An Integration of UPLC-Q-TOF-MS, GC-MS, Electronic Nose, Electronic Tongue, and Molecular Docking for the Study of the Chemical Properties and Flavor Profiles of Moringa oleifera Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Reagents
2.3. Apparatus
2.4. E-Tongue Analysis
2.5. E-Nose Analysis
2.6. Molecular Docking Study
2.7. Statistical Analysis
3. Results
3.1. Identification of Chemical Properties Based on UPLC-Q-TOF-MS and GC-MS
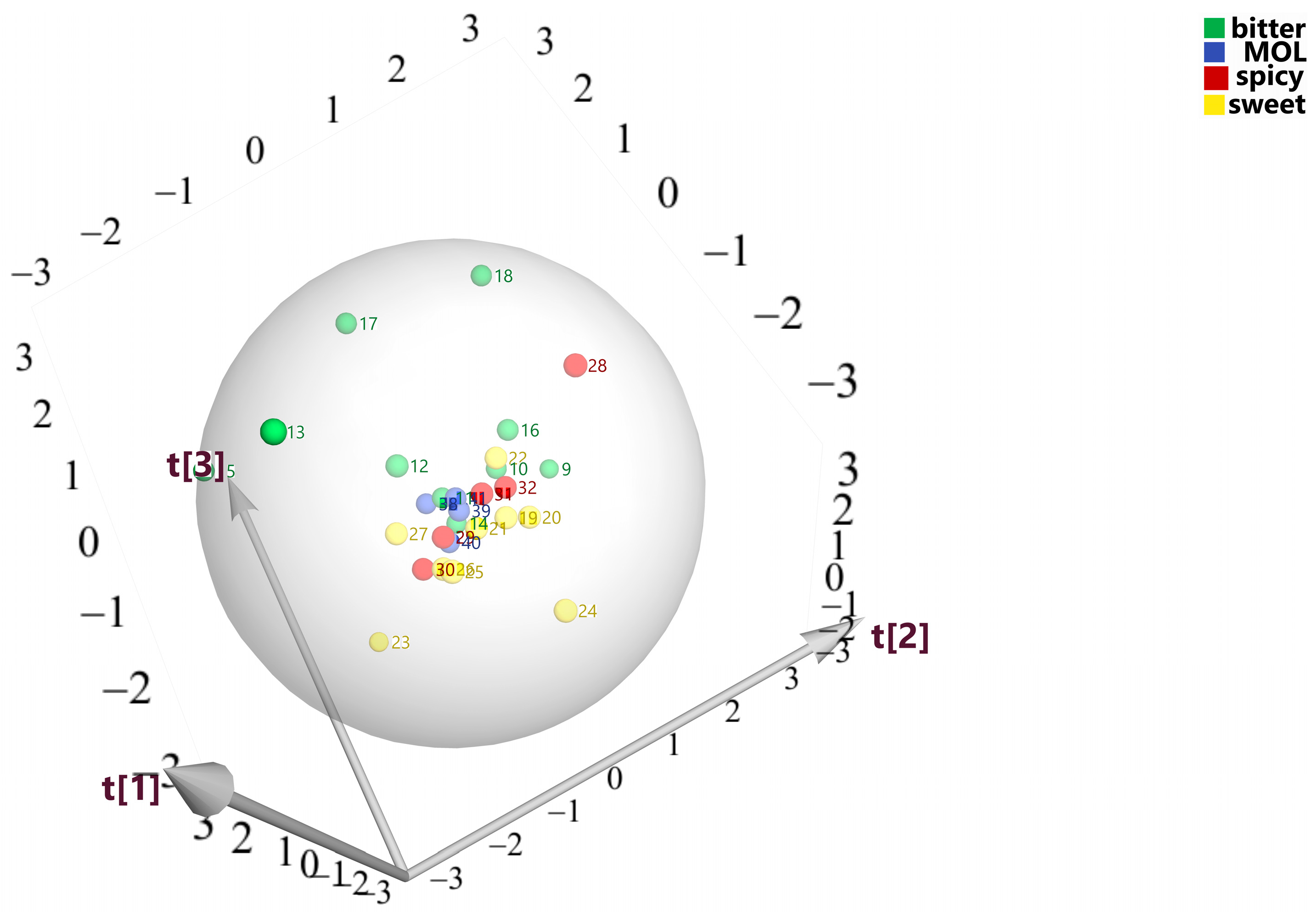
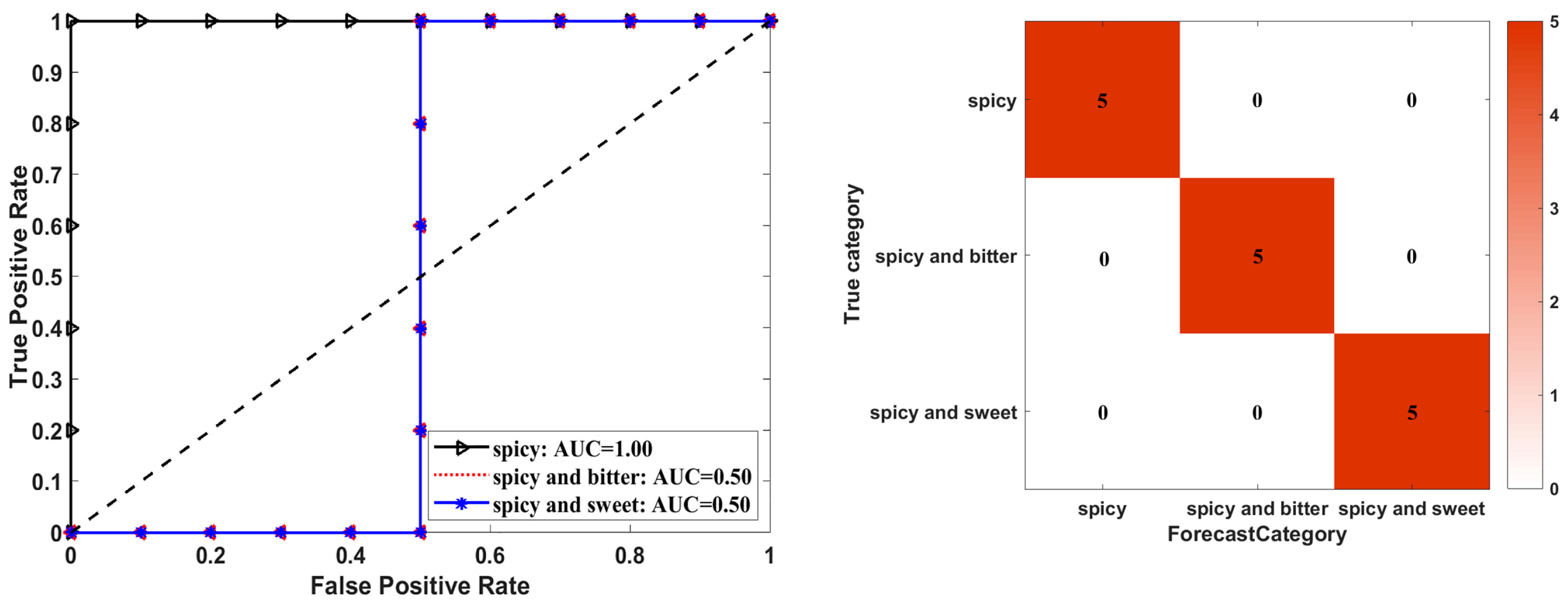
3.2. Taste Analysis Based on E-Tongue by PLS-DA and ANN
3.3. Odor Analysis Based on E-Nose by PLS-DA and ANN
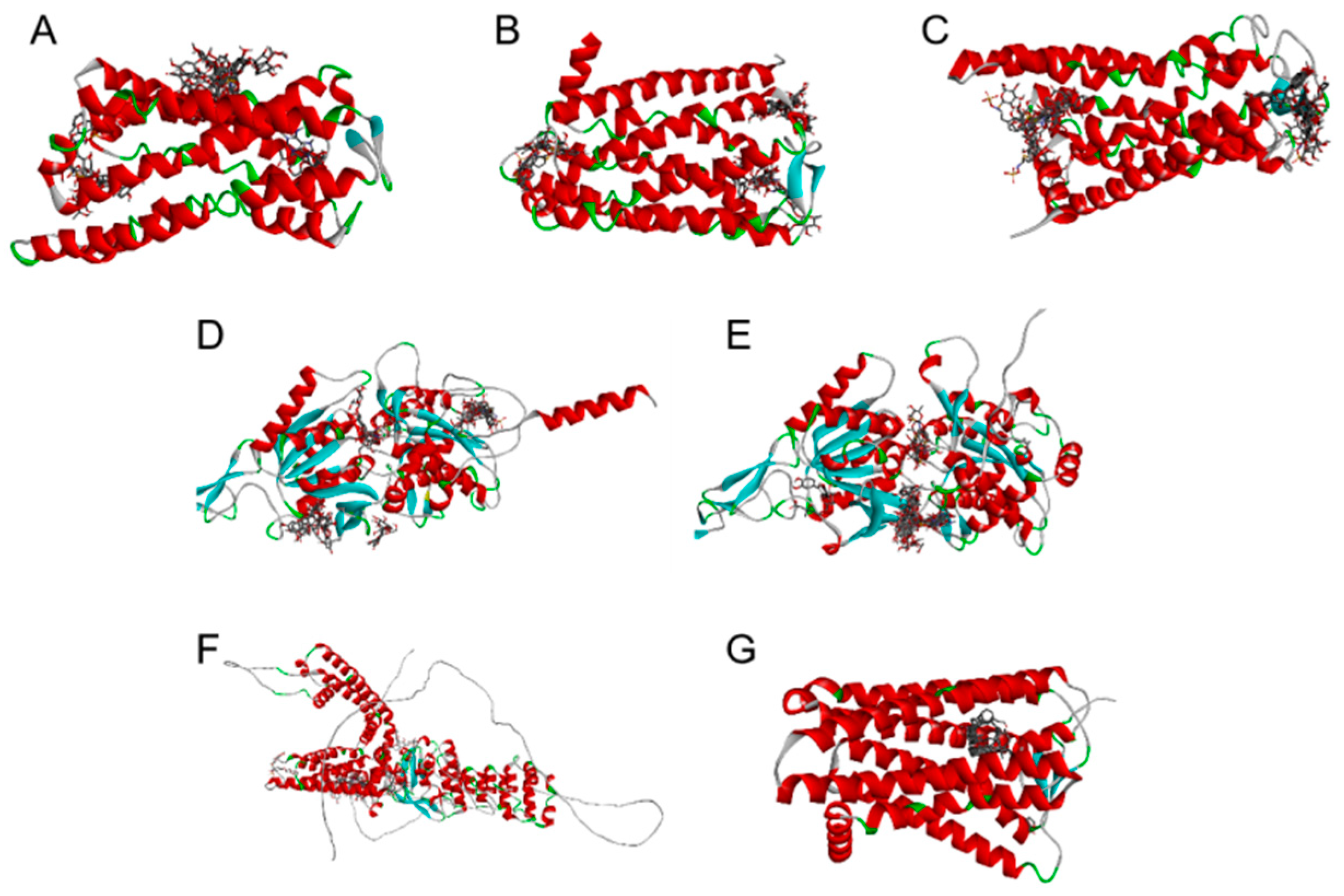
3.4. Results of Molecular Docking Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stohs, S.J.; Hartman, M.J. Review of the Safety and Efficacy of Moringa oleifera. Phytother. Res. 2015, 29, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Tiwari, P.; Sahu, P.; Kumar, S. A review of the phytochemical and pharmacological characteristics of Moringa oleifera. J. Pharm. Bioallied Sci. 2018, 10, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Sonewane, K.; Chouhan, S.S.; Rajan, M.; Chauhan, N.S.; Rout, O.P.; Kumar, A.; Baghel, G.S.; Gupta, P.K. Pharmacological, ethnomedicinal, and evidence-based comparative review of Moringa oleifera Lam. (Shigru) and its potential role in the management of malnutrition in Tribal Regions of India, especially Chhattisgarh. World J. Tradit. Chin. Med. 2022, 8, 314–338. [Google Scholar] [CrossRef]
- Setyani, W.; Murwanti, R.; Sulaiman, T.N.S.; Hertiani, T. Flavonoid from Moringa oleifera leaves revisited: A review article on in vitro, in vivo, and in silico studies of antidiabetic insulin-resistant activity. J. Adv. Pharm. Technol. Res. 2023, 14, 283–288. [Google Scholar] [CrossRef]
- Wei, P.; Zhang, Y.; Wang, Y.-Y.; Dong, J.-F.; Lin, Z.-H.; Li, W.; Liu, L.; Hu, S.-L.; Zhang, L.; Lou, W.-Y.; et al. Efficient extraction and excellent activity of flavonoid from Moringa oleifera leaves and its microencapsulation. LWT 2023, 184, 115021. [Google Scholar] [CrossRef]
