Study of the Influence of Thermal Annealing of Ga-Doped ZnO Thin Films on NO2 Sensing at ppb Level
Abstract
1. Introduction
2. Materials and Methods
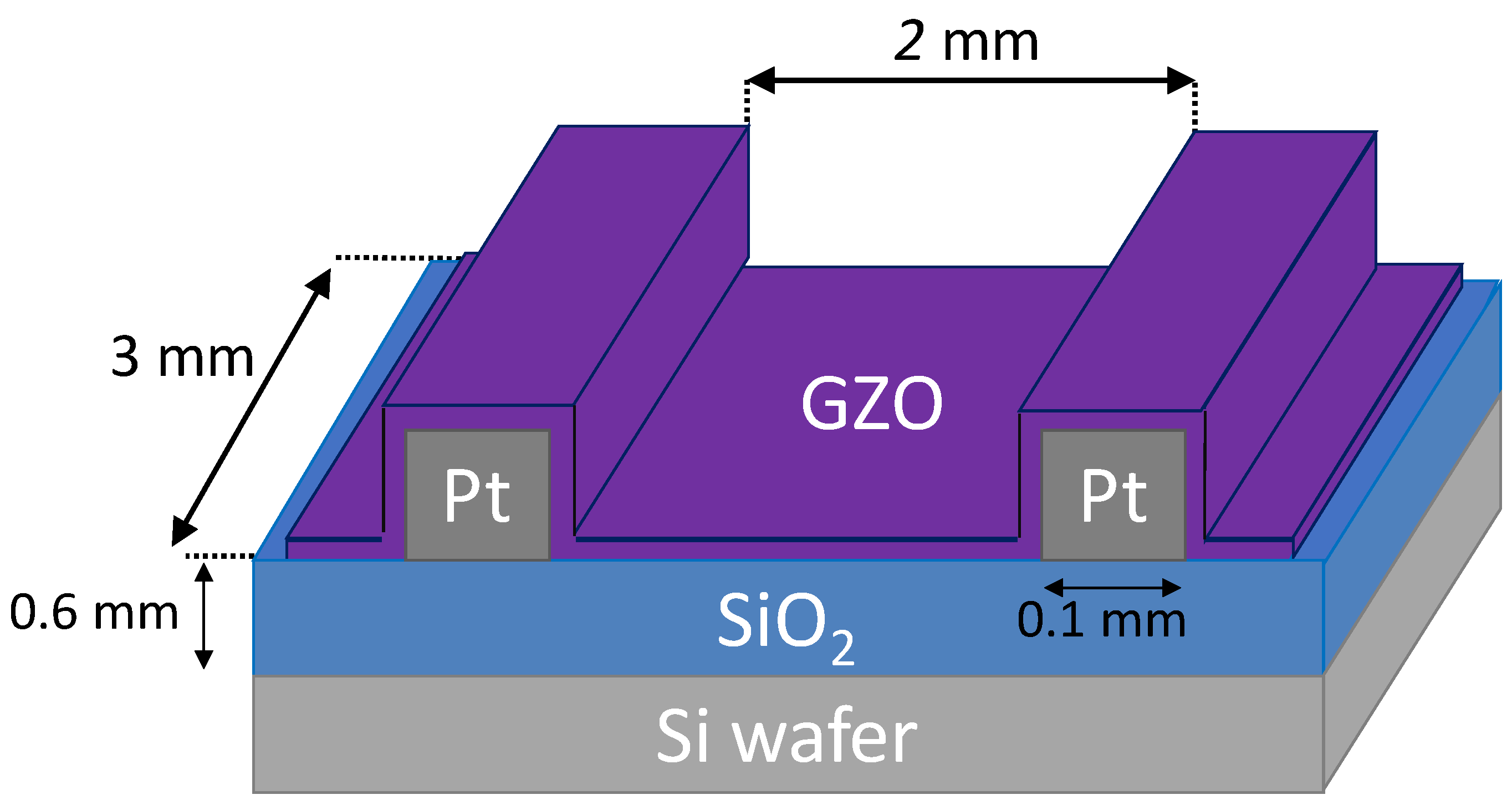
2.1. Preparation of Gas Sensing Elements
2.2. Microstructural and Structural Characterization
2.3. Gas Sensing
3. Results and Discussion
3.1. Chemical Composition
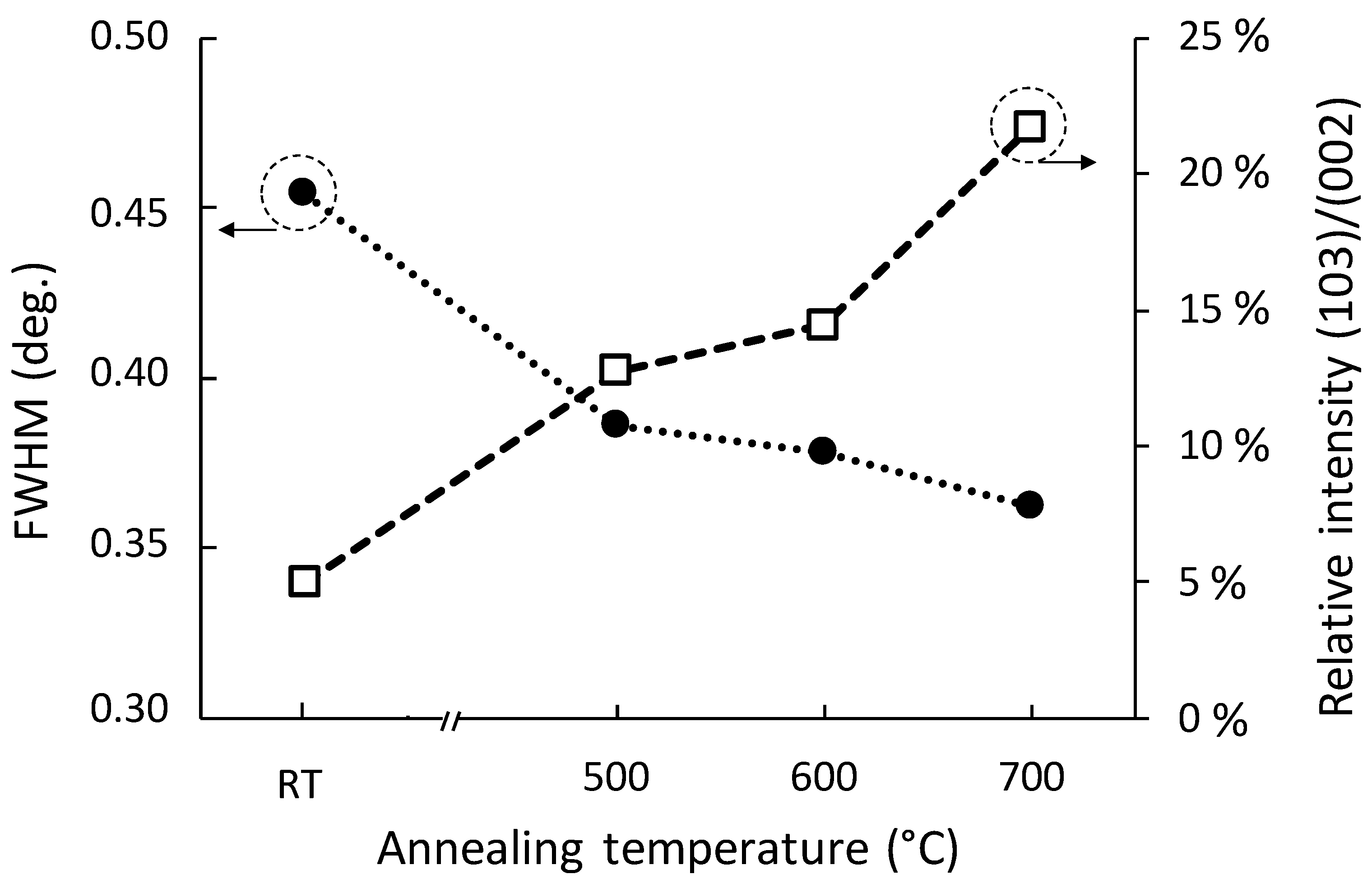
3.2. Influence of Annealing on Film Structure
3.3. Influence of Annealing on Microstructural Properties
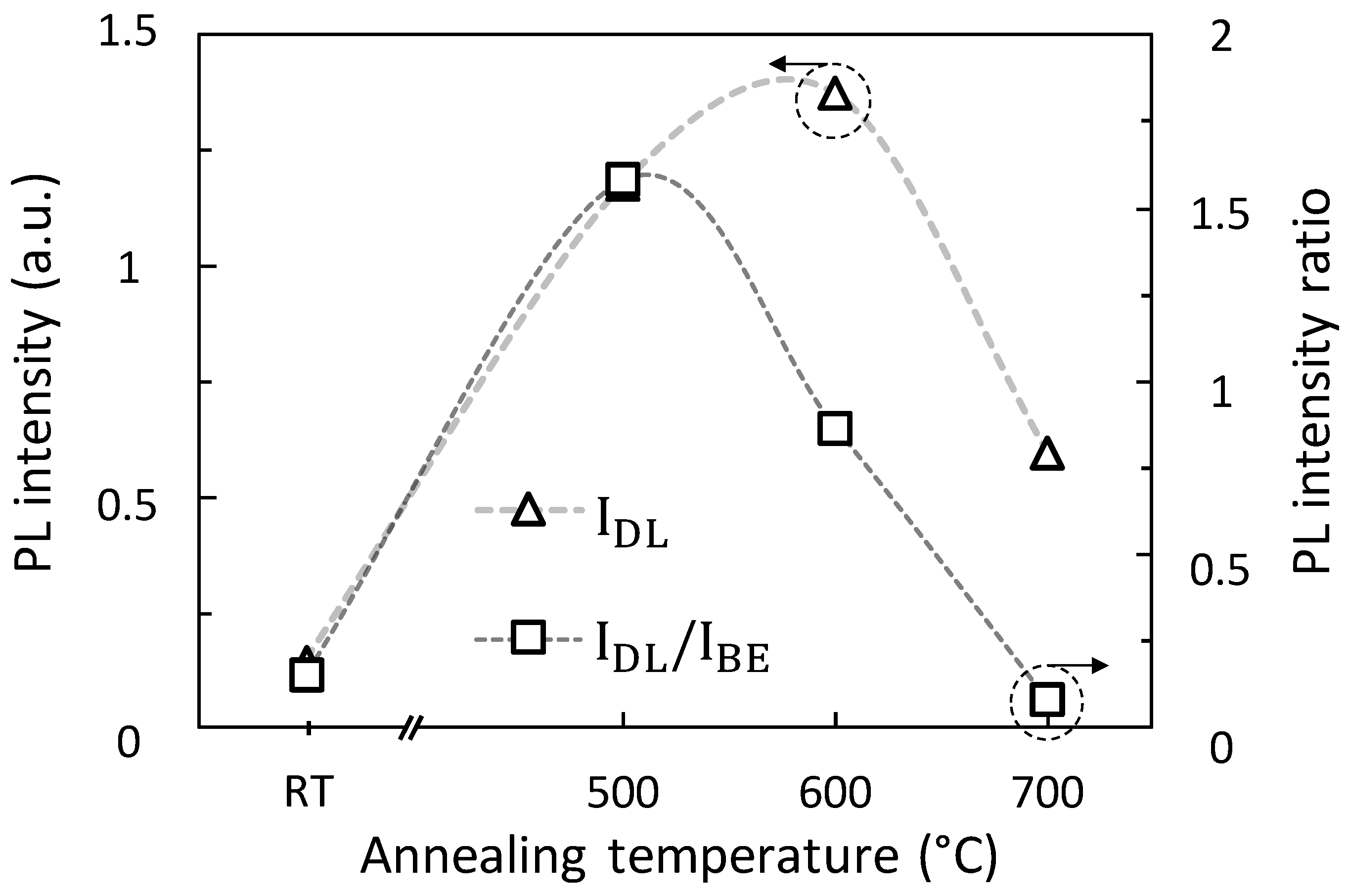
3.4. Photoluminescence Measurements
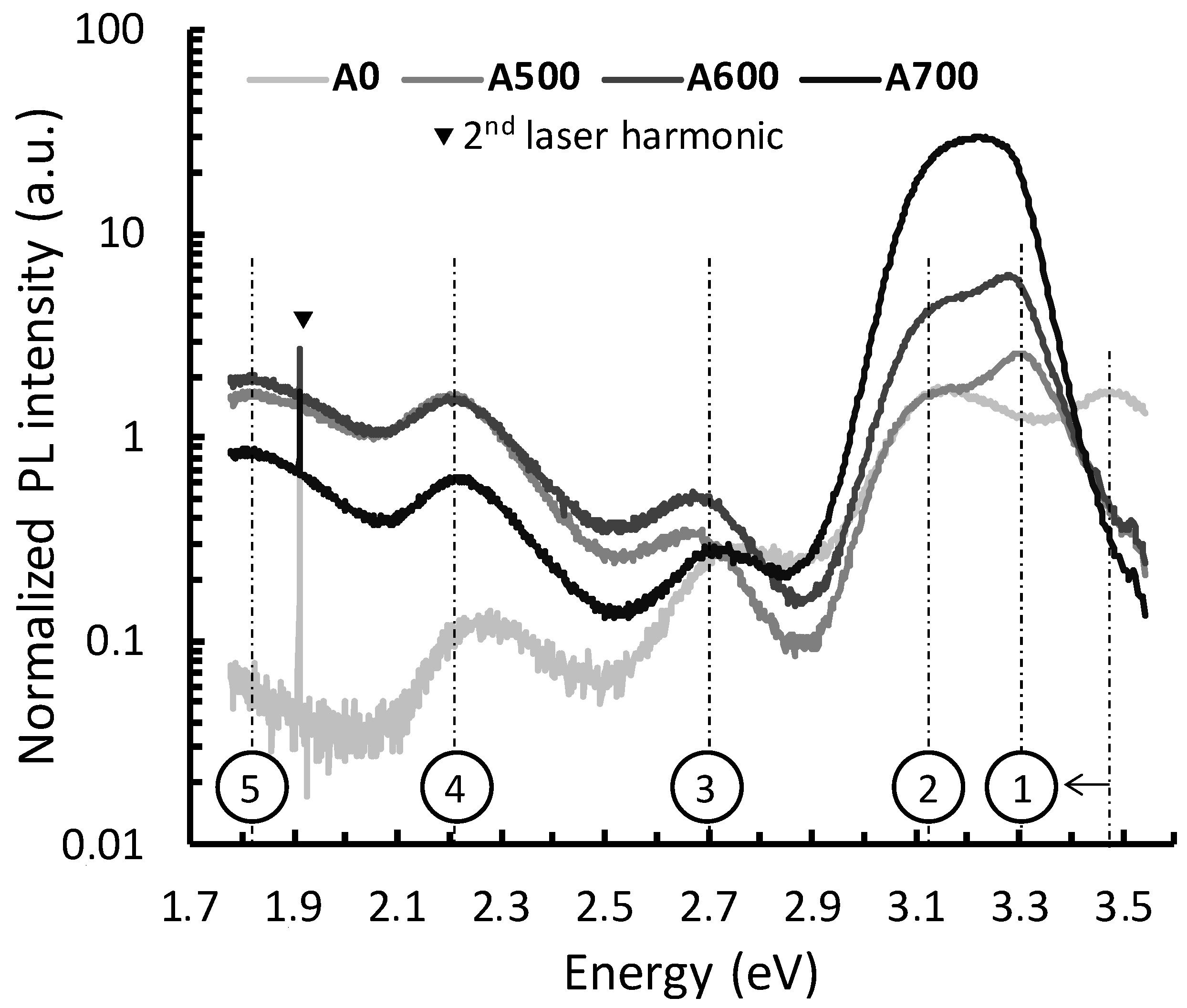
