Optimization by Hydrogen Plasma Treatment of a-CH and Hydrogen/Nitrogen-Assisted a-CH Layers for SAW Sensors
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasma Synthesis of a-CH Thin Films
2.2. Characterization of the Deposited a-CH Layers
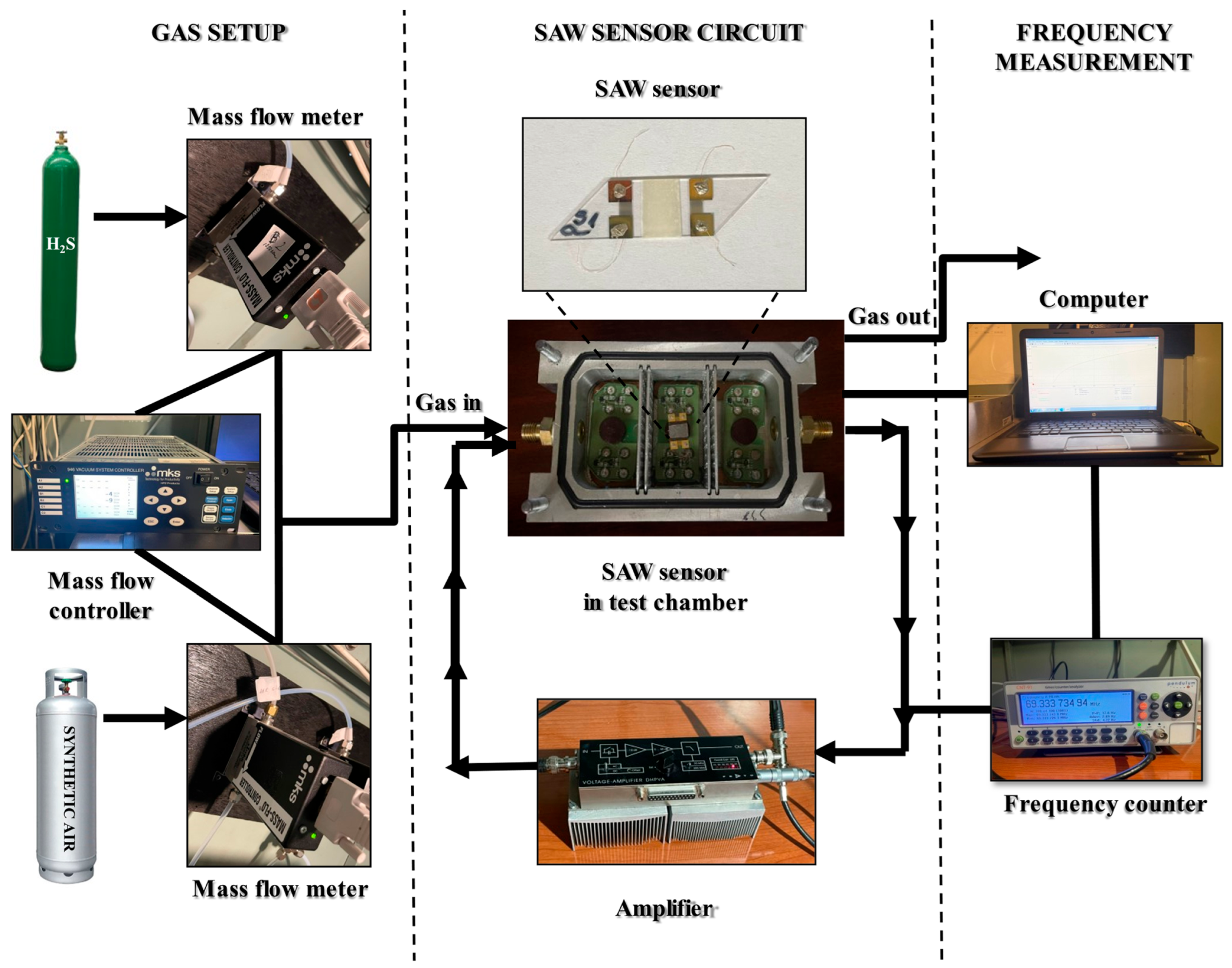
2.3. Sensor Structure and Testing
3. Results and Discussion
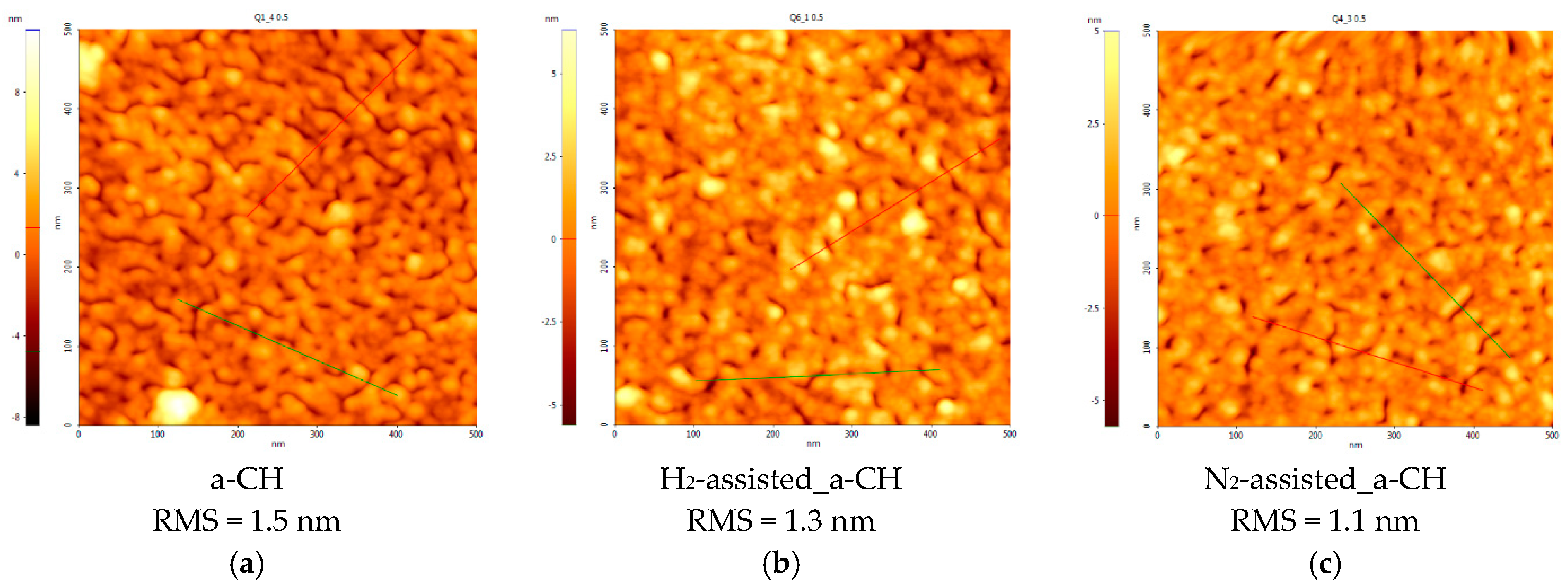
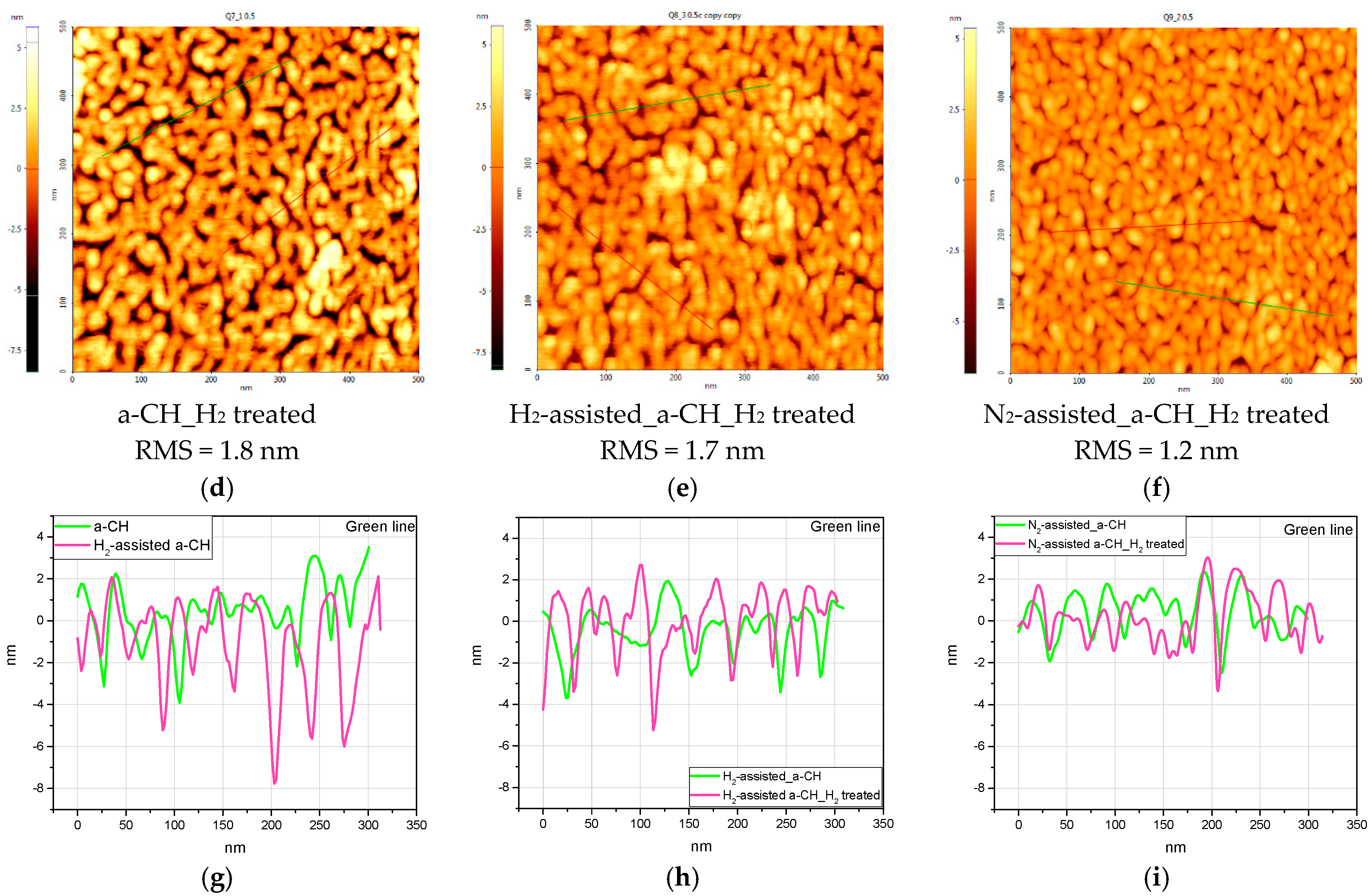
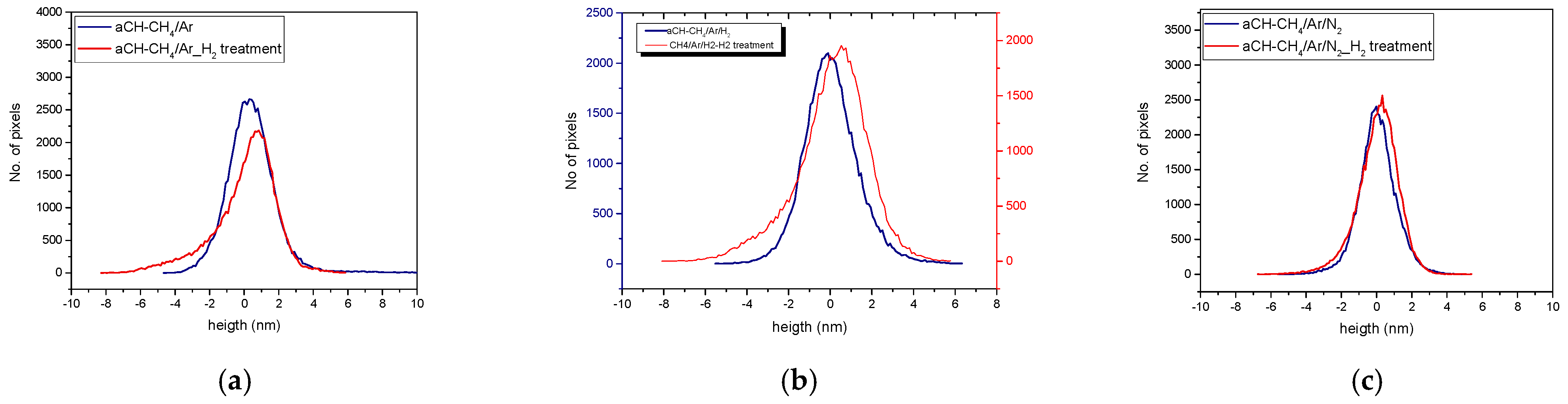
3.1. Topographical Characterization
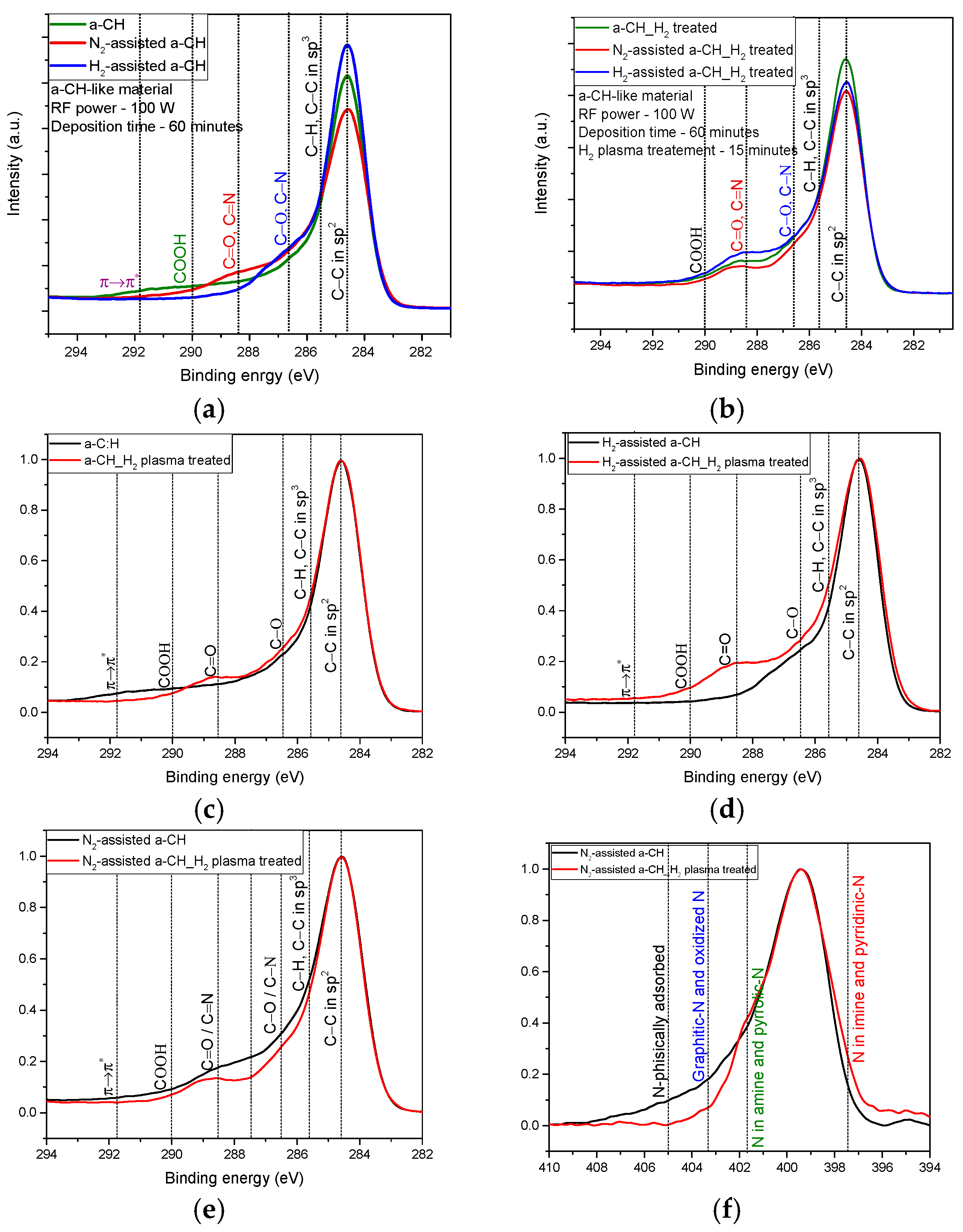
3.2. Chemical Composition
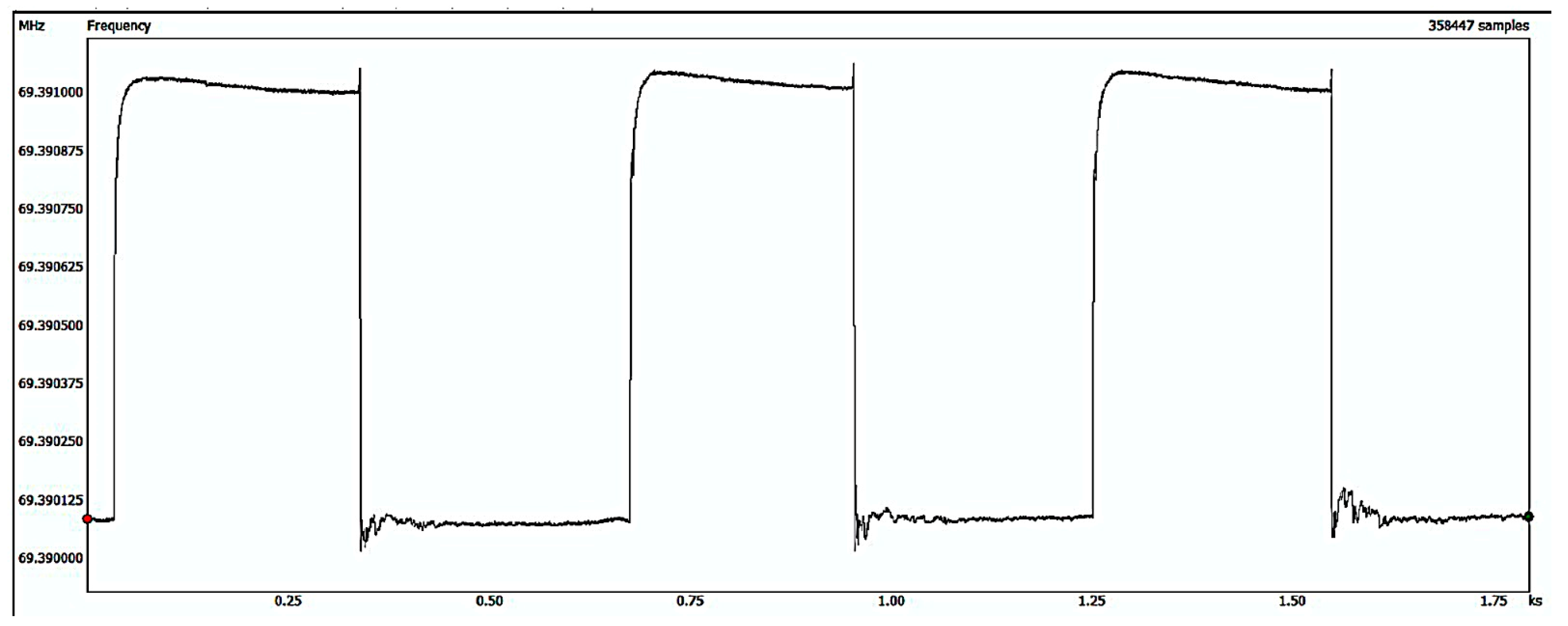
3.3. Gas Sensing Performance of the Sensors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Public Health England. Compendium of Chemical Hazards: Hydrogen Sulphide, Hydrogen Sulphide Toxicological Overview; Public Health England: London, UK, 2016; p. 2014790. [Google Scholar]
- Minier-Matar, J.; Janson, A.; Hussain, A.; Adham, S. Application of Membrane Contactors to Remove Hydrogen Sulfide from Sour Water. J. Membr. Sci. 2017, 541, 378–385. [Google Scholar] [CrossRef]
- Peluso, A.; Gargiulo, N.; Aprea, P.; Pepe, F.; Caputo, D. Nanoporous Materials as H2S Adsorbents for Biogas Purification: A Review. Sep. Purif. Rev. 2018, 48, 78–89. [Google Scholar] [CrossRef]
- Coe, L.C.C. Ambient Hydrogen Sulfide Levels at a Wastewater Treatment Plant. Environ. Monit. Assess. 1985, 5, 101–108. [Google Scholar] [CrossRef]
