Chemiluminescence Immunoassay for Sensitive Detection of C-reactive Protein Using Graphene Oxide–Gold Nanoparticle–Luminol Hybrids as Enhanced Luminogenic Molecules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
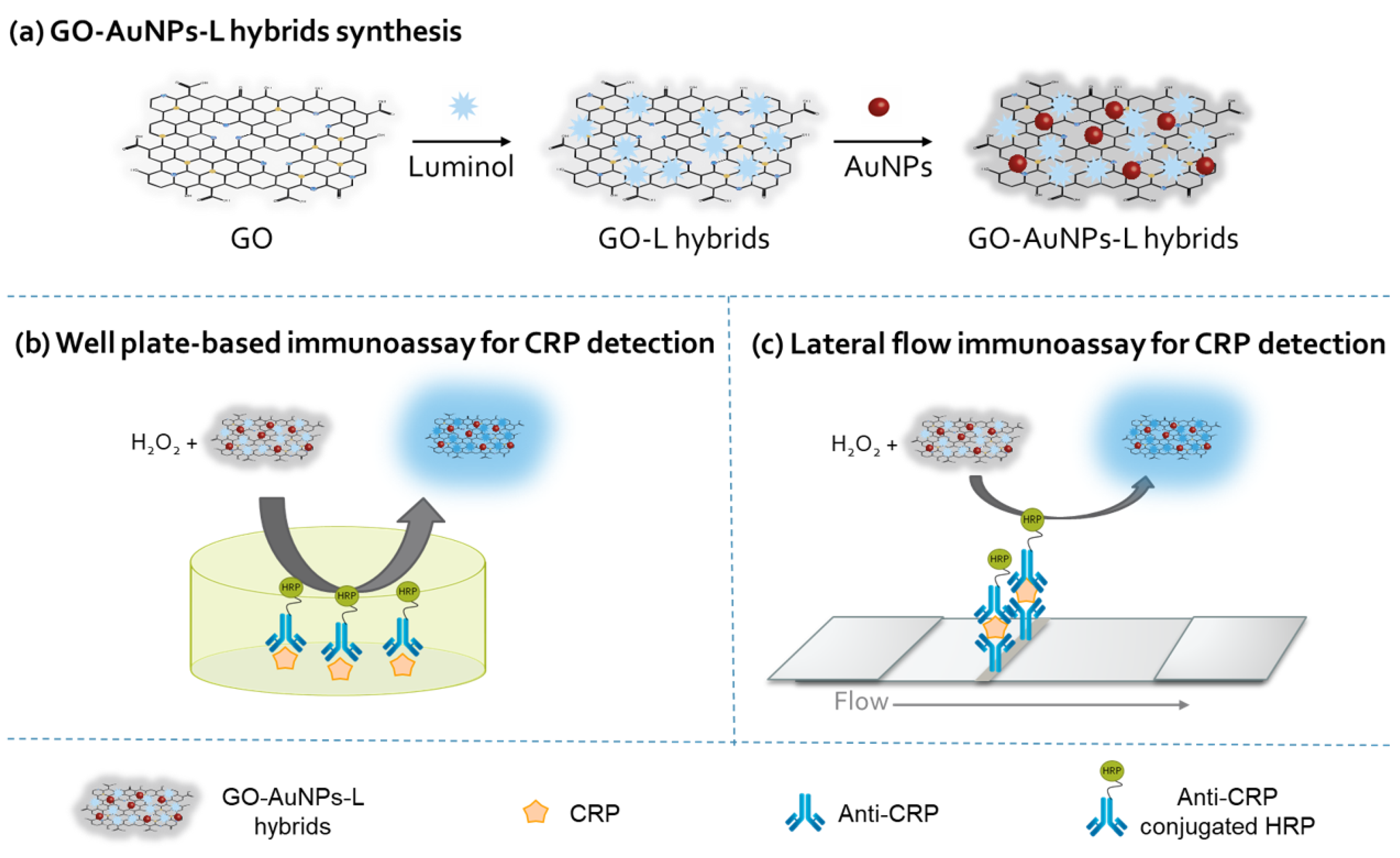
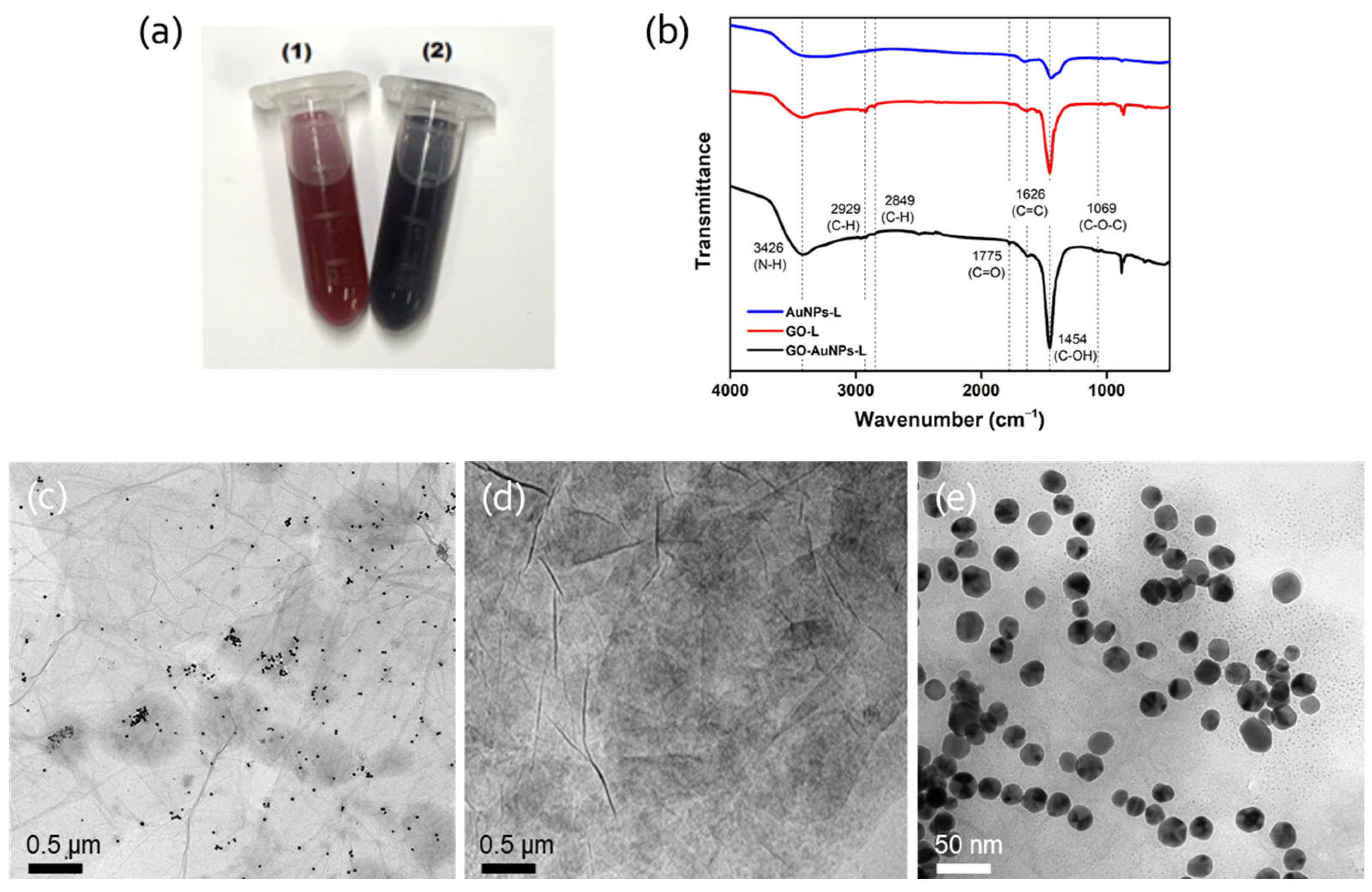
2.2. Synthesis and Characterization of GO-AuNPs-L Hybrids
2.3. CL Measurements
2.4. CL Immunoassays for CRP Detection
2.4.1. Well Plate-Based Immunoassay for CRP
2.4.2. LFIA for CRP
3. Results and Discussion
3.1. Construction of GO-AuNPs-L Hybrids
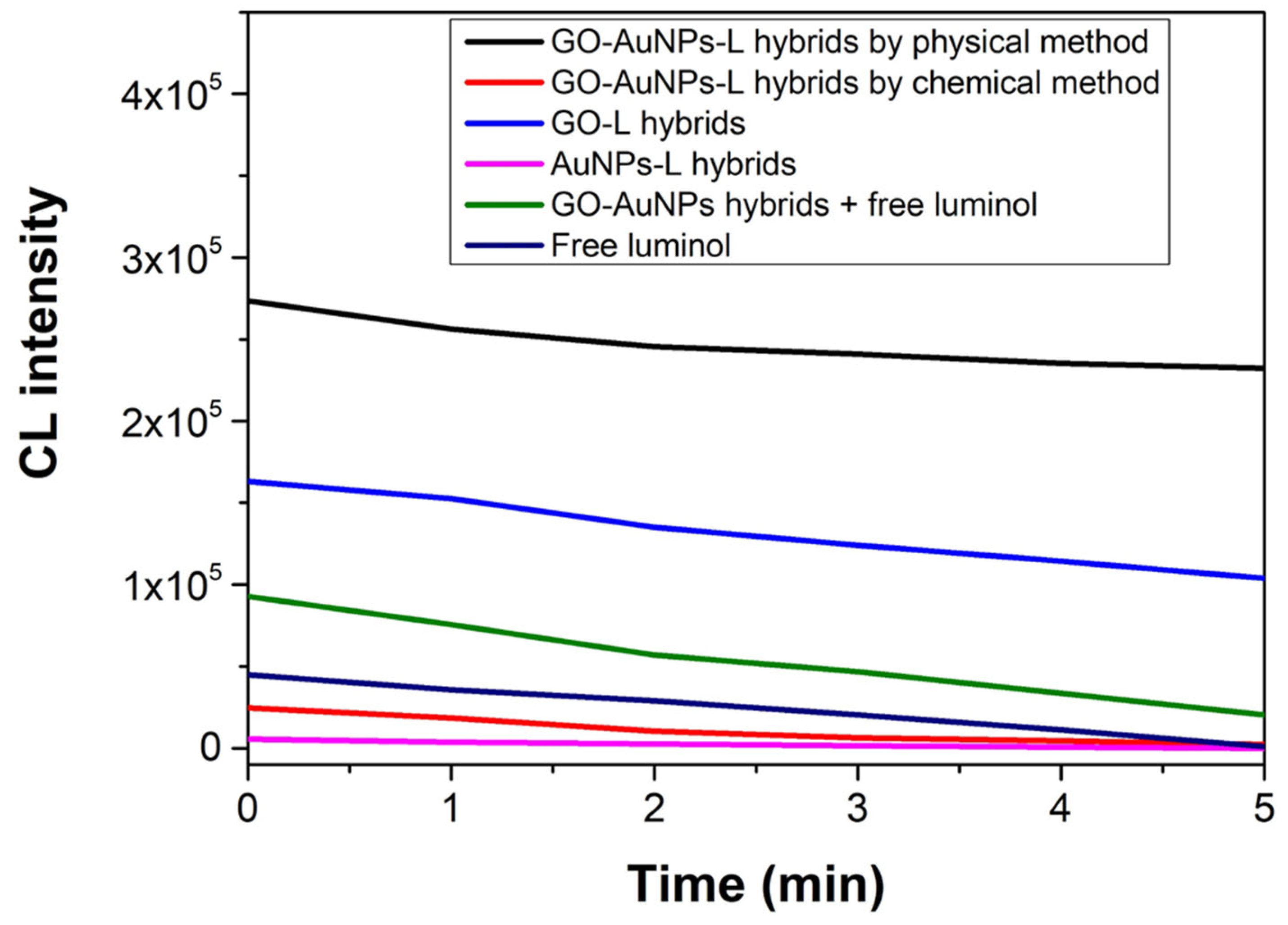
3.2. Enhanced CL of GO-AuNPs-L Hybrids
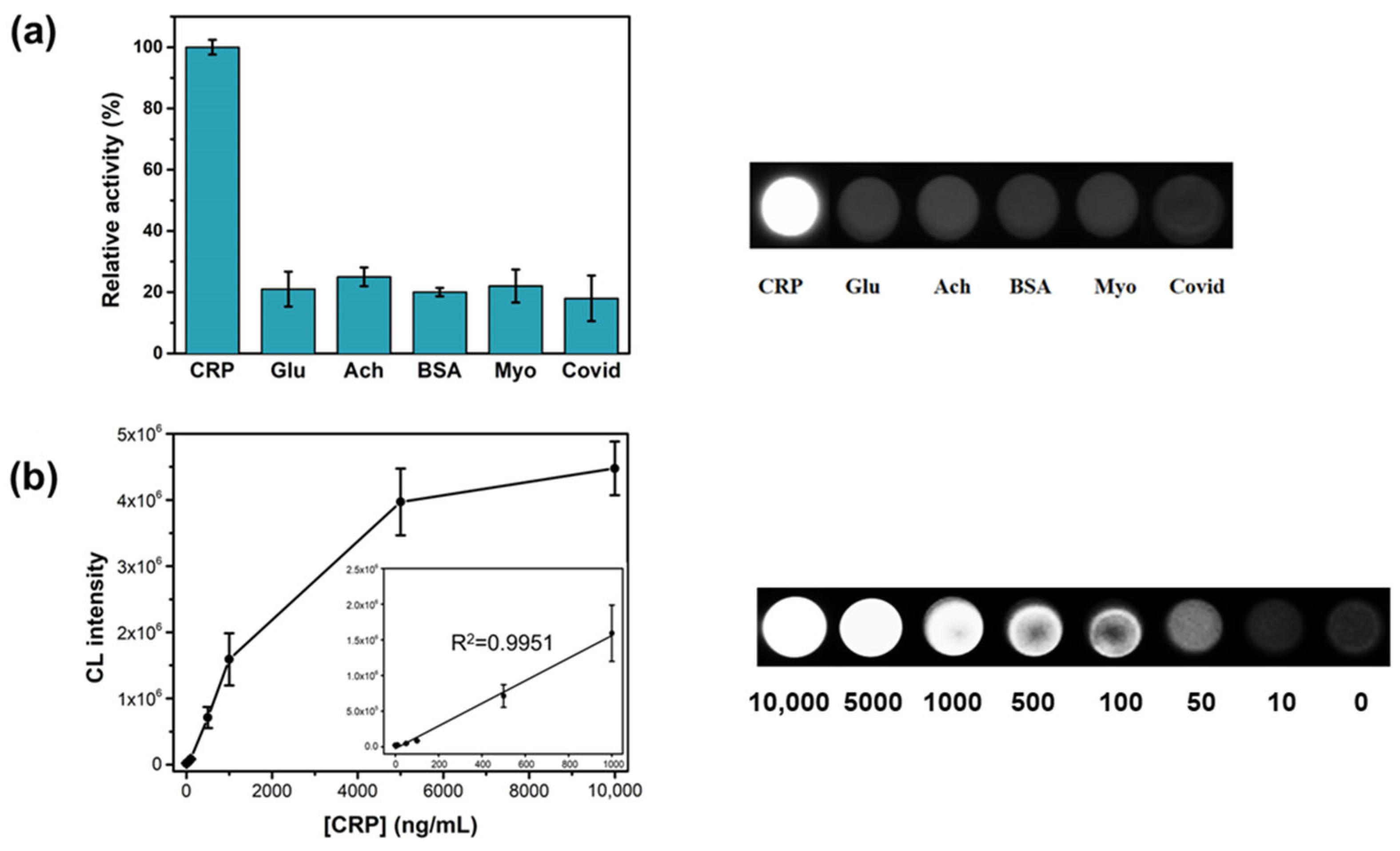
3.3. CL Immunoassay for CRP Using GO-AuNPs-L Hybrids as Luminogenic Reagents
3.3.1. Well Plate-Based Immunoassay for CRP
3.3.2. LFIA for CRP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ridker, P.M.; Glynn, R.J.; Hennekens, C.H. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. Circulation 1998, 97, 2007–2011. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M. High-sensitivity C-reactive protein, inflammation, and cardiovascular risk: From concept to clinical practice to clinical benefit. Am. Heart J. 2004, 148, S19–S26. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, T.; Quispe, R.; Das, T.; Juraschek, S.P.; Martin, S.S.; Michos, E.D. The Use of Blood Biomarkers in Precision Medicine for the Primary Prevention of Atherosclerotic Cardiovascular Disease: A Review. Expert. Rev. Precis. Med. Drug Dev. 2021, 6, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.Y.; Heo, N.S.; Kang, J.W.; Lee, J.B.; Kim, H.J.; Kim, M.I. Nanoceria-based lateral flow immunoassay for hydrogen peroxide-free colorimetric biosensing for C-reactive protein. Anal. Bioanal. Chem. 2022, 414, 3257–3265. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. Diagnoses based on C-reactive protein point-of-care tests. Biosensors 2022, 12, 344. [Google Scholar] [CrossRef]
- Siddiqui, M.F.; Khan, Z.A.; Park, S. Detection of C-reactive protein using histag-HRP functionalized nanoconjugate with signal amplified immunoassay. Nanomaterials 2020, 10, 1240. [Google Scholar] [CrossRef]
- Zhang, Z.; Lai, J.; Wu, K.; Huang, X.; Guo, S.; Zhang, L.; Liu, J. Peroxidase-catalyzed chemiluminescence system and its application in immunoassay. Talanta 2018, 180, 260–270. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, X.; Xiong, S.; Li, X.; Zhan, S.; Zeng, L.; Xiong, Y. Dual-mode fluorescent and colorimetric immunoassay for the ultrasensitive detection of alpha-fetoprotein in serum samples. Anal. Chim. Acta 2018, 1038, 112–119. [Google Scholar] [CrossRef]
- Darwish, I.A.; Alzoman, N.Z.; Khalil, N.N. A Novel Highly Sensitive Chemiluminescence Enzyme Immunoassay with Signal Enhancement Using Horseradish Peroxidase-Luminol-Hydrogen Peroxide Reaction for the Quantitation of Monoclonal Antibodies Used for Cancer Immunotherapy. Chemosensors 2023, 11, 245. [Google Scholar] [CrossRef]
