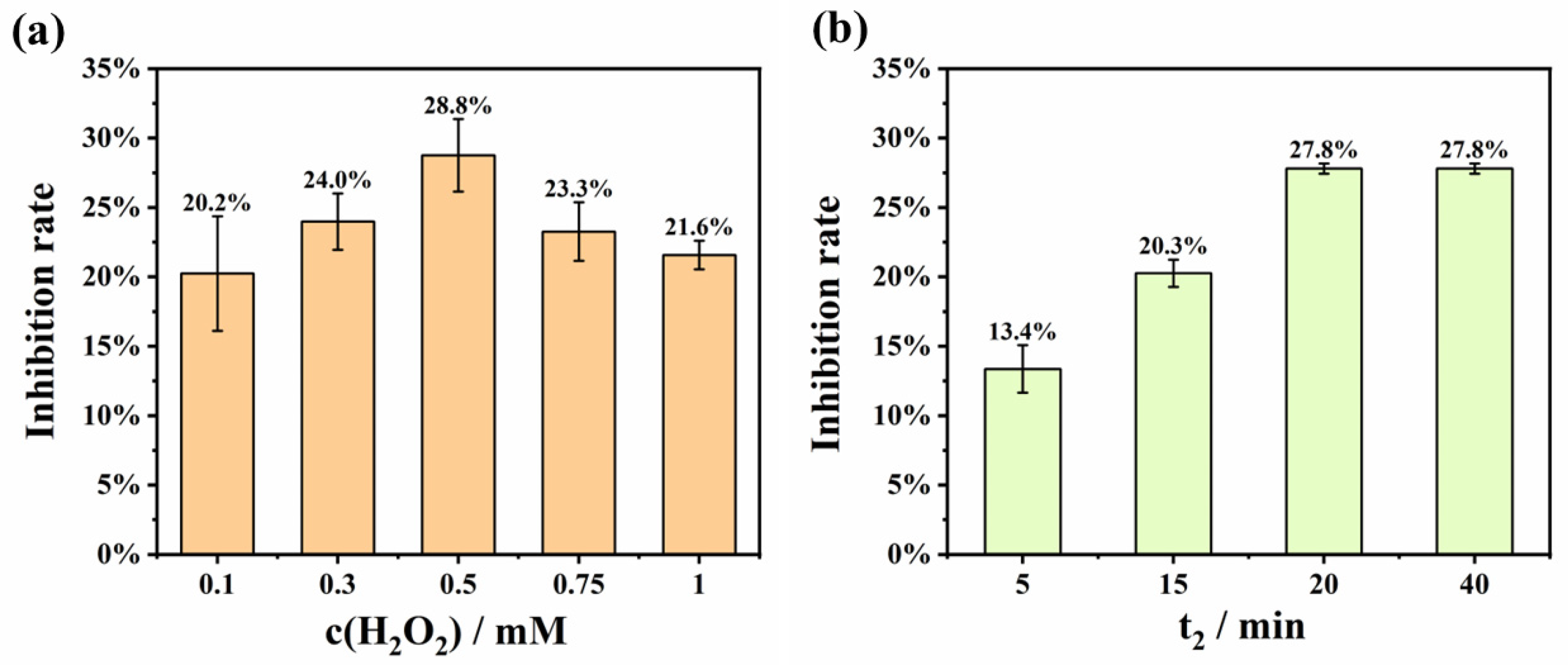
3.3.2. Optimization of H2O2 Concentration and H2O2 Reaction Time (t2)
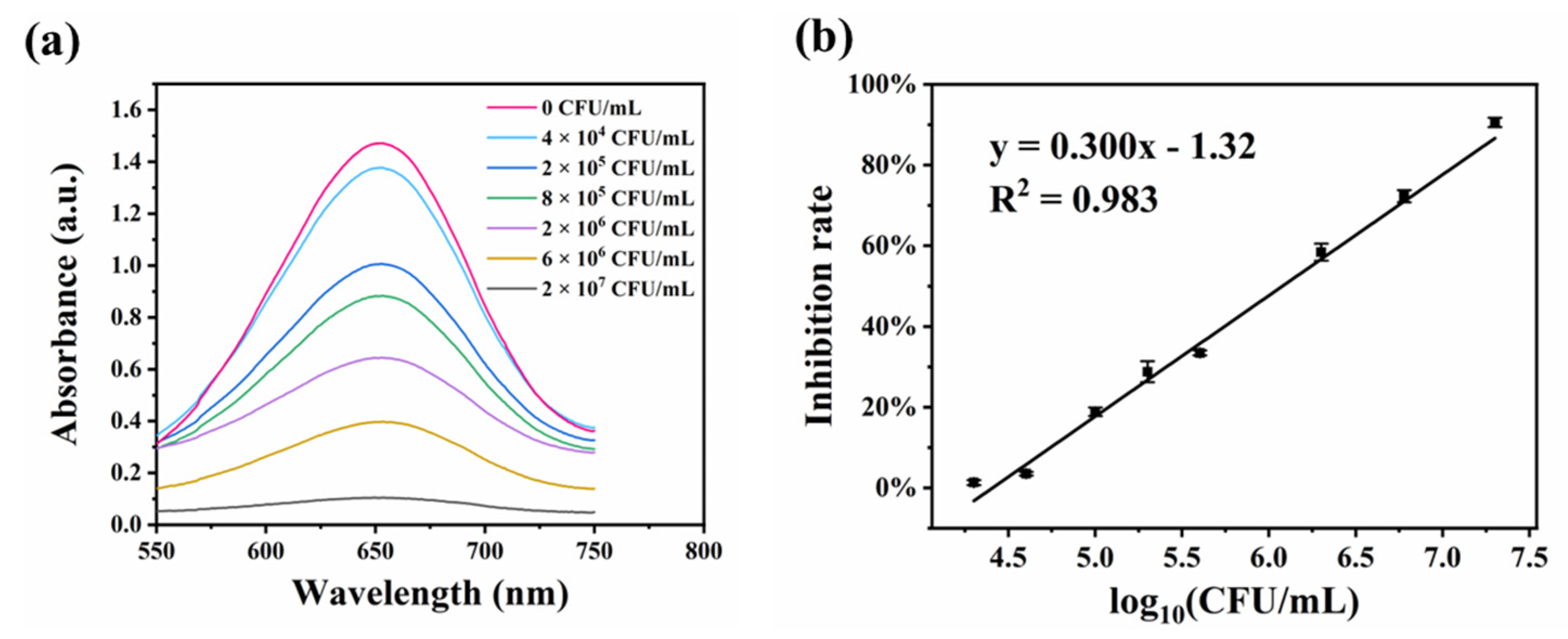
3.4. Application of the As-Prepared Colorimetric Biosensor for S. aureus Detection
3.5. Assessment of Anti-Interference Ability
3.6. Detection of S. aureus in Real Water Samples
3.6.1. Single Bacterial Test
3.6.2. Mixed Bacterial Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed]
- Knox, J.; Uhlemann, A.-C.; Lowy, F.D. Staphylococcus aureus infections: Transmission within households and the community. Trends Microbiol. 2015, 23, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Trilla, A.; Miro, J.M. Identifying high risk patients for Staphylococcus aureus infections: Skin and soft tissue infections. J. Chemother. 1995, 7, 37–43. [Google Scholar] [PubMed]
- Nicolau, D.P.; Marangos, M.N.; Nightingale, C.H.; Quintiliani, R. Influence of aspirin on development and treatment of experimental Staphylococcus aureus endocarditis. Antimicrob. Agents Chemother. 1995, 39, 1748–1751. [Google Scholar] [CrossRef]
- Robertson, L.; Caley, J.P.; Moore, J. Importance of Staphylococcus aureus in pneumonia in the 1957 epidemic of influenza A. Lancet 1958, 272, 233–236. [Google Scholar] [CrossRef]
- Kim, J.H.; van der Horst, C.; Mulrow, C.D.; Corey, G.R. Staphylococcus aureus Meningitis: Review of 28 Cases. Clin. Infect. Dis. 1989, 11, 698–706. [Google Scholar] [CrossRef]
- Solberg, C.O. Spread of Staphylococcus aureus in Hospitals: Causes and prevention. Scand. J. Infect. Dis. 2000, 32, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Strommenger, B.; Layer, F.; Werner, G. Staphylococcus aureus and Methicillin-Resistant Staphylococcus aureus in Workers in the Food Industry. In Staphylococcus aureus; Fetsch, A., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 163–188. [Google Scholar]