- Jiang, J.; Chan, A.; Ali, S.; Moon, C.; Vasdev, S.; Mantha, S.; Kartha, V.; Mukherjee, S.D. Hydrogen Sulfide—Mechanisms of Toxicity and Development of an Antidote. Sci. Rep. 2016, 6, 20831. [Google Scholar] [CrossRef]
- Wu, W.; Long, J.; Guo, Y.; Zu, X.; Li, S.; Xiang, X. P-CuO/n-TiO2 Heterojunction Nanostructure-Based Surface Acoustic Wave Sensor with Strong Electric Loading Effect for Highly Sensitive H2S Gas Sensing. Sens. Actuators B Chem. 2023, 394, 134380. [Google Scholar] [CrossRef]
- Li, D.; Wang, L.; Zhao, Z.; Ma, J.; Liu, Y.; Zhang, H.; Ma, J. High Humidity Enhanced Surface Acoustic Wave (SAW) H2S Sensors Based on Sol–Gel CuO Films. Sens. Actuators B Chem. 2019, 294, 55–61. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, W.; Wang, B.; Dai, X.; Xie, W.; Yang, Y.; Zhang, R.; Shi, X.; Zhu, H.; Luo, J.; et al. H2S Gas Sensing Performance and Mechanisms Using CuO-Al2O3 Composite Films Based on Both Surface Acoustic Wave and Chemiresistor Techniques. Sens. Actuators B Chem. 2020, 325, 128742. [Google Scholar] [CrossRef]
- Shin, J.; Lee, G.; Choi, M.; Jang, H.; Lim, Y.; Kim, G.-S.; Nam, S.-H.; Baek, S.-H.; Song, H.-C.; Kim, J.; et al. Atomically Mixed Catalysts on a 3D Thin-Shell TiO2 for Dual-Modal Chemical Detection and Neutralization. J. Mater. Chem. A 2023, 11, 18195–18206. [Google Scholar] [CrossRef]
- Maier, K.; Helwig, A.; Müller, G.; Hille, P.; Eickhoff, M. Effect of Water Vapor and Surface Morphology on the Low Temperature Response of Metal Oxide Semiconductor Gas Sensors. Materials 2015, 8, 6570–6588. [Google Scholar] [CrossRef]
