Microneedle-Based Sensors for Wearable Diagnostics
Abstract
1. Introduction
2. MN Sensors Applied to Human Health
2.1. Real-Time Sensing of Two Metabolites (Lactate and Glucose or Alcohol and Glucose)
2.2. Transdermal Tracing of Electrolytes
2.3. Rapid Detection of Methotrexate
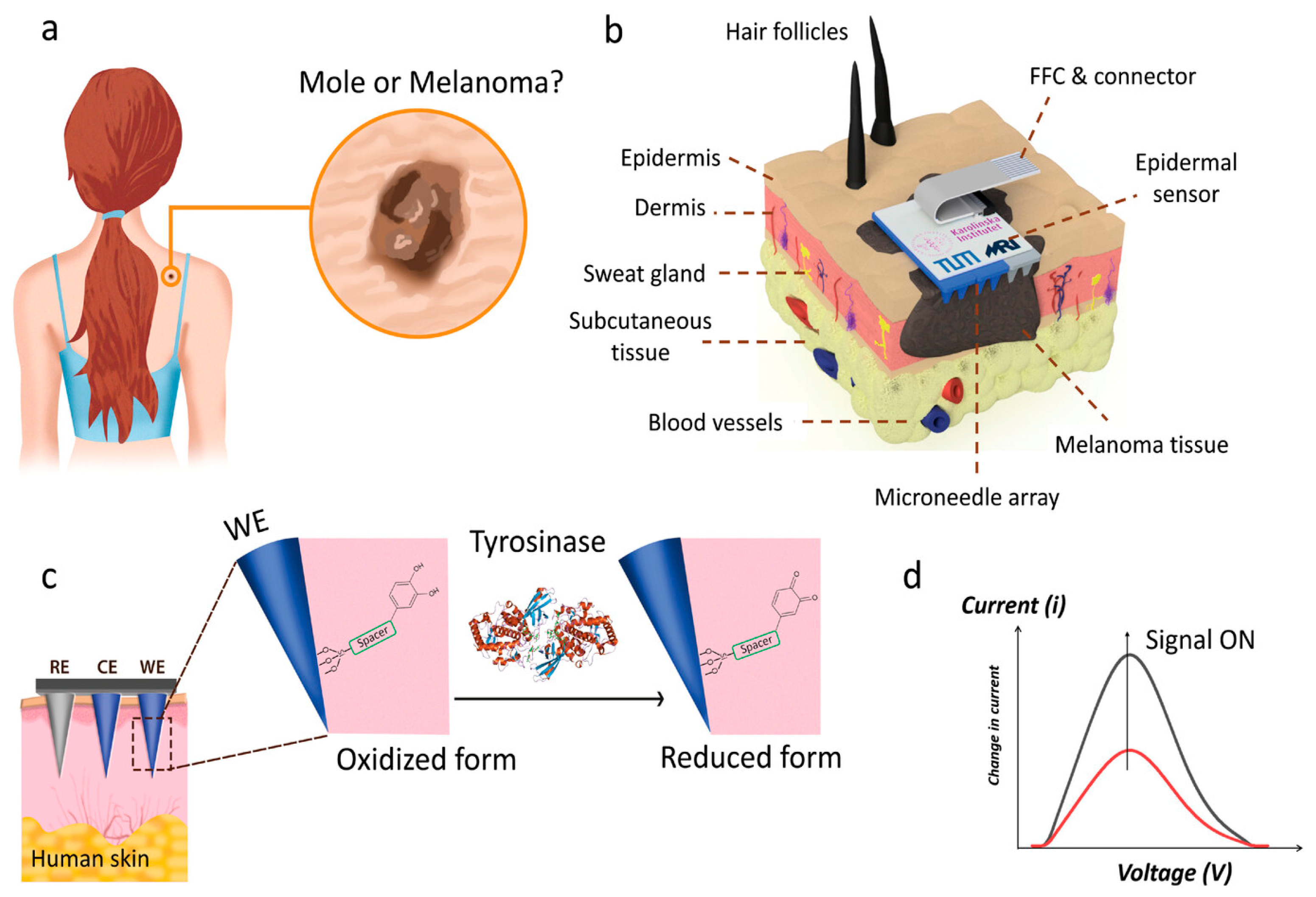
2.4. Transdermal Sensing of Tyrosinase Enzyme as Melanoma Biomarker
2.5. Real-Time cfDNA Extraction and Monitoring
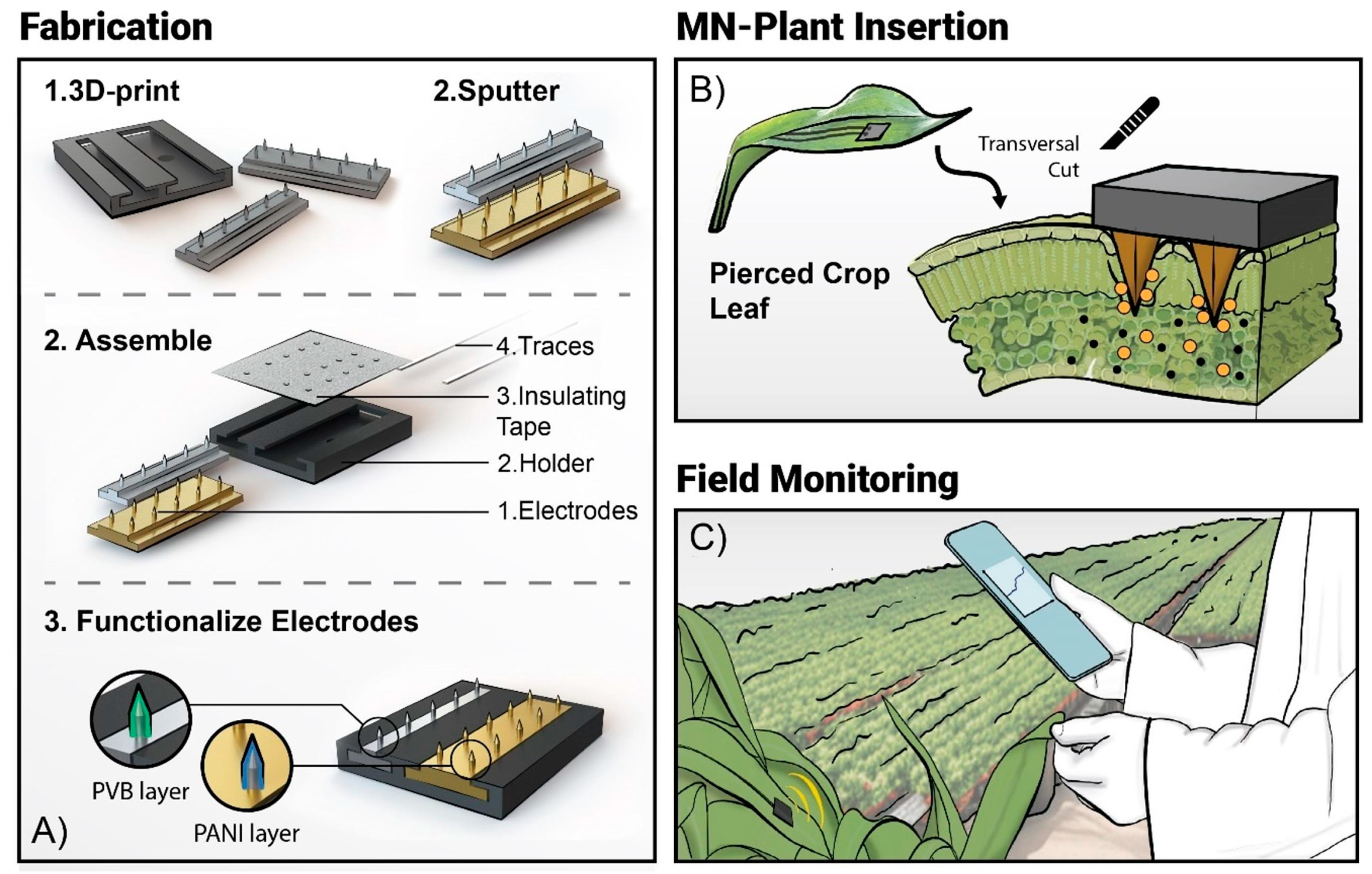
3. MN Sensors Applied to Precision Agriculture
4. MN Devices on the Market
5. Challenges
5.1. User Comfort and Longevity
5.2. Biofouling and Biocompatibility
5.3. Optimized Design and Multiplexing
5.4. Standardization and Regulatory Approval
5.5. Necessary Interdisciplinarity
Author Contributions
Funding
Conflicts of Interest
References
- Henry, S.; McAllister, D.V.; Allen, M.G.; Prausnitz, M.G. Microfabricated Microneedles: A Novel Approach to Transdermal Drug Delivery. J. Pharm. Sci. 1998, 87, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.A.; Li, R.; Tse, Z.T.H. Reshaping healthcare with wearable biosensors. Sci. Rep. 2023, 13, 4998. [Google Scholar] [CrossRef] [PubMed]
- Ometov, A.; Shubina, V.; Klus, L.; Skibińska, J.; Saafi, S.; Pascacio, P.; Flueratoru, L.; Gaibor, D.Q.; Chukhno, N.; Chukhno, O. A survey on wearable technology: History, state-of-the-art and current challenges. Comput. Netw. 2021, 193, 108074. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Mao, S.; Chen, S.; Ma, J.; Jaffrezic-Renault, N.; Guo, Z. Opportunities and challenges of microneedle electrochemical sensors for interstitial fluid detection. TrAC Trends Anal. Chem. 2024, 180, 117891. [Google Scholar] [CrossRef]