- Kadariya, J.; Smith, T.C.; Thapaliya, D. Staphylococcus aureus and Staphylococcal Food-Borne Disease: An Ongoing Challenge in Public Health. BioMed Res. Int. 2014, 2014, 827965. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, C.; Zhong, J.; Jin, S. Research advance in rapid detection of foodborne Staphylococcus aureus. Biotechnol. Biotechnol. Equip. 2016, 30, 827–833. [Google Scholar] [CrossRef]
- Warren, D.K.; Liao, R.S.; Merz, L.R.; Eveland, M.; Dunne, W.M. Detection of Methicillin-Resistant Staphylococcus aureus Directly from Nasal Swab Specimens by a Real-Time PCR Assay. J. Clin. Microbiol. 2004, 42, 5578–5581. [Google Scholar] [CrossRef]
- Kim, J.-K.; Yun, H.-Y.; Kim, J.-S.; Kim, W.; Lee, C.-S.; Kim, B.-G.; Jeong, H.-J. Development of fluorescence-linked immunosorbent assay for rapid detection of Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2023, 108, 2. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.H.; Kim, M.I. Nanomaterial-mediated paper-based biosensors for colorimetric pathogen detection. TrAC Trends Anal. Chem. 2020, 132, 116038. [Google Scholar] [CrossRef]
- Zhang, R.; Belwal, T.; Li, L.; Lin, X.; Xu, Y.; Luo, Z. Nanomaterial-based biosensors for sensing key foodborne pathogens: Advances from recent decades. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1465–1487. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Li, J.; Pang, B.; Wang, X.; Shi, Y.; Song, X.; Xu, K.; Wang, J.; Zhao, C. Colorimetric immunoassay for rapid detection of Staphylococcus aureus based on etching-enhanced peroxidase-like catalytic activity of gold nanoparticles. Microchim. Acta 2020, 187, 504. [Google Scholar] [CrossRef]
- Zhou, W.; Li, Y.; Zhu, G.; Xu, X.; Hu, Q.; Yang, Z.; Gu, X. High-sensitivity and high-specificity colorimetric detection of viable Staphylococcus aureus in ready-to-eat foods using a temperate-bacteriophage-based system with peroxidase-like activity. Sens. Actuators B Chem. 2024, 399, 134810. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, M.; Qiao, W.; Cong, S.; Zhang, Y.; Wang, L.; Hu, Z.; Liu, F.; Wang, D.; Wang, P.; et al. Multicolor colorimetric visual detection of Staphylococcus aureus based on Fe3O4-Ag-MnO2 composites nano-oxidative mimetic enzyme. Anal. Chim. Acta 2023, 1239, 340654. [Google Scholar] [CrossRef] [PubMed]