- Constantinoiu, I.; Viespe, C. Hydrogen Detection with SAW Polymer/Quantum Dots Sensitive Films. Sensors 2019, 19, 4481. [Google Scholar] [CrossRef]
- Abdullah, A.N.; Kamarudin, K.; Kamarudin, L.M.; Adom, A.H.; Mamduh, S.M.; Mohd Juffry, Z.H.; Bennetts, V.H. Correction Model for Metal Oxide Sensor Drift Caused by Ambient Temperature and Humidity. Sensors 2022, 22, 3301. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Zhang, S.; Li, J.; Gao, N.; Tong, K. Mid-Infrared Tunable Laser-Based Broadband Fingerprint Absorption Spectroscopy for Trace Gas Sensing: A Review. Appl. Sci. 2019, 9, 338. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, S.; Liu, J.; Sun, Y.; Zhang, X. Pd/Ni Nanowire Film Coated SAW Hydrogen Sensor with Fast Response. Sens. Actuators B Chem. 2021, 345, 130952. [Google Scholar] [CrossRef]
- Liu, X.; Wang, W.; Zhang, Y.; Pan, Y.; Liang, Y.; Li, J. Enhanced Sensitivity of a Hydrogen Sulfide Sensor Based on Surface Acoustic Waves at Room Temperature. Sensors 2018, 18, 3796. [Google Scholar] [CrossRef]
- Nicolae, I.; Viespe, C.; Grigoriu, C. Nanocomposite Sensitive Polymeric Films for SAW Sensors Deposited by the MAPLE Direct Write Technique. Sens. Actuators B Chem. 2011, 158, 418–422. [Google Scholar] [CrossRef]
- Ballantine, D.S.; White, R.M.; Martin, S.J.; Ricco, A.J.; Zellers, E.T.; Frye, G.C.; Wohtjen, H. Acoustic Wave Sensors, Theory, Design, and Physico-Chemical Applications; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- Falconer, R.S. A Versatile SAW-Based Sensor System for Investigating Gas-Sensitive Coatings. Sens. Actuators B Chem. 1995, 24–25, 54–57. [Google Scholar] [CrossRef]