- Tehrani, F.; Teymourian, H.; Wuerstle, B.; Kavner, J.; Patel, R.; Furmidge, A.; Aghavali, R.; Hosseini-Toudeshki, H.; Brown, C.; Zhang, F.; et al. An Integrated Wearable Microneedle Array for the Continuous Monitoring of Multiple Biomarkers in Interstitial Fluid. Nat. Biomed. Eng. 2022, 6, 1214–1224. [Google Scholar] [CrossRef]
- Lin, S.; Cheng, X.; Zhu, J.; Wang, B.; Jelinek, D.; Zhao, Y.; Wu, T.-Y.; Horrillo, A.; Tan, J.; Yeung, J.; et al. Wearable Microneedle-Based Electrochemical Aptamer Biosensing for Precision Dosing of Drugs with Narrow Therapeutic Windows. Sci. Adv. 2022, 8, eabq4539. [Google Scholar] [CrossRef]
- Parrilla, M.; Vanhooydonck, A.; Johns, M.; Watts, R.; De Wael, K. 3D-Printed Microneedle-Based Potentiometric Sensor for PH Monitoring in Skin Interstitial Fluid. Sens. Actuators B Chem. 2023, 378, 133159. [Google Scholar] [CrossRef]
- Goud, K.Y.; Mahato, K.; Teymourian, H.; Longardner, K.; Litvan, I.; Wang, J. Wearable Electrochemical Microneedle Sensing Platform for Real-Time Continuous Interstitial Fluid Monitoring of Apomorphine: Toward Parkinson Management. Sens. Actuators B Chem. 2022, 354, 131234. [Google Scholar] [CrossRef]
- Parrilla, M.; Detamornrat, U.; Dominguez-Robles, J.; Donnelly, R.F.; De Wael, K. Wearable Hollow Microneedle Sensing Patches for the Transdermal Electrochemical Monitoring of Glucose. Talanta 2022, 249, 123695. [Google Scholar] [CrossRef]