- Bukharin, O.V.; Cherkasov, S.V.; Sgibnev, A.V.; Zabirova, T.M.; Ivanov, Y.B. Effects of microbial metabolites on catalase activity and growth of Staphylococcus aureus 6538 P. Bull. Exp. Biol. Med. 2000, 130, 679–681. [Google Scholar] [CrossRef] [PubMed]
- Bukharin, O.V.; Sgibnev, A.V.; Cherkasov, S.V.; Ivanov, Y.B. The Effect of the Intra- and Extracellular Metabolites of Microorganisms Isolated from Various Ecotopes on the Catalase Activity of Staphylococcus aureus ATCC 6538 P. Microbiology 2002, 71, 183–186. [Google Scholar] [CrossRef]
- Guarín, P.; Cristancho, J.; Castillo, J.J. Rapid electrochemical detection of Staphylococcus aureus based on screen-printed gold electrodes modified with cysteine and Guinea grass (Panicum maximum) peroxidase. Rev. Acad. Colomb. Cienc. Exactas Físicas Nat. 2020, 44, 835–844. [Google Scholar] [CrossRef]
- Majumdar, T.; Chakraborty, R.; Raychaudhuri, U. Rapid Electrochemical Quantification of Food Borne Pathogen Staphylococcus aureus Based on Hydrogen Peroxide Degradation by Catalase. J. Electrochem. Soc. 2013, 160, G75–G78. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Wu, H.-F. Selective biosensing of Staphylococcus aureus using chitosan quantum dots. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 188, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Gunasekaran, S. Nanozymes-based biosensors for food quality and safety. TrAC Trends Anal. Chem. 2020, 126, 115841. [Google Scholar] [CrossRef]
- Baranwal, A.; Shukla, R.; Bansal, V. Nanozyme-enhanced paper-based biosensor technologies. TrAC Trends Anal. Chem. 2024, 172, 117573. [Google Scholar] [CrossRef]
- Wang, X.; Dong, S.; Wei, H. Recent Advances on Nanozyme-based Electrochemical Biosensors. Electroanalysis 2022, 35, e202100684. [Google Scholar] [CrossRef]
- Everse, J. Heme Proteins. In Encyclopedia of Biological Chemistry, 2nd ed.; Lennarz, W.J., Lane, M.D., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 532–538. [Google Scholar]