- Yoo, B.K.; Park, Y.W.; Kang, C.Y.; Yoon, S.J.; Kim, J.S. Surface Acoustic Wave Sensors to Detect Volatile Gases by Measuring Output Phase Shift. J. Electroceram. 2006, 17, 1013–1017. [Google Scholar] [CrossRef]
- Marcu, A.; Nicolae, I.; Viespe, C. Active Surface Geometrical Control of Noise in Nanowire-SAW Sensors. Sens. Actuators B Chem. 2016, 231, 469–473. [Google Scholar] [CrossRef]
- Marcu, A.; Viespe, C. Surface Acoustic Wave Sensors for Hydrogen and Deuterium Detection. Sensors 2017, 17, 1417. [Google Scholar] [CrossRef]
- Zhu, H.; Xie, D.; Lin, S.; Zhang, W.; Yang, Y.; Zhang, R.; Shi, X.; Wang, H.; Zhang, Z.; Zu, X.; et al. Elastic Loading Enhanced NH3 Sensing for Surface Acoustic Wave Sensor with Highly Porous Nitrogen-Doped Diamond-like Carbon Film. Sens. Actuators B Chem. 2021, 344, 130175. [Google Scholar] [CrossRef]
- Kalinin, V. Wireless Physical SAW Sensors for Automotive Applications. In Proceedings of the 2011 IEEE International Ultrasonics Symposium (IUS), Orlando, FL, USA, 18–21 October 2011; pp. 212–221. [Google Scholar] [CrossRef]
- Wilson, W.C.; Malocha, D.C.; Kozlovski, N.; Gallagher, D.R.; Fisher, B.; Pavlina, J.; Saldanha, N.; Puccio, D.; Atkinson, G.M. Orthogonal Frequency Coded SAW Sensors for Aerospace SHM Applications. IEEE Sens. J. 2009, 9, 1546–1556. [Google Scholar] [CrossRef]