- Zheng, L.; Zhu, D.; Wang, W.; Liu, J.; Thng, S.T.G.; Chen, P. A Silk-Microneedle Patch to Detect Glucose in the Interstitial Fluid of Skin or Plant Tissue. Sens. Actuators B Chem. 2022, 372, 132626. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, Y.; Lee, J.; Hoover, A.; King, S.A.; Chen, L.; Zhao, J.; Lin, Q.; Yu, C.; Zhu, L. Continuous glucose monitoring enabled by fluorescent nanodiamond boronic hydrogel. Adv. Sci. 2023, 10, 2203943. [Google Scholar] [CrossRef]
- He, R.; Liu, H.; Fang, T.; Niu, Y.; Zhang, H.; Han, F.; Gao, B.; Li, F.; Xu, F. A Colorimetric Dermal Tattoo Biosensor Fabricated by Microneedle Patch for Multiplexed Detection of Health-Related Biomarkers. Adv. Sci. 2021, 8, 2103030. [Google Scholar] [CrossRef] [PubMed]
- Friedel, M.; Thompson, I.A.P.; Kasting, G.; Polsky, R.; Cunningham, D.; Soh, H.T.; Heikenfeld, J. Opportunities and Challenges in the Diagnostic Utility of Dermal Interstitial Fluid. Nat. Biomed. Eng. 2023, 7, 1541–1555. [Google Scholar] [CrossRef]
- Vora, L.K.; Sabri, A.H.; McKenna, P.E.; Himawan, A.; Hutton, A.R.J.; Detamornrat, A.; Paredes, A.J.; Larraneta, E.; Donnelly, R.F. Microneedle-based biosensing. Nat. Rev. Bioeng. 2024, 2, 64–81. [Google Scholar] [CrossRef]
- Li, J.; Wei, M.; Gao, B. A review of recent advances in microneedle-based sensing within the dermal ISF that could transform medical testing. ACS Sens. 2024, 9, 1149–1161. [Google Scholar] [CrossRef] [PubMed]
- Poudineh, M. Microneedle Assays for Continuous Health Monitoring: Challenges and Solutions. ACS Sens. 2024, 9, 535–542. [Google Scholar] [CrossRef]