- Ye, Y.; Zou, J.; Wu, W.; Wang, Z.; Wen, S.; Liang, Z.; Liu, S.; Lin, Y.; Chen, X.; Luo, T.; et al. Advanced nanozymes possess peroxidase-like catalytic activities in biomedical and antibacterial fields: Review and progress. Nanoscale 2024, 16, 3324–3346. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Sun, X.; Su, D.S.; Centi, G.; Perathoner, S. Catalysis by hybrid sp2/sp3 nanodiamonds and their role in the design of advanced nanocarbon materials. Chem. Soc. Rev. 2018, 47, 8438–8473. [Google Scholar] [CrossRef] [PubMed]
- Zeiger, M.; Jäckel, N.; Mochalin, V.N.; Presser, V. Review: Carbon onions for electrochemical energy storage. J. Mater. Chem. A 2016, 4, 3172–3196. [Google Scholar] [CrossRef]
- Dhand, V.; Yadav, M.; Kim, S.H.; Rhee, K.Y. A comprehensive review on the prospects of multi-functional carbon nano onions as an effective, high-performance energy storage material. Carbon 2021, 175, 534–575. [Google Scholar] [CrossRef]
- Su, D.S.; Maksimova, N.; Delgado, J.J.; Keller, N.; Mestl, G.; Ledoux, M.J.; Schlögl, R. Nanocarbons in selective oxidative dehydrogenation reaction. Catal. Today 2005, 102–103, 110–114. [Google Scholar] [CrossRef]
- Su, D.; Maksimova, N.I.; Mestl, G.; Kuznetsov, V.L.; Keller, V.; Schlögl, R.; Keller, N. Oxidative dehydrogenation of ethylbenzene to styrene over ultra-dispersed diamond and onion-like carbon. Carbon 2007, 45, 2145–2151. [Google Scholar] [CrossRef]
- Szymański, G.S.; Wiśniewski, M.; Olejnik, P.; Koter, S.; Castro, E.; Echegoyen, L.; Terzyk, A.P.; Plonska-Brzezinska, M.E. Correlation between the catalytic and electrocatalytic properties of nitrogen-doped carbon nanoonions and the polarity of the carbon surface: Experimental and theoretical investigations. Carbon 2019, 151, 120–129. [Google Scholar] [CrossRef]
- Butsyk, O.; Olejnik, P.; Romero, E.; Plonska-Brzezinska, M.E. Postsynthetic treatment of carbon nano-onions: Surface modification by heteroatoms to enhance their capacitive and electrocatalytic properties. Carbon 2019, 147, 90–104. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, Q.; Guan, B.; Li, J.; Gao, G.; Zhi, J. Onion-like carbon with controllable carbon atom hybridization embedded in BiVO4 photoanode for enhanced photoelectrochemical water splitting. Appl. Surf. Sci. 2023, 614, 156120. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, Q.; Li, J.; Gao, G.; Zhi, J. Onion-liked carbon-embedded graphitic carbon nitride for enhanced photocatalytic hydrogen evolution and dye degradation. Appl. Catal. B Environ. 2022, 308, 121216. [Google Scholar] [CrossRef]
- Ghanbari, M.H.; Norouzi, Z. A new nanostructure consisting of nitrogen-doped carbon nanoonions for an electrochemical sensor to the determination of doxorubicin. Microchem. J. 2020, 157, 105098. [Google Scholar] [CrossRef]
- Sok, V.; Fragoso, A. Amperometric biosensor for glyphosate based on the inhibition of tyrosinase conjugated to carbon nano-onions in a chitosan matrix on a screen-printed electrode. Microchim. Acta 2019, 186, 569. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kang, E. A graphitic nano-onion/molybdenum disulfide nanosheet composite as a platform for HPV-associated cancer-detecting DNA biosensors. J. Nanobiotechnol. 2023, 21, 187. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Hazmi, M.; Lim, S.A.; Ahmed, M.U. A highly sensitive electrochemical detection of human chorionic gonadotropin on a carbon nano-onions/gold nanoparticles/polyethylene glycol nanocomposite modified glassy carbon electrode. J. Electroanal. Chem. 2019, 833, 462–470. [Google Scholar] [CrossRef]
- Yoon, J.H.; Han, Y.D.; Lee, P.C.; Yoon, H.C. Lysostaphin-mediated fragmentation of microbial peptidoglycans for label-free electrochemical impedance immunoanalysis of Staphylococcus aureus. BioChip J. 2013, 7, 344–352. [Google Scholar] [CrossRef]
- Wang, M.; Fan, E.; Wu, Y.; Fu, Z. Daptomycin-modified magnetic beads integrated with lysostaphin for selective analysis of Staphylococcus. J. Pharm. Biomed. Anal. 2019, 175, 112785. [Google Scholar] [CrossRef]