- Tittmann, B.R. Recent Results and Trends in Health Monitoring with Surface Acoustic Waves (SAWs). In Testing, Reliability, and Application of Micro- and Nano-Material Systems; Meyendorf, N., Baaklini, G.Y., Michel, B., Eds.; SPIE: Bellingham, WA, USA, 2003; Volume 5045, pp. 37–46. [Google Scholar] [CrossRef]
- Chevallier, E.; Scorsone, E.; Bergonzo, P. New Sensitive Coating Based on Modified Diamond Nanoparticles for Chemical SAW Sensors. Sens. Actuators B Chem. 2011, 154, 238–244. [Google Scholar] [CrossRef]
- Mohasin Sulthana, N.; Ganesan, K.; Ajikumar, P.K. Enhanced Sensitivity of Partial O-Terminated H-Diamond for H2S Detection at Room Temperature. Diam. Relat. Mater. 2023, 140, 110568. [Google Scholar] [CrossRef]
- Rao, X.; Yang, J.; Chen, Z.; Yuan, Y.; Chen, Q.; Feng, X.; Qin, L.; Zhang, Y. Tuning C–C sp2/sp3 Ratio of DLC Films in FCVA System for Biomedical Application. Bioact. Mater. 2020, 5, 192–200. [Google Scholar] [CrossRef]
- Zia, A.W.; Hussain, S.A.; Rasul, S.; Bae, D.; Pitchaimuthu, S. Progress in Diamond-Like Carbon Coatings for Lithium-Based Batteries. J. Energy Storage 2023, 72, 108803. [Google Scholar] [CrossRef]
- Luo, W.; Fu, Q.; Deng, J.; Yan, G.; Zhou, D.; Gong, S.; Hu, Y. An Integrated Passive Impedance-Loaded SAW Sensor. Sens. Actuators B Chem. 2013, 187, 215–220. [Google Scholar] [CrossRef]
- Nilkar, M.; Ghodsi, F.E.; Jafari, S.; Thiry, D.; Snyders, R. Effects of Nitrogen Incorporation on N-Doped DLC Thin Film Electrodes Fabricated by Dielectric Barrier Discharge Plasma: Structural Evolution and Electrochemical Performances. J. Alloys Compd. 2021, 853, 157298. [Google Scholar] [CrossRef]