- Liu, Z.J.; Wang, X.Y.; Ren, X.X.; Li, W.B.; Sun, J.F.; Wang, X.W.; Huang, Y.Q.; Guo, Y.G.; Zeng, H.W. Novel Fluorescence Immunoassay for the Detection of Zearalenone Using Hrp-Mediated Fluorescence Quenching of Gold-Silver Bimetallic Nanoclusters. Food Chem. 2021, 355, 129633. [Google Scholar] [CrossRef]
- Zhang, C.; Su, Y.; Liang, Y.; Lai, W.; Jiang, J.; Wu, H.; Mao, X.; Zheng, L.; Zhang, R. Chemiluminescence and Its Biomedical Applications. In Nanophotonics in Biomedical Engineering; Zhao, X., Lu, M., Eds.; Springer: Singapore, 2021; pp. 143–195. [Google Scholar]
- Li, F.; You, M.; Li, S.; Hu, J.; Liu, C.; Gong, Y.; Yang, H.; Xu, F. Paper based point-of-care immunoassays: Recent advances and emerging trends. Biotechnol. Adv. 2020, 39, 107442. [Google Scholar] [CrossRef]
- Zhao, L.; Xu, J.; Xiong, L.; Wang, S.; Yu, C.; Lv, J.; Lin, J.-M. Recent development of chemiluminescence for bioanalysis. TrAC Trends Anal. Chem. 2023, 166, 117213. [Google Scholar] [CrossRef]
- Islam, M.S.; Kang, S.H. Chemiluminescence detection of label-free C-reactive protein based on catalytic activity of gold nanoparticles. Talanta 2011, 84, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhang, B.; Chen, M.; Jiang, T.; Zhou, D.; Huang, J.; Fu, W. Sensitive and rapid quantification of C-reactive protein using quantum dot-labeled microplate immunoassay. J. Transl. Med. 2012, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Ghavamipour, F.; Rahmani, H.; Shanehsaz, M.; Khajeh, K.; Mirshahi, M.; Sajedi, R.H. Enhanced Sensitivity of VEGF Detection Using Catalase-Mediated Chemiluminescence Immunoassay Based on CdTe QD/H2O2 System. J. Nanobiotechnol. 2020, 18, 93. [Google Scholar] [CrossRef]