- Lee, H.Y.; Atlasevich, N.; Granzotto, C.; Schultz, J.; Loike, J.; Arslanoglu, J. Development and application of an ELISA method for the analysis of protein-based binding media of artworks. Anal. Methods 2015, 7, 187–196. [Google Scholar] [CrossRef]
- Li, M.; Su, H.; Tu, Y.; Shang, Y.; Liu, Y.; Peng, C.; Liu, H. Development and Application of an Efficient Medium for Chromogenic Catalysis of Tetramethylbenzidine with Horseradish Peroxidase. ACS Omega 2019, 4, 5459–5470. [Google Scholar] [CrossRef]
- Ma, W.; Xue, Y.; Guo, S.; Jiang, Y.; Wu, F.; Yu, P.; Mao, L. Graphdiyne oxide: A new carbon nanozyme. Chem. Commun. 2020, 56, 5115–5118. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhen, M.; Guan, M.; Chen, D.; Zhang, G.; Ge, J.; Gong, P.; Wang, C.; Shu, C. A novel glucose colorimetric sensor based on intrinsic peroxidase-like activity of C60-carboxyfullerenes. Biosens. Bioelectron. 2013, 47, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Qu, K.; Zhao, C.; Ren, J.; Qu, X. Graphene Oxide: Intrinsic Peroxidase Catalytic Activity and Its Application to Glucose Detection. Adv. Mater. 2010, 22, 2206–2210. [Google Scholar] [CrossRef]
- Sepasi Tehrani, H.; Moosavi-Movahedi, A.A. Catalase and its mysteries. Prog. Biophys. Mol. Biol. 2018, 140, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, J.; Mehandia, S.; Singh, G.; Raina, A.; Arya, S.K. Catalase enzyme: Application in bioremediation and food industry. Biocatal. Agric. Biotechnol. 2018, 16, 192–199. [Google Scholar] [CrossRef]
- Galasso, M.; Gambino, S.; Romanelli, M.G.; Donadelli, M.; Scupoli, M.T. Browsing the oldest antioxidant enzyme: Catalase and its multiple regulation in cancer. Free Radic. Biol. Med. 2021, 172, 264–272. [Google Scholar] [CrossRef]
- Kirkman, H.N.; Gaetani, G.F. Mammalian catalase: A venerable enzyme with new mysteries. Trends Biochem. Sci. 2007, 32, 44–50. [Google Scholar] [CrossRef]
- Josephy, P.D.; Eling, T.; Mason, R.P. The horseradish peroxidase-catalyzed oxidation of 3,5,3′,5′-tetramethylbenzidine. Free radical and charge-transfer complex intermediates. J. Biol. Chem. 1982, 257, 3669–3675. [Google Scholar] [CrossRef]


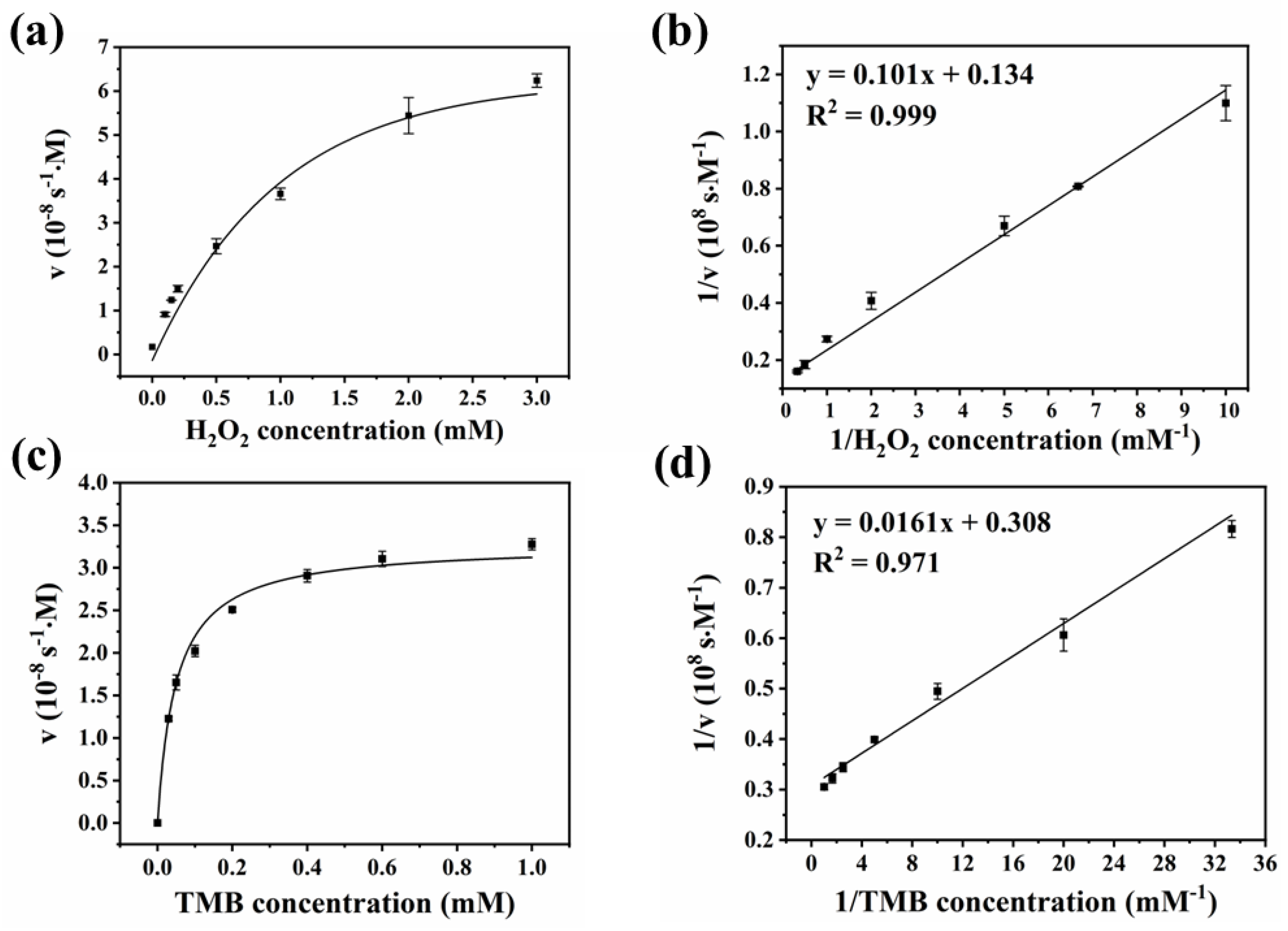