- Li, O.L.; Chiba, S.; Wada, Y.; Panomsuwan, G.; Ishizaki, T. Synthesis of Graphitic-N and Amino-N in Nitrogen-Doped Carbon via a Solution Plasma Process and Exploration of Their Synergic Effect for Advanced Oxygen Reduction Reaction. J. Mater. Chem. A 2017, 5, 2073. [Google Scholar] [CrossRef]
- Chen, Z.; Jaworski, A.; Chen, J.; Budnyak, T.M.; Szewczyk, I.; Rokicińska, A.; Dronskowski, R.; Hedin, N.; Kuśtrowski, P.; Slabon, A. Graphitic Nitrogen in Carbon Catalysts Is Important for the Reduction of Nitrite as Revealed by Naturally Abundant 15N NMR Spectroscopy. Dalton Trans. 2021, 50, 6857–6866. [Google Scholar] [CrossRef]
- Niu, K.; Gao, J.; Wu, L.; Lu, X.; Chen, J. Nitrogen-Doped Graphdiyne as a Robust Electrochemical Biosensing Platform for Ultrasensitive Detection of Environmental Pollutants. Anal. Chem. 2021, 93, 8656–8662. [Google Scholar] [CrossRef]
- Vizireanu, S.; Constantinoiu, I.; Satulu, V.; Stoica, S.D.; Viespe, C. High-Sensitivity H2 and CH4 SAW Sensors with Carbon Nanowalls and Improvement in Their Performance after Plasma Treatment. Chemosensors 2023, 11, 566. [Google Scholar] [CrossRef]
- MalekAlaie, M.; Jahangiri, M.; Rashidi, A.M.; HaghighiAsl, A.; Izadi, N. Selective Hydrogen Sulfide (H2S) Sensors Based on Molybdenum Trioxide (MoO3) Nanoparticle Decorated Reduced Graphene Oxide. Mater. Sci. Semicond. Process. 2015, 38, 93–100. [Google Scholar] [CrossRef]