3.4.1. Description and Identification
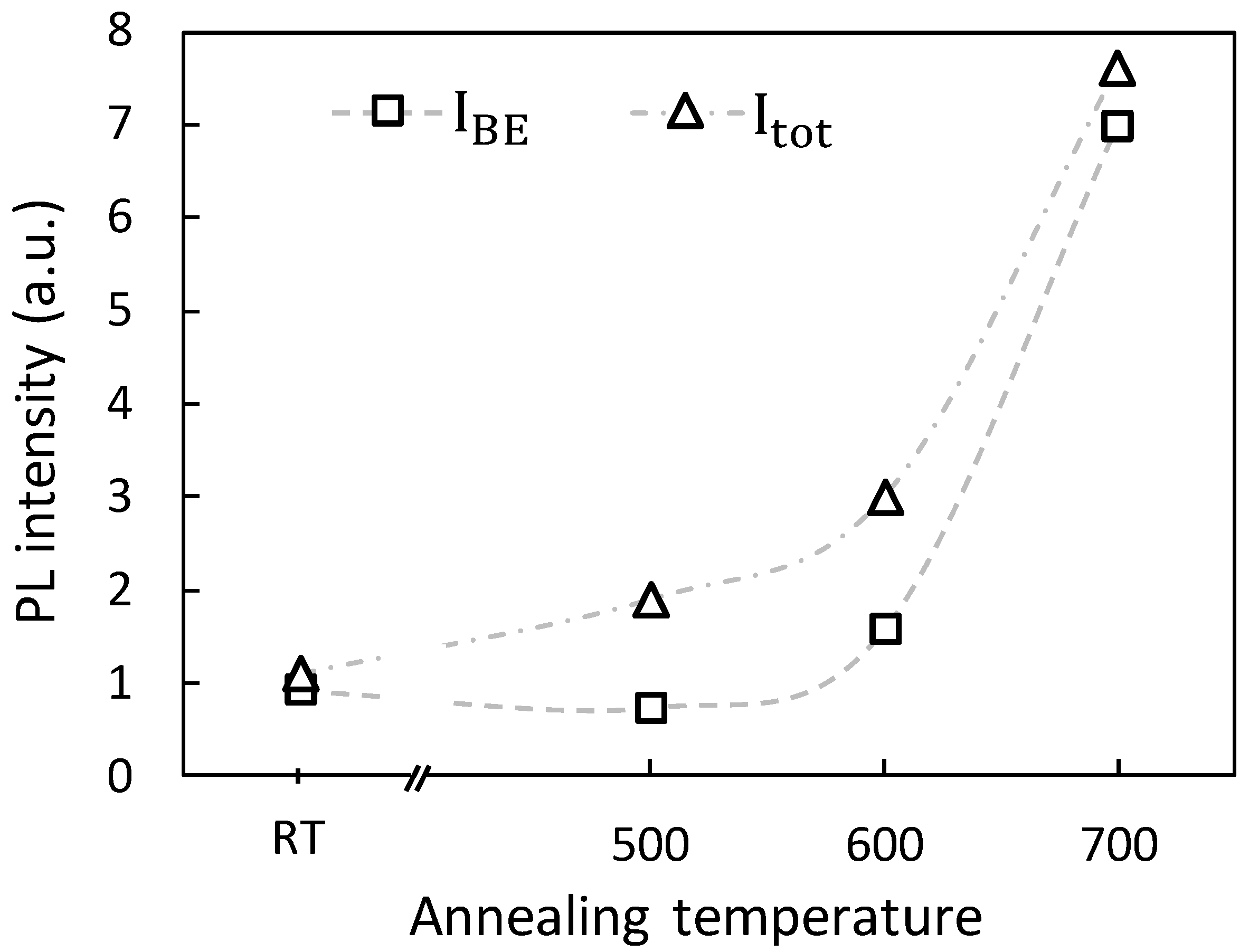
3.4.2. Cristallinity/nRAD
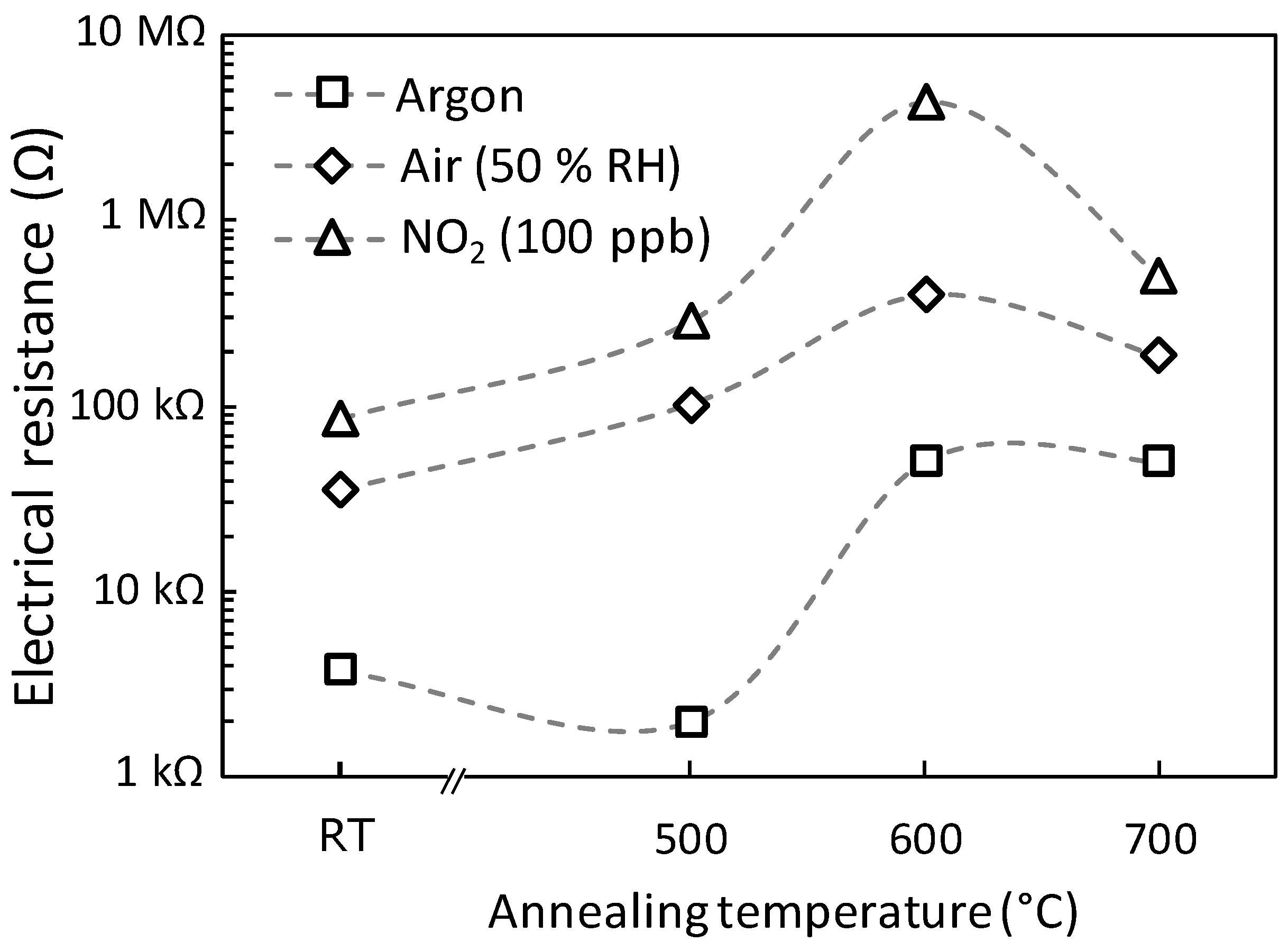
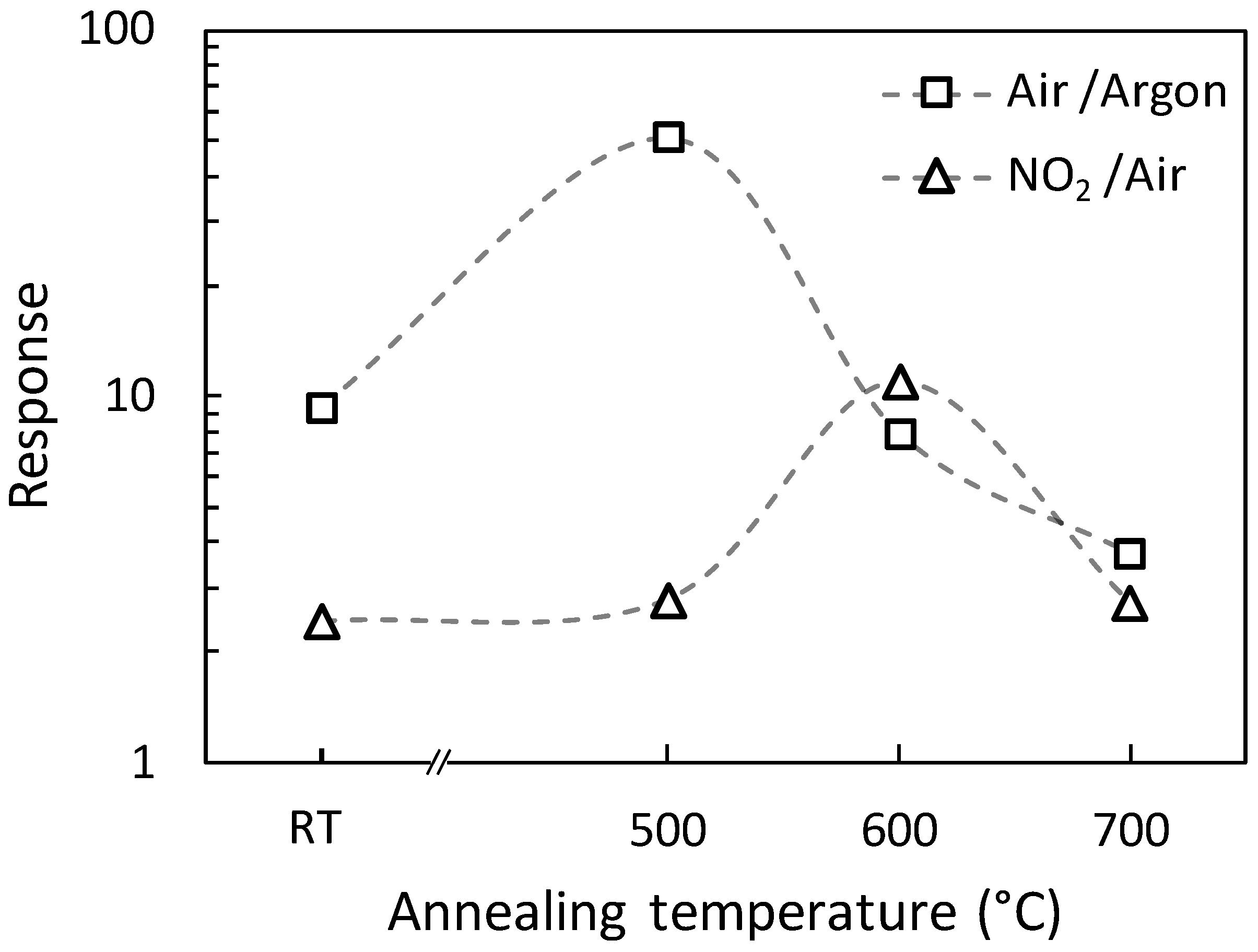
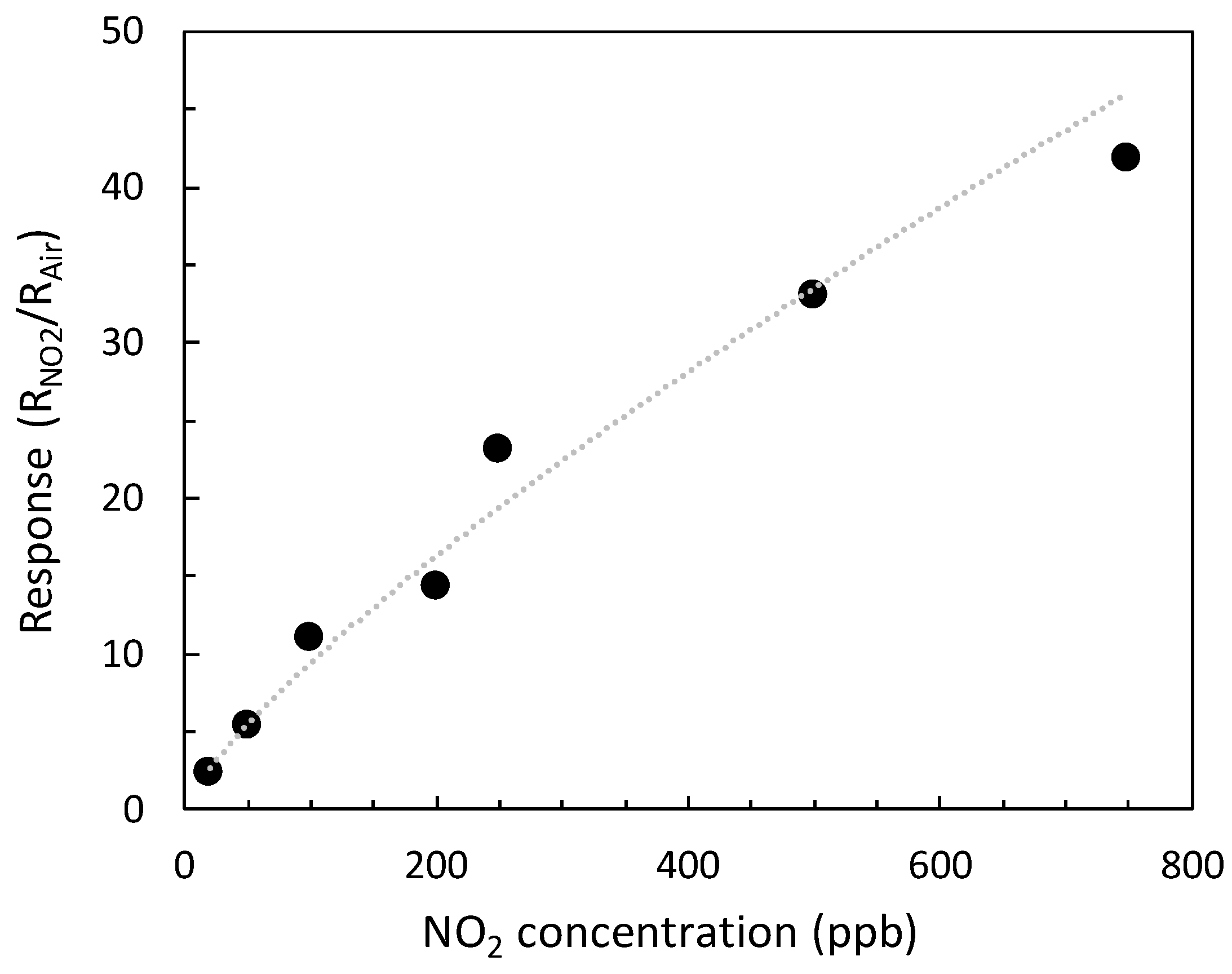
3.5. Gas Sensing Tests and Response to 100 ppb of NO2
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choudhari, U.; Jagtap, S. A Panoramic View of NOx and NH3 Gas Sensors. Nano-Struct. Nano-Objects 2023, 35, 100995. [Google Scholar] [CrossRef]
- Ji, H.; Zeng, W.; Li, Y. Gas Sensing Mechanisms of Metal Oxide Semiconductors: A Focus Review. Nanoscale 2019, 11, 22664–22684. [Google Scholar] [CrossRef]