| Nanozyme | KM (mM) | Vmax (10−8 M s−1) | Reference | ||
|---|---|---|---|---|---|
| H2O2 | TMB | H2O2 | TMB | ||
| graphdiyne oxide | 2.59 | 0.62 | 1.77 | 1.92 | [45] |
| C60[C(COOH)2]2 | 24.58 | 0.23 | 0.40 | 0.35 | [46] |
| GO-COOH | 3.99 | 0.02 | 3.85 | 3.45 | [47] |
| OLC | 0.77 | 0.05 | 7.48 | 3.25 | This work |
| Materials | OLC | Nanodiamonds | Fe3O4 Nanoparticles |
|---|---|---|---|
| Absorbance of experimental group | 1.18 | 0.13 | 0.45 |
| Absorbance of blank group | 1.45 | 0.14 | 0.53 |
| Inhibition rates | 18.6% | 7.1% | 15.1% |
| Spiked Concentration of S. aureus (CFU/mL) | Inhibition Rate | Found Concentration (CFU/mL) | Recovery Rate | RSD |
|---|---|---|---|---|
| 5 × 104 | 9.14% | 5.16 × 104 | 103.20% | 2.71% |
| 2 × 105 | 27.16% | 2.06 × 105 | 103.00% | 6.45% |
| 2 × 107 | 88.17% | 2.24 × 107 | 112.00% | 3.94% |
| Inhibition Rate | RSD | |
|---|---|---|
| E. coli | 3.74% | 0.97% |
| B. subtilis | 1.25% | 0.55% |
| Spiked Samples | Inhibition Rate | Found Concentration (CFU/mL) | Recovery Rate | RSD |
|---|---|---|---|---|
| S. aureus + E. coli | 26.93% | 2.03 × 105 | 101.50% | 4.94% |
| S. aureus + B. subtilis | 26.20% | 1.92 × 105 | 96.00% | 2.42% |
| S. aureus + E. coli + B. subtilis | 26.14% | 1.91 × 105 | 95.50% | 4.00% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Gao, G.; Zhi, J. A Novel Colorimetric Biosensor for the Detection of Catalase-Positive Staphylococcus aureus Based on an Onion-like Carbon Nanozyme. Chemosensors 2024, 12, 176. https://doi.org/10.3390/chemosensors12090176
Fan Y, Gao G, Zhi J. A Novel Colorimetric Biosensor for the Detection of Catalase-Positive Staphylococcus aureus Based on an Onion-like Carbon Nanozyme. Chemosensors. 2024; 12(9):176. https://doi.org/10.3390/chemosensors12090176
Chicago/Turabian StyleFan, Yining, Guanyue Gao, and Jinfang Zhi. 2024. "A Novel Colorimetric Biosensor for the Detection of Catalase-Positive Staphylococcus aureus Based on an Onion-like Carbon Nanozyme" Chemosensors 12, no. 9: 176. https://doi.org/10.3390/chemosensors12090176