- Al Yahyai, I.; Al-Lawati, H.A. A review of recent developments based on chemiluminescence detection systems for pesticides analysis. Luminescence 2021, 36, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Wang, W.; Wang, Z.; Cao, X.; Zhu, J.; Niu, L.; Wu, X.; Jiang, H.; Shen, J. Development of a highly sensitive chemiluminescence enzyme immunoassay using enhanced luminol as substrate. Luminescence 2014, 29, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Khan, P.; Idrees, D.; Moxley, M.A.; Corbett, J.A.; Ahmad, F.; von Figura, G.; Sly, W.S.; Waheed, A.; Hassan, M.I. Luminol-based chemiluminescent signals: Clinical and non-clinical application and future uses. Appl. Biochem. Biotechnol. 2014, 173, 333–355. [Google Scholar] [CrossRef]
- Lin, K.L.; Yang, T.; Zhang, F.F.; Lei, G.; Zou, H.Y.; Li, Y.F.; Huang, C.Z. Luminol and gold nanoparticle-co-precipitated reduced graphene oxide hybrids with long-persistent chemiluminescence for cholesterol detection. J. Mater. Chem. B 2017, 5, 7335–7341. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Q.; Shen, Q.; Liu, X.; Li, W.; Nie, Z.; Yao, S. Nitrogen doped graphene quantum dots based long-persistent chemiluminescence system for ascorbic acid imaging. Biosens. Bioelectron. 2017, 91, 878–884. [Google Scholar] [CrossRef]