- Barzan, G.; Sacco, A.; Giovannozzi, A.M.; Portesi, C.; Schiavone, C.; Salafranca, J.; Wrona, M.; Nerín, C.; Rossi, A.M. Development of innovative antioxidant food packaging systems based on natural extracts from food industry waste and Moringa oleifera leaves. Food Chem. 2024, 432, 137088. [Google Scholar] [CrossRef]
- Nudel, A.; Cohen, R.; Abbo, S.; Kerem, Z. Developing a nutrient-rich and functional wheat bread by incorporating Moringa oleifera leaf powder and gluten. LWT 2023, 187, 115343. [Google Scholar] [CrossRef]
- Kotsou, K.; Chatzimitakos, T.; Athanasiadis, V.; Bozinou, E.; Rumbos, C.I.; Athanassiou, C.G.; Lalas, S.I. Enhancing the Nutritional Profile of Tenebrio molitor Using the Leaves of Moringa oleifera. Foods 2023, 12, 2612. [Google Scholar] [CrossRef]
- Yang, M.; Tao, L.; Kang, X.-R.; Wang, Z.-L.; Su, L.-Y.; Li, L.-F.; Gu, F.; Zhao, C.-C.; Sheng, J.; Tian, Y. Moringa oleifera Lam. leaves as new raw food material: A review of its nutritional composition, functional properties, and comprehensive application. Trends Food Sci. Technol. 2023, 138, 399–416. [Google Scholar] [CrossRef]
- Sivakumar, D.; Bautista-Baños, S. A review on the use of essential oils for postharvest decay control and maintenance of fruit quality during storage. Crop. Prot. 2014, 64, 27–37. [Google Scholar] [CrossRef]
- Jha, V.; Gharat, K.; Kaur, D.; Kasbe, S.; Maroo, K.; Jhangiani, A.; Parulekar, O.; Dhamapurkar, V.; Thakur, K.; Marick, A.; et al. GC-MS Analysis, Thermal Characterization and Biomedical Applications of Essential Oil from Cymbopogon martinii: In vitro Approach. Adv. Res. 2022, 23, 50–69. [Google Scholar] [CrossRef]
- Bonik, S.K.; Tamanna, S.T.; Happy, T.A.; Haque, M.N.; Islam, S.; Faruque, M.O. Formulation and evaluation of cereal-based breads fortified with natural prebiotics from green banana, moringa leaves powder and soya powder. Appl. Food Res. 2024, 4, 100377. [Google Scholar] [CrossRef]