- Kumar, R.; Al-Dossary, O.; Kumar, G.; Umar, A. Zinc Oxide Nanostructures for NO2 Gas–Sensor Applications: A Review. Nano-Micro Lett. 2015, 7, 97–120. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.A.; Conti, P.P.; Andre, R.S.; Correa, D.S. A Review on Chemiresistive ZnO Gas Sensors. Sens. Actuators Rep. 2022, 4, 100100. [Google Scholar] [CrossRef]
- Rothschild, A.; Komem, Y. The Effect of Grain Size on the Sensitivity of Nanocrystalline Metal-Oxide Gas Sensors. J. Appl. Phys. 2004, 95, 6374–6380. [Google Scholar] [CrossRef]
- Yamazoe, N.; Sakai, G.; Shimanoe, K. Oxide Semiconductor Gas Sensors. Catal. Surv. Asia. 2003, 7, 63–75. [Google Scholar] [CrossRef]
- Ellmer, K. Resistivity of Polycrystalline Zinc Oxide Films: Current Status and Physical Limit. J. Phys. D Appl. Phys. 2001, 34, 3097. [Google Scholar] [CrossRef]
- Demchenko, D.O.; Earles, B.; Liu, H.Y.; Avrutin, V.; Izyumskaya, N.; Özgür, Ü.; Morkoç, H. Impurity Complexes and Conductivity of Ga-Doped ZnO. Phys. Rev. B 2011, 84, 075201. [Google Scholar] [CrossRef]
- Wang, T.; Chen, J.; Chen, J.; Yao, X.; Chen, G.; Jiao, Z.; Zhao, J.-T.; Cheng, S.; Yang, X.-C.; Li, Q. UV-Light Enhanced Gas Sensor Based on Ga Doped ZnO for Ultra-High Sensitive and Selective n-Butanol Detection. Appl. Surf. Sci. 2023, 641, 158551. [Google Scholar] [CrossRef]
- Anders, A. A Structure Zone Diagram Including Plasma-Based Deposition and Ion Etching. Thin Solid Films 2010, 518, 4087–4090. [Google Scholar] [CrossRef]
- Gunasekaran, V. Intégration et Caractérisation de Couches Sensibles de ZnO:Ga et de Cobaltites Spinelles (CoxFe3-xO4; 1<=x<=3) Sur Véhicules de Test Pour La Détection de Dioxyde d’azote Sub-ppm et d’éthanol. Ph.D. Thesis, Université de Toulouse, Université Toulouse III—Paul Sabatier, Toulouse, France, 2021. Available online: https://theses.fr/2021TOU30105 (accessed on 1 November 2024).