- Choi, S.J.; Jang, B.H.; Lee, S.J.; Min, B.K.; Rothschild, A.; Kim, I.D. Selective Detection of Acetone and Hydrogen Sulfide for the Diagnosis of Diabetes and Halitosis Using SnO2 Nanofibers Functionalized with Reduced Graphene Oxide Nanosheets. ACS Appl. Mater. Interfaces 2014, 6, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Li, J.; Aslan, H.; Li, Q.; Li, Y.; Chen, M.; Huang, Y.; Froning, J.P.; Otyepka, M.; Zboril, R.; et al. A High Efficiency H2S Gas Sensor Material: Paper-Like Fe2O3/Graphene Nanosheets and Structural Alignment Dependency of Device Efficiency. J. Mater. Chem. A 2014, 2, 6714–6717. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Guo, Y. Cuprous Oxide Nanowires/Nanoparticles Decorated on Reduced Graphene Oxide Nanosheets: Sensitive and Selective H2S Detection at Low Temperature. Mater. Lett. 2019, 254, 336–339. [Google Scholar] [CrossRef]

| Samples | Plasma Deposition Environment | Plasma Exposure Time (min) | Plasma Treatment Environment |
|---|---|---|---|
| a-CH | CH4/Ar—50/25 | 60 | - |
| H2-assisted a-CH | CH4/Ar/H2—50/25/5 | 60 | - |
| N2-assisted a-CH | CH4/Ar/N2—50/25/5 | 60 | |
| a-CH_H2 treated | 15 | Ar/H2—100/25 | |
| H2-assisted a-CH_H2 treated | 15 | Ar/H2—100/25 | |
| N2-assisted a-CH_H2 treated | 15 | Ar/H2—100/25 |
| Sample/Element | PP At. % | |||
|---|---|---|---|---|
| C1s | O1s | N1s | O/C Ratio | |
| a-CH | 86.07 | 13.93 | - | 0.16 |
| a-CH_H2 treatment | 61.09 | 38.91 | - | 0.64 |
| H2-assisted a-CH | 91.15 | 8.85 | - | 0.10 |
| H2-assisted a-CH_H2 treatment | 61.66 | 38.34 | - | 0.62 |
| N2-assisted a-CH | 86.81 | 10.96 | 2.24 | 0.13 |