- Gao, J.-W.; Chen, M.-M.; Wen, W.; Zhang, X.; Wang, S.; Huang, W.-H. Au-Luminol-decorated porous carbon nanospheres for the electrochemiluminescence biosensing of MUC1. Nanoscale 2019, 11, 16860–16867. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Huang, X.; Deng, Y.; Chen, H.; Fan, M.; Gong, Z. Applications of nanomaterial-based chemiluminescence sensors in environmental analysis. Trac-Trend. Anal. Chem. 2022, 158, 116879. [Google Scholar] [CrossRef]
- Teng, X.; Qi, L.; Liu, T.; Li, L.; Lu, C. Nanomaterial-based chemiluminescence systems for tracing of reactive oxygen species in biosensors. Trac-Trend. Anal. Chem. 2023, 162, 117020. [Google Scholar] [CrossRef]
- Jiang, X.; Ruan, G.; Huang, Y.; Chen, Z.; Yuan, H.; Du, F. Assembly and application advancement of organic-functionalized graphene-based materials: A review. J. Sep. Sci. 2020, 43, 1544–1557. [Google Scholar] [CrossRef] [PubMed]
- Shahriari, S.; Sastry, M.; Panjikar, S.; Singh Raman, R. Graphene and Graphene Oxide as a Support for Biomolecules in the Development of Biosensors. Nanotechnol. Sci. Appl. 2021, 14, 197–220. [Google Scholar] [CrossRef]
- Gosai, A.; Khondakar, K.R.; Ma, X.; Ali, M.A. Application of functionalized graphene oxide based biosensors for health monitoring: Simple graphene derivatives to 3D printed platforms. Biosensors 2021, 11, 384. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, R.; Liu, B.; Wang, J.; Wang, S.; Han, M.Y.; Zhang, Z. π-Conjugated carbon radicals at graphene oxide to initiate ultrastrong chemiluminescence. Angew. Chem. Int. Ed. 2014, 53, 10109–10113. [Google Scholar] [CrossRef]