- Gomes, O.J.; Leitão, A.; Gaspar, M.C.; Vitorino, C.; Sousa, J.J.; de Sousa, H.C.; Braga, M.E.; Gando-Ferreira, L.M. Fortified chocolate mousse with powder and extract from Moringa oleifera leaves for nutritional value improvement. Food Chem. 2024, 441, 138338. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, X.; Hu, Y.; Hu, X.; Zhang, Y.; An, T.; Lv, B.; Tao, S.; Liu, Q.; Jiang, G. Moringa oleifera leaf supplementation relieves oxidative stress and regulates intestinal flora to ameliorate polycystic ovary syndrome in letrozole-induced rats. Food Sci. Nutr. 2023, 11, 5137–5156. [Google Scholar] [CrossRef]
- Boumaza-Hamladji, S.; Benhabyles, N.; Toubal, S.; El Haddad, D.; Bouchenak, O.; Bellalemi, N.; Berrichi, D.; Meziani, I. Flavonoic content and antibacterial evaluation of Moringa oleifera Lam. leaves grow in Algeria. J. Adv. Pharm. Technol. Res. 2023, 14, 191–195. [Google Scholar] [CrossRef]
- Khan, S.; Ibrar, D.; Hasnain, Z.; Nawaz, M.; Rais, A.; Ullah, S.; Gul, S.; Siddiqui, M.H.; Irshad, S. Moringa Leaf Extract Mitigates the Adverse Impacts of Drought and Improves the Yield and Grain Quality of Rice through Enhanced Physiological, Biochemical, and Antioxidant Activities. Plants 2023, 12, 2511. [Google Scholar] [CrossRef]
- Noreen, S.; Saleem, S.; Iqbal, U.; Mahmood, S.; Akhter, M.S.; Akbar, N.; El-Sheikh, M.; Kaushik, P. Moringa olifera leaf extract increases physio-biochemical properties, growth and yield of Pisum sativum grown under salinity stress. J. King Saud Univ. Sci. 2024, 36, 103056. [Google Scholar] [CrossRef]
- Ghadimi, M.; Najafi, A.; Sharifi, S.D.; Mohammadi-Sangcheshmeh, A.; Mehr, M.R.-A. Effects of dietary Moringa oleifera leaf extract on semen characteristics, fertility, and hatchability in aged broiler breeder roosters. Poult. Sci. 2024, 103, 103491. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, J.; Zhou, Z.; Yu, L.; Yu, L.; He, J.; Zhu, K.; Luo, Y.; Wang, H.; Du, X.; et al. Moringa oleifera leaf improves meat quality by modulating intestinal microbes in white feather broilers. Food Chem. X 2023, 20, 100938. [Google Scholar] [CrossRef]