- Yoboué, P.; Konaté, A.; Asseu, O.; Menini, P. An Industrializable Silicon-Based Microhotplate for Metal Oxides Gas Sensors. Phys. Chem. News 2014, 72, 34–41. [Google Scholar]
- Quy, C.; Thai, N.; Duc Hoa, N.; Thi Thanh Le, D.; Hung, C.; Duy, N.; Hieu, N. C2H5OH and NO2 Sensing Properties of ZnO Nanostructures: Correlation between Crystal Size, Defect Level and Sensing Performance. RSC Adv. 2018, 8, 5629–5639. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, H.; Meng, L.; Sun, Z.; Chen, Z.; Huang, X.; Qin, Y. Adjustment of Oxygen Vacancy States in ZnO and Its Application in Ppb-Level NO2 Gas Sensor. Sci. Bull. 2020, 65, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Guo, F.; Liu, S.; Yang, B.; Jiang, Y.; Qi, L.; Fan, X. Both Oxygen Vacancies Defects and Porosity Facilitated NO2 Gas Sensing Response in 2D ZnO Nanowalls at Room Temperature. J. Alloy Compd. 2016, 682, 352–356. [Google Scholar] [CrossRef]
- Kumar, R.R.; Murugesan, T.; Chang, T.-W.; Lin, H.-N. Defect Controlled Adsorption/Desorption Kinetics of ZnO Nanorods for UV-Activated NO2 Gas Sensing at Room Temperature. Mater. Lett. 2021, 287, 129257. [Google Scholar] [CrossRef]
- Zhang, C.; Geng, X.; Li, J.; Luo, Y.; Lu, P. Role of Oxygen Vacancy in Tuning of Optical, Electrical and NO2 Sensing Properties of ZnO1-x Coatings at Room Temperature. Sens. Actuat. B-Chem. 2017, 248, 886–893. [Google Scholar] [CrossRef]
- Sundara Venkatesh, P.; Dharmaraj, P.; Purushothaman, V.; Ramakrishnan, V.; Jeganathan, K. Point Defects Assisted NH3 Gas Sensing Properties in ZnO Nanostructures. Sens. Actuat. B-Chem. 2015, 212, 10–17. [Google Scholar] [CrossRef]
- Spencer, M.J.S.; Yarovsky, I. ZnO Nanostructures for Gas Sensing: Interaction of NO2, NO, O, and N with the ZnO() Surface. J. Phys. Chem. C 2010, 114, 10881–10893. [Google Scholar] [CrossRef]
- An, W.; Wu, X.; Zeng, X.C. Adsorption of O2, H2, CO, NH3, and NO2 on ZnO Nanotube: A Density Functional Theory Study. J. Phys. Chem. C 2008, 112, 5747–5755. [Google Scholar] [CrossRef]
- Djurisic, A.; Leung, Y.; Tam, K.; Ding, L.; Ge, W.; Chen, H.-Y.; Gwo, S. Green, Yellow, and Orange Defect Emission from ZnO Nanostructures: Influence of Excitation Wavelength. Appl. Phys. Lett. 2006, 88, 103107. [Google Scholar] [CrossRef]
- Lyons, J.L.; Varley, J.B.; Steiauf, D.; Janotti, A.; Van de Walle, C.G. First-Principles Characterization of Native-Defect-Related Optical Transitions in ZnO. J. Appl. Phys. 2017, 122, 035704. [Google Scholar] [CrossRef]
- Janotti, A.; Van De Walle, C.G. Fundamentals of Zinc Oxide as a Semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef]
- Vanheusden, K.; Warren, W.L.; Seager, C.H.; Tallant, D.R.; Voigt, J.A.; Gnade, B.E. Mechanisms behind Green Photoluminescence in ZnO Phosphor Powders. J. Appl. Phys. 1996, 79, 7983–7990. [Google Scholar] [CrossRef]
- Lin, B.; Fu, Z.; Jia, Y. Green Luminescent Center in Undoped Zinc Oxide Films Deposited on Silicon Substrates. Appl. Phys. Lett. 2001, 79, 943–945. [Google Scholar] [CrossRef]
- Röhr, J.A.; Sá, J.; Konezny, S.J. The Role of Adsorbates in the Green Emission and Conductivity of Zinc Oxide. Commun. Chem. 2019, 2, 52. [Google Scholar] [CrossRef]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doǧan, S.; Avrutin, V.; Cho, S.J.; Morkç, H. A Comprehensive Review of ZnO Materials and Devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Liao, Z.-M.; Zhang, H.-Z.; Zhou, Y.-B.; Xu, J.; Zhang, J.-M.; Yu, D.-P. Surface Effects on Photoluminescence of Single ZnO Nanowires. Phys. Lett. A 2008, 372, 4505–4509. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kato, H.; Miyamoto, K.; Sano, M.; Zhukov, E.A.; Yao, T. Correlation between Grain Size and Optical Properties in Zinc Oxide Thin Films. Appl. Phys. Lett. 2002, 81, 1231–1233. [Google Scholar] [CrossRef]
- Shalish, I.; Temkin, H.; Narayanamurti, V. Size-Dependent Surface Luminescence in ZnO Nanowires. Phys. Rev. B 2004, 69, 245401. [Google Scholar] [CrossRef]
- Sawada, H.; Wang, R.; Sleight, A.W. An Electron Density Residual Study of Zinc Oxide. J. Solid State Chem. 1996, 122, 148–150. [Google Scholar] [CrossRef]
- Bura, M.; Singh, G.; Gupta, D.; Malik, N.; Salim, A.; Kumar, A.; Singhal, R.; Kumar, S.; Aggarwal, S. Transition in the Preferred Orientation of RF Sputtered ZnO/Si Thin Films by Thermal Annealing: Structural, Morphological, and Optical Characteristics. Opt. Mater. 2022, 133, 113024. [Google Scholar] [CrossRef]
- Gupta, V.; Mansingh, A. Influence of Postdeposition Annealing on the Structural and Optical Properties of Sputtered Zinc Oxide Film. J. Appl. Phys. 1996, 80, 1063–1073. [Google Scholar] [CrossRef]