- Shams, N.; Lim, H.N.; Hajian, R.; Yusof, N.A.; Abdullah, J.; Sulaiman, Y.; Ibrahim, I.; Huang, N.M. Electrochemical sensor based on gold nanoparticles/ethylenediamine-reduced graphene oxide for trace determination of fenitrothion in water. RSC Adv. 2016, 6, 89430–89439. [Google Scholar] [CrossRef]
- Lou, F.; Wang, A.; Jin, J.; Li, Q.; Zhang, S. One-pot synthesis of popcorn-like Au@Polyluminol nanoflowers for sensitive solid-state electrochemiluminescent sensor. Electrochim. Acta 2018, 278, 255–262. [Google Scholar] [CrossRef]
- Guo, J.; Chen, S.; Tian, S.; Liu, K.; Ma, X.; Guo, J. A sensitive and quantitative prognosis of C-reactive protein at picogram level using mesoporous silica encapsulated core-shell up-conversion nanoparticle based lateral flow strip assay. Talanta 2021, 230, 122335. [Google Scholar] [CrossRef]
- Jin, B.; Du, Z.; Zhang, C.; Yu, Z.; Wang, X.; Hu, J.; Li, Z. Eu-chelate polystyrene microsphere-based lateral flow immunoassay platform for hs-CRP detection. Biosensors 2022, 12, 977. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Zhou, S.; Chen, T.; Li, J.; Shen, H.; Chai, Y.; Li, L.S. Quantitative and rapid detection of C-reactive protein using quantum dot-based lateral flow test strip. Anal. Chim. Acta 2018, 1008, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ren, X.; Chen, L.; Zou, J.; Li, T.; Tan, L.; Fu, C.; Wu, Q.; Li, C.; Wang, J.; et al. Fluorescent hollow ZrO2@CdTe nanoparticles-based lateral flow assay for simultaneous detection of C-reactive protein and troponin T. Microchim. Acta 2021, 188, 209. [Google Scholar] [CrossRef] [PubMed]