- Istiqomah, N.I.; Budianti, S.I.; Cuana, R.; Puspitarum, D.L.; Mahardhika, L.J.; Suharyadi, E. Magnetically separable and reusable Fe3O4/chitosan nanocomposites green synthesized utilizing Moringa oleifera extract for rapid photocatalytic degradation of methylene blue. Results Chem. 2024, 7, 101245. [Google Scholar] [CrossRef]
- Sari, E.K.; Tumbelaka, R.M.; Ardiyanti, H.; Istiqomah, N.I.; Suharyadi, E. Green synthesis of magnetically separable and reusable Fe3O4/Cdots nanocomposites photocatalyst utilizing Moringa oleifera extract and watermelon peel for rapid dye degradation. Carbon Resour. Convers. 2023, 6, 274–286. [Google Scholar] [CrossRef]
- Weng, Z.; Sun, L.; Wang, F.; Sui, X.; Fang, Y.; Tang, X.; Shen, X. Assessment the flavor of soybean meal hydrolyzed with Alcalase enzyme under different hydrolysis conditions by E-nose, E-tongue and HS-SPME-GC–MS. Food Chem. X 2021, 12, 100141. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.-J.; Cao, L.-G.; Tan, D.-P.; Qin, L.; Lu, Y.-L.; Zhao, Y.-X.; Qian, Y.; Bai, C.-J.; Yang, J.-Y.; Ling, H.; et al. UPLC-Q/TOF-MS coupled with multivariate analysis for comparative analysis of metabolomic in Dendrobium nobile from different growth altitudes. Arab. J. Chem. 2022, 15, 104208. [Google Scholar] [CrossRef]
- Rong, Y.; Xie, J.; Yuan, H.; Wang, L.; Liu, F.; Deng, Y.; Jiang, Y.; Yang, Y. Characterization of volatile metabolites in Pu-erh teas with different storage years by combining GC-E-Nose, GC–MS, and GC-IMS. Food Chem. X 2023, 18, 100693. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Sun, L.; Yongliang, Z. UPLC-Q-Orbitrap-MS2 analysis of Moringa oleifera leaf extract and its antioxidant, antibacterial and anti-inflammatory activities. Nat. Prod. Res. 2020, 34, 2090–2094. [Google Scholar] [CrossRef]
- Wang, J.; Du, Y.; Jiang, L.; Li, J.; Yu, B.; Ren, C.; Yan, T.; Jia, Y.; He, B. LC-MS/MS-based chemical profiling of water extracts of Moringa oleifera leaves and pharmacokinetics of their major constituents in rat plasma. Food Chem. X 2024, 23, 101585. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, H.; Yu, X.; Luo, H.; Lu, Y.; Yang, H.; Li, X.; Li, Z.; Tang, L.; Wang, Z. Determination of Bitterness of Andrographis Herba Based on Electronic Tongue Technology and Discovery of the Key Compounds of Bitter Substances. Molecules 2018, 23, 3362. [Google Scholar] [CrossRef]