| N2-assisted a-CH_H2 treatment | 48.38 | 50.39 | 1.23 | 1.04 |
| Sensor | Sensitivity (Δf/c) (Hz/ppm) | LOD (3xn)/(Δf/c) (ppm) |
|---|---|---|
| a-CH | 53 | 1.14 |
| a-CH_H2 treated | 68 | 0.9 |
| H2-assisted a-CH | 49 | 1.2 |
| H2-assisted a-CH_H2 treated | 68 | 0.9 |
| N2-assisted a-CH | 45 | 1.3 |
| N2-assisted a-CH_H2 treated | 65 | 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satulu, V.; Paunica, M.; Brajnicov, S.; Vizireanu, S.; Dinescu, G.; Mitu, B.; Viespe, C. Optimization by Hydrogen Plasma Treatment of a-CH and Hydrogen/Nitrogen-Assisted a-CH Layers for SAW Sensors. Chemosensors 2024, 12, 194. https://doi.org/10.3390/chemosensors12090194
Satulu V, Paunica M, Brajnicov S, Vizireanu S, Dinescu G, Mitu B, Viespe C. Optimization by Hydrogen Plasma Treatment of a-CH and Hydrogen/Nitrogen-Assisted a-CH Layers for SAW Sensors. Chemosensors. 2024; 12(9):194. https://doi.org/10.3390/chemosensors12090194
Chicago/Turabian StyleSatulu, Veronica, Mihai Paunica, Simona Brajnicov, Sorin Vizireanu, Gheorghe Dinescu, Bogdana Mitu, and Cristian Viespe. 2024. "Optimization by Hydrogen Plasma Treatment of a-CH and Hydrogen/Nitrogen-Assisted a-CH Layers for SAW Sensors" Chemosensors 12, no. 9: 194. https://doi.org/10.3390/chemosensors12090194
APA StyleSatulu, V., Paunica, M., Brajnicov, S., Vizireanu, S., Dinescu, G., Mitu, B., & Viespe, C. (2024). Optimization by Hydrogen Plasma Treatment of a-CH and Hydrogen/Nitrogen-Assisted a-CH Layers for SAW Sensors. Chemosensors, 12(9), 194. https://doi.org/10.3390/chemosensors12090194