- Gonçalves, G.; Elamurugu, E.; Barquinha, P.; Pereira, L.; Martins, R.; Fortunato, E. Influence of Post-Annealing Temperature on the Properties Exhibited by ITO IZO and GZO Thin Films. Thin Solid Films 2007, 515, 8562–8566. [Google Scholar] [CrossRef]
- Valdez, L.A.; Caravaca, M.A.; Casali, R.A. Ab-Initio Study of Elastic Anisotropy, Hardness and Volumetric Thermal Expansion Coefficient of ZnO, ZnS, ZnSe in Wurtzite and Zinc Blende Phases. J. Phys. Chem. Solids 2019, 134, 245–254. [Google Scholar] [CrossRef]
- Watanabe, H.; Yamada, N.; Okaji, M. Linear Thermal Expansion Coefficient of Silicon from 293 to 1000 K. Int. J. Thermophys. 2004, 25, 221–236. [Google Scholar] [CrossRef]
- Ibach, H. Thermal Expansion of Silicon and Zinc Oxide (II). Phys. Status Solidi B 1969, 33, 257–265. [Google Scholar] [CrossRef]
- Van Dijken, A.; Meulenkamp, E.A.; Vanmaekelbergh, D.; Meijerink, A. The Luminescence of Nanocrystalline ZnO Particles: The Mechanism of the Ultraviolet and Visible Emission. J. Lumin. 2000, 87–89, 454–456. [Google Scholar] [CrossRef]
- Wang, Y.G.; Lau, S.P.; Lee, H.W.; Yu, S.F.; Tay, B.K.; Zhang, X.H.; Hng, H.H. Photoluminescence Study of ZnO Films Prepared by Thermal Oxidation of Zn Metallic Films in Air. J. Appl. Phys. 2003, 94, 354–358. [Google Scholar] [CrossRef]
- Burstein, E. Anomalous Optical Absorption Limit in InSb. Phys. Rev. 1954, 93, 632–633. [Google Scholar] [CrossRef]
- Shan, F.K.; Liu, G.X.; Lee, W.J.; Shin, B.C.; Kim, S.C. Nanoscale Phenomena of Gallium-Doped ZnO Thin Films on Sapphire Substrates. J. Electroceram. 2006, 17, 287–292. [Google Scholar] [CrossRef]
- Wang, L.-W.; Chu, S.-Y. Effects of Post-Annealing on the Properties of ZnO:Ga Films with High Transparency (94%) and Low Sheet Resistance (29 Ω/Square). Materials 2023, 16, 6463. [Google Scholar] [CrossRef] [PubMed]
- Sim, K.U.; Shin, S.W.; Moholkar, A.V.; Yun, J.H.; Moon, J.H.; Kim, J.H. Effects of Dopant (Al, Ga, and In) on the Characteristics of ZnO Thin Films Prepared by RF Magnetron Sputtering System. Curr. Appl. Phys. 2010, 10, S463–S467. [Google Scholar] [CrossRef]
- Du Ahn, B.; Hoon Oh, S.; Hee Lee, C.; Hee Kim, G.; Jae Kim, H.; Yeol Lee, S. Influence of Thermal Annealing Ambient on Ga-Doped ZnO Thin Films. J. Cryst. Growth 2007, 309, 128–133. [Google Scholar] [CrossRef]
- McCluskey, M.D.; Corolewski, C.D.; Lv, J.; Tarun, M.C.; Teklemichael, S.T.; Walter, E.D.; Norton, M.G.; Harrison, K.W.; Ha, S. Acceptors in ZnO. J. Appl. Phys. 2015, 117, 112802. [Google Scholar] [CrossRef]
- Willander, M.; Nur, O.; Sadaf, J.R.; Qadir, M.I.; Zaman, S.; Zainelabdin, A.; Bano, N.; Hussain, I. Luminescence from Zinc Oxide Nanostructures and Polymers and Their Hybrid Devices. Materials 2010, 3, 2643–2667. [Google Scholar] [CrossRef]
- Gupta, H.; Singh, J.; Dutt, R.N.; Ojha, S.; Kar, S.; Kumar, R.; Reddy, V.R.; Singh, F. Defect-Induced Photoluminescence from Gallium-Doped Zinc Oxide Thin Films: Influence of Doping and Energetic Ion Irradiation. Phys. Chem. Chem. Phys. 2019, 21, 15019–15029. [Google Scholar] [CrossRef] [PubMed]
- Tam, K.H.; Cheung, C.K.; Leung, Y.H.; Djurišić, A.B.; Ling, C.C.; Beling, C.D.; Fung, S.; Kwok, W.M.; Chan, W.K.; Phillips, D.L.; et al. Defects in ZnO Nanorods Prepared by a Hydrothermal Method. J. Phys. Chem. B 2006, 110, 20865–20871. [Google Scholar] [CrossRef]
- Baek, M.; Kim, D.; Yong, K. Simple but Effective Way To Enhance Photoelectrochemical Solar-Water-Splitting Performance of ZnO Nanorod Arrays: Charge-Trapping Zn(OH)2 Annihilation and Oxygen Vacancy Generation by Vacuum Annealing. ACS Appl. Mater. Inter. 2017, 9, 2317–2325. [Google Scholar] [CrossRef]
- Xie, R.; Li, D.; Yang, D.; Sekiguchi, T.; Jiang, M. Thermal-Desorption Induced Enhancement and Patterning of Ultraviolet Emission in Chemically Grown ZnO. Nanotechnology 2006, 17, 2789. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Leung, Y.H.; Tam, K.H.; Hsu, Y.F.; Ding, L.; Ge, W.K.; Zhong, Y.C.; Wong, K.S.; Chan, W.K.; Tam, H.L.; et al. Defect Emissions in ZnO Nanostructures. Nanotechnology 2007, 18, 095702. [Google Scholar] [CrossRef]
- Meyer, B.K.; Sann, J.; Hofmann, D.M.; Neumann, C.; Zeuner, A. Shallow Donors and Acceptors in ZnO. Semicond. Sci. Technol. 2005, 20, S62. [Google Scholar] [CrossRef]
- Shan, W.; Walukiewicz, W.; Ager, J.W., III; Yu, K.M.; Yuan, H.B.; Xin, H.P.; Cantwell, G.; Song, J.J. Nature of Room-Temperature Photoluminescence in ZnO. Appl. Phys. Lett. 2005, 86, 191911. [Google Scholar] [CrossRef]
- Teklemichael, S.T.; McCluskey, M.D. Acceptor and Surface States of ZnO Nanocrystals: A Unified Model. Nanotechnology 2011, 22, 475703. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, Z.; Han, D.; Gu, F.; Guo, G. Porous ZnO Polygonal Nanoflakes: Synthesis, Use in High-Sensitivity NO2 Gas Sensor, and Proposed Mechanism of Gas Sensing. J. Phys. Chem. C 2011, 115, 12763–12773. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Peng, Y.-C.; Lu, J.-H.; Zhu, Y.-R.; Wu, H.-C. Electronic and Optical Properties of Ga-Doped ZnO. Thin Solid Films 2014, 570, 464–470. [Google Scholar] [CrossRef]