- Zou, G.; Xiao, Y.; Wang, M.; Zhang, H. Detection of bitterness and astringency of green tea with different taste by electronic nose and tongue. PLoS ONE 2018, 13, e0206517. [Google Scholar] [CrossRef]
- Tan, J.; Xu, J. Applications of electronic nose (e-nose) and electronic tongue (e-tongue) in food quality-related properties determination: A review. Artif. Intell. Agric. 2020, 4, 104–115. [Google Scholar] [CrossRef]
- Pu, D.; Meng, R.; Qiao, K.; Cao, B.; Shi, Y.; Wang, Y.; Zhang, Y. Electronic tongue, proton-transfer-reaction mass spectrometry, spectral analysis, and molecular docking characterization for determining the effect of α-amylase on flavor perception. Food Res. Int. 2024, 181, 114078. [Google Scholar] [CrossRef]
- Cui, H.; Li, H.; Wu, Y.; Hu, X. Identification, flavor characteristics and molecular docking of umami taste peptides of Xuanwei ham. Food Res. Int. 2023, 173, 113211. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Zhang, Y.; Li, X.; Dong, Y.; Dou, Z.; Yang, Z.; Zhang, M.; Wang, H. The material basis of bitter constituents in Carbonized Typhae Pollen, based on the integration strategy of constituent analysis, taste sensing system and molecular docking. J. Pharm. Biomed. Anal. 2024, 242, 116028. [Google Scholar] [CrossRef]
- Zhang, M.X.; Li, H.; Chen, N.; Xiang, J.J.; Lin, L.J.; Li, Z.Y.; Yang, B. Mechanism of Moringa Folium in Treatment of Constipation Based on UPLC-Q-TOF-MS and GC-MS and Network Pharmacology. Chin. J. Exp. Tradit. Med. Formulae 2022, 28, 182–188. [Google Scholar] [CrossRef]
- Coppin, J.P.; Xu, Y.; Chen, H.; Pan, M.-H.; Ho, C.-T.; Juliani, R.; Simon, J.E.; Wu, Q. Determination of flavonoids by LC/MS and anti-inflammatory activity in Moringa oleifera. J. Funct. Foods 2013, 5, 1892–1899. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Ahmed, F.A.; Kurimoto, S.-I.; Kim, S.-Y.; Shibata, H.; Fujioka, T.; Takaishi, Y. New α-glucosides of caffeoyl quinic acid from the leaves of Moringa oleifera Lam. J. Nat. Med. 2011, 66, 217–221. [Google Scholar] [CrossRef]