- Kokorina, A.A.; Rashchevskaya, R.O.; Goryacheva, I.Y. Nets of biotin-derived gold nanoparticles as a label for the C-reactive protein immunoassay. Anal. Bioanal. Chem. 2021, 413, 6867–6875. [Google Scholar] [CrossRef]
- Panferov, V.G.; Byzova, N.A.; Zherdev, A.V.; Dzantiev, B.B. Peroxidase-mimicking nanozyme with surface-dispersed Pt atoms for the colorimetric lateral flow immunoassay of C-reactive protein. Microchim. Acta 2021, 188, 309. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.M.; Nguyen, P.T.; Kim, J.; Song, S.H.; Park, J.W.; Kim, M.I. Chemiluminescence Immunoassay for Sensitive Detection of C-reactive Protein Using Graphene Oxide–Gold Nanoparticle–Luminol Hybrids as Enhanced Luminogenic Molecules. Chemosensors 2024, 12, 193. https://doi.org/10.3390/chemosensors12090193
Kim KM, Nguyen PT, Kim J, Song SH, Park JW, Kim MI. Chemiluminescence Immunoassay for Sensitive Detection of C-reactive Protein Using Graphene Oxide–Gold Nanoparticle–Luminol Hybrids as Enhanced Luminogenic Molecules. Chemosensors. 2024; 12(9):193. https://doi.org/10.3390/chemosensors12090193
Chicago/Turabian StyleKim, Kyung Mi, Phuong Thy Nguyen, Jeemin Kim, Seung Hoo Song, Jin Woo Park, and Moon Il Kim. 2024. "Chemiluminescence Immunoassay for Sensitive Detection of C-reactive Protein Using Graphene Oxide–Gold Nanoparticle–Luminol Hybrids as Enhanced Luminogenic Molecules" Chemosensors 12, no. 9: 193. https://doi.org/10.3390/chemosensors12090193