- Alvi, N.H.; ul Hasan, K.; Nur, O.; Willander, M. The Origin of the Red Emission in n-ZnO Nanotubes/p-GaN White Light Emitting Diodes. Nanoscale Res. Lett. 2011, 6, 130. [Google Scholar] [CrossRef]
- Kumar, V.; Swart, H.; Ntwaeaborwa, O.; Kroon, R.; Terblans, J.; Shaat, S.; Mohmmed, A.; Duvenhage, M.-M. Origin of the Red Emission in Zinc Oxide Nanophosphors. Mater. Lett. 2013, 101, 57. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, X.; Li, R.; Li, Q. Planar-Defect-Rich Zinc Oxide Nanoparticles Assembled on Carbon Nanotube Films as Ultraviolet Emitters and Photocatalysts. Sci. Rep. 2014, 4, 4728. [Google Scholar] [CrossRef]
- Van Duy, L.; Nguyet, T.T.; Hung, C.M.; Thanh Le, D.T.; Van Duy, N.; Hoa, N.D.; Biasioli, F.; Tonezzer, M.; Di Natale, C. Ultrasensitive NO2 Gas Sensing Performance of Two Dimensional ZnO Nanomaterials: Nanosheets and Nanoplates. Ceram. Int. 2021, 47, 28811–28820. [Google Scholar] [CrossRef]
- Patil, V.L.; Bhosale, S.R.; Bhosale, R.R.; Tarwal, N.L.; Vanalakar, S.A.; Dhavale, R.P.; Sharma, K.K.; Patil, P.S. Low Temperature Chemiresistive Gas Sensing Performance towards Oxidising Gas Based on Chemically Prepared Ga Doped ZnO Nanorods. Inorg. Chem. Commun. 2023, 152, 110691. [Google Scholar] [CrossRef]
- Vorobyeva, N.; Rumyantseva, M.; Filatova, D.; Spiridonov, F.; Zaytsev, V.; Zaytseva, A.; Gaskov, A. Highly Sensitive ZnO(Ga,In) for Sub-ppm Level NO2 Detection: Effect of Indium Content. Chemosensors 2017, 5, 18. [Google Scholar] [CrossRef]
- Rigon, M.; Paolucci, V.; Sturaro, M.; Emamjomeh, S.M.; Cantalini, C.; Martucci, A. Effect of Pt Nanoparticles on the Plasmonic and Chemoresistive Gas Sensing Properties of ZnO:Ga Film. Proceedings 2018, 2, 997. [Google Scholar] [CrossRef]
- Ramola, R.C.; Negi, S.; Rawat, M.; Singh, R.C.; Singh, F. Annealing Effects on Gas Sensing Response of Ga-Doped ZnO Thin Films. ACS Omega 2021, 6, 11660–11668. [Google Scholar] [CrossRef]





| Deposition Parameters | Value |
|---|---|
| Target material | ZnO:Ga (4%at) |
| Power per surface unit | 1.14 W/cm2 |
| Magnetron | Yes |
| Argon pressure | 2 Pa |
| Target to substrate distance | 7 cm |
| Mean free path | 0.3 cm |
| Deposition speed | 2 nm/min |
| Sample Name | Atomic Concentration (%) | ||||
|---|---|---|---|---|---|
| O | Zn | Ga | Si | O/(Zn + Ga) | |
| A0 | 49.00 ± 0.03 | 47.58 ± 0.05 | 2.18 ± 0.01 | 1.25 ± 0.05 | 0.985 ± 0.002 |
| A500 | 48.58 ± 0.03 | 47.99 ± 0.03 | 2.18 ± 0.01 | 1.25 ± 0.06 | 0.968 ± 0.001 |
| A600 | 48.61 ± 0.07 | 47.50 ± 0.04 | 2.17 ± 0.01 | 1.73 ± 0.09 | 0.979 ± 0.002 |
| A700 | 48.37 ± 0.02 | 48.17 ± 0.04 | 2.20 ± 0.01 | 1.26 ± 0.05 | 0.960 ± 0.001 |
| Sample Name | 2θ (002) (°) | d(002) (Å) |
|---|---|---|
| ZnO:Ga 4%at reference [34] | 2.602 | |
| A0 | 34.30 | 2.612 |
| A500 | 34.50 | 2.597 |
| A600 | 34.54 | 2.594 |
| A700 | 34.56 | 2.593 |
| Sample Name | Average Grain Diameter (nm) |
|---|---|
| A0 | 14.6 ± 1.3 |
| A500 | 15.6 ± 1.4 |
| A600 | 16.1 ± 1.3 |
| A700 | 15.9 ± 1.4 |
| Line No. 3 | Line No. 4 | Line No. 5 |
|---|---|---|
| 2.7 eV | 2.2 eV | 1.8 eV |
| [28] | [29] | [57,58] (unspecified charge) |
| [26] | [46] | |
| - [46,47,48,49,50,51] | [8] |
| Sample Name | RAir/RArgon | RNO2/RAir | RNO2/RArgon |
|---|---|---|---|
| A0 | 9 | 2.4 | 23 |
| A500 | 51 | 2.8 | 142 |
| A600 | 8 | 10.9 | 90 |
| A700 | 4 | 2.7 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paret, B.; Monflier, R.; Menini, P.; Camps, T.; Thimont, Y.; Barnabé, A.; Presmanes, L. Study of the Influence of Thermal Annealing of Ga-Doped ZnO Thin Films on NO2 Sensing at ppb Level. Chemosensors 2025, 13, 1. https://doi.org/10.3390/chemosensors13010001
Paret B, Monflier R, Menini P, Camps T, Thimont Y, Barnabé A, Presmanes L. Study of the Influence of Thermal Annealing of Ga-Doped ZnO Thin Films on NO2 Sensing at ppb Level. Chemosensors. 2025; 13(1):1. https://doi.org/10.3390/chemosensors13010001
Chicago/Turabian StyleParet, Benjamin, Richard Monflier, Philippe Menini, Thierry Camps, Yohann Thimont, Antoine Barnabé, and Lionel Presmanes. 2025. "Study of the Influence of Thermal Annealing of Ga-Doped ZnO Thin Films on NO2 Sensing at ppb Level" Chemosensors 13, no. 1: 1. https://doi.org/10.3390/chemosensors13010001
APA StyleParet, B., Monflier, R., Menini, P., Camps, T., Thimont, Y., Barnabé, A., & Presmanes, L. (2025). Study of the Influence of Thermal Annealing of Ga-Doped ZnO Thin Films on NO2 Sensing at ppb Level. Chemosensors, 13(1), 1. https://doi.org/10.3390/chemosensors13010001