- Al-Ghanayem, A.A.; Alhussaini, M.S.; Asad, M.; Joseph, B. Moringa oleifera Leaf Extract Promotes Healing of Infected Wounds in Diabetic Rats: Evidence of Antimicrobial, Antioxidant and Proliferative Properties. Pharmaceuticals 2022, 15, 528. [Google Scholar] [CrossRef]
- Li, X.; Shi, C.; Wang, S.; Wang, S.; Wang, X.; Lü, X. Uncovering the effect of Moringa oleifera Lam. leaf addition to Fuzhuan Brick Tea on sensory properties, volatile profiles and anti-obesity activity. Food Funct. 2023, 14, 2404–2415. [Google Scholar] [CrossRef]
- Chen, X.; Xu, Y.; Meng, L.; Chen, X.; Yuan, L.; Cai, Q.; Shi, W.; Huang, G. Non-parametric partial least squares–discriminant analysis model based on sum of ranking difference algorithm for tea grade identification using electronic tongue data. Sens. Actuators B Chem. 2020, 311, 127924. [Google Scholar] [CrossRef]
- Romani, S.; Cevoli, C.; Fabbri, A.; Alessandrini, L.; Rosa, M.D. Evaluation of Coffee Roasting Degree by Using Electronic Nose and Artificial Neural Network for Off-line Quality Control. J. Food Sci. 2012, 77, C960–C965. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, X.; Gao, Y.; Jin, Z.; Guo, S.; Li, Z.; Wang, M.; Zhao, R.; Zhou, W.; Wu, J. Study on the mechanism of Shujin Tongluo granules in treating cervical spondylosis based on network pharmacology and molecular docking. Medicine 2023, 102, e34030. [Google Scholar] [CrossRef]
- Yuan, Y.; Yiasmin, M.N.; Tristanto, N.A.; Chen, Y.; Liu, Y.; Guan, S.; Wang, Z.; Hua, X. Computational simulations on the taste mechanism of steviol glycosides based on their interactions with receptor proteins. Int. J. Biol. Macromol. 2024, 255, 128110. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Pan, Y.; Li, Y.; Zhou, Y.; Liu, H.; Liu, X. Molecular mechanisms of bitterness and astringency in the oral cavity induced by soyasaponin. Food Sci. Hum. Wellness 2024, 13, 1–19. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, X.; Lin, Y.; Ke, Q.; Niu, Y.; Zhang, J.; Yang, E.; Shen, T.; Sun, Z.; Xiao, Z. Unraveling the characteristic chestnut aroma compounds in MeiTanCuiYa green tea and their interaction mechanisms with broad-spectrum olfactory receptors using molecular docking. LWT 2024, 194, 115785. [Google Scholar] [CrossRef]
- Xiao, Z.; Qu, H.; Mao, C.; Niu, Y. Study on the sweetening mechanism of aroma compounds in yangshan peach using sensory analysis, molecular docking, and molecular dynamics simulation techniques. LWT 2024, 191, 115562. [Google Scholar] [CrossRef]
- Meyerhof, W.; Batram, C.; Kuhn, C.; Brockhoff, A.; Chudoba, E.; Bufe, B.; Appendino, G.; Behrens, M. The Molecular Receptive Ranges of Human TAS2R Bitter Taste Receptors. Chem. Senses 2010, 35, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Dhaka, A.; Viswanath, V.; Patapoutian, A. Trp Ion Channels and Temperature Sensation. Annu. Rev. Neurosci. 2006, 29, 135–161. [Google Scholar] [CrossRef]
- Pang, G.; Xie, J.; Chen, Q.; Hu, Z. How functional foods play critical roles in human health. Food Sci. Hum. Wellness 2012, 1, 26–60. [Google Scholar] [CrossRef]
- Xiao, Y.; Liang, J.; Gu, F.; Du, D.S.; Chen, F.X. Berberine activates bitter taste responses of enteroendocrine STC-1 cells. Mol. Cell. Biochem. 2018, 447, 21–32. [Google Scholar] [CrossRef]
- Chae, S.H.; Lee, O.N.; Park, H.Y.; Ku, K.-M. Seasonal Effects of Glucosinolate and Sugar Content Determine the Pungency of Small-Type (Altari) Radishes (Raphanus sativus L.). Plants 2022, 11, 312. [Google Scholar] [CrossRef]
- Yuan, J.; Liang, W.; Yuan, Y.; Zhou, M.; Liu, Y.; Wang, M.; Hu, Q.; Chang, Z.; Zhang, Q.; Zhang, L. Research progress on chemical constituents and pharmacological activities of Moringa oleifera leaves. Chin. Tradit. Herb. Drugs 2021, 52, 4422–4432. [Google Scholar] [CrossRef]
- Chen, G.L.; Xu, Y.-B.; Wu, J.-L.; Li, N.; Guo, M.-Q. Hypoglycemic and hypolipidemic effects of Moringa oleifera leaves and their functional chemical constituents. Food Chem. 2020, 333, 127478. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Wei, Z.; Liu, Y.; Wang, F.; Zhang, S.; Serrano, C.; Li, L.; Sun, B. Characterization, Large-Scale HSCCC Separation and Neuroprotective Effects of Polyphenols from Moringa oleifera Leaves. Molecules 2022, 27, 678. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.; Mohamed, M.F.; Elhalwagi, A.; El-Itriby, H.A.; Shawki, H.H.; Abdelhamid, I.A. Moringa peregrina Leaves Extracts Induce Apoptosis and Cell Cycle Arrest of Hepatocellular Carcinoma. BioMed Res. Int. 2019, 2019, 2698570. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Guo, M.; Chen, N.; Tang, Z.; Xiang, J.; Yang, L.; Wang, G.; Yang, B.; Li, H. An Integration of UPLC-Q-TOF-MS, GC-MS, Electronic Nose, Electronic Tongue, and Molecular Docking for the Study of the Chemical Properties and Flavor Profiles of Moringa oleifera Leaves. Chemosensors 2024, 12, 199. https://doi.org/10.3390/chemosensors12090199
Zhang M, Guo M, Chen N, Tang Z, Xiang J, Yang L, Wang G, Yang B, Li H. An Integration of UPLC-Q-TOF-MS, GC-MS, Electronic Nose, Electronic Tongue, and Molecular Docking for the Study of the Chemical Properties and Flavor Profiles of Moringa oleifera Leaves. Chemosensors. 2024; 12(9):199. https://doi.org/10.3390/chemosensors12090199
Chicago/Turabian StyleZhang, Mingxiao, Mengjia Guo, Na Chen, Zhuqian Tang, Junjie Xiang, Lixin Yang, Guohua Wang, Bin Yang, and Hua Li. 2024. "An Integration of UPLC-Q-TOF-MS, GC-MS, Electronic Nose, Electronic Tongue, and Molecular Docking for the Study of the Chemical Properties and Flavor Profiles of Moringa oleifera Leaves" Chemosensors 12, no. 9: 199. https://doi.org/10.3390/chemosensors12090199
APA StyleZhang, M., Guo, M., Chen, N., Tang, Z., Xiang, J., Yang, L., Wang, G., Yang, B., & Li, H. (2024). An Integration of UPLC-Q-TOF-MS, GC-MS, Electronic Nose, Electronic Tongue, and Molecular Docking for the Study of the Chemical Properties and Flavor Profiles of Moringa oleifera Leaves. Chemosensors, 12(9), 199. https://doi.org/10.3390/chemosensors12090199