- Chinnadayyala, S.R.; Park, J.; Satti, A.T.; Kim, D.; Cho, S. Minimally invasive and continuous glucose monitoring sensor based on non-enzymatic porous platinum black-coated gold microneedles. Electrochim. Acta 2021, 369, 137691. [Google Scholar] [CrossRef]
- Ma, T.Q.; He, Q.Y.; Li, X.Y.; Zhang, B.; Li, Y.L.; Chen, L.J.; You, X.Q.; Liu, J. Wearable microneedle sensor with replaceable sensor base for multi-purpose detecting in skin interstitial fluid. Microchem. J. 2024, 203, 110933. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, S.; Ma, D.; Zhang, T.; Huang, X.; Huang, S.; Chen, H.J.; Wang, J.; Jiang, L.; Xie, X. Masticatory system–inspired microneedle theranostic platform for intelligent and precise diabetic management. Sci. Adv. 2022, 8, eabo6900. [Google Scholar] [CrossRef]
- Molinero-Fernández, A.; Casanova, A.; Wang, Q.; Cuartero, M.; Crespo, G.A. In Vivo Transdermal Multi-Ion Monitoring with a Potentiometric Microneedle-Based Sensor Patch. ACS Sens. 2023, 8, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Parrilla, M.; Detamornrat, U.; Domínguez-Robles, J.; Tunca, S.; Donnelly, R.F.; De Wael, K. Wearable microneedle-based array patches for continuous electrochemical monitoring and drug delivery: Toward a closed-loop system for methotrexate treatment. ACS Sens. 2023, 8, 4161–4170. [Google Scholar] [CrossRef] [PubMed]
- Poursharifi, N.; Hassanpouramiri, M.; Zink, A.; Ucuncu, M.; Parlak, O. Transdermal Sensing of Enzyme Biomarker Enabled by Chemo-Responsive Probe-Modified Epidermal Microneedle Patch in Human Skin Tissue. Adv. Mater. 2024, 36, 2403758. [Google Scholar] [CrossRef]
- Yang, B.; Kong, J.; Fang, X. Programmable CRISPR-Cas9 microneedle patch for long-term capture and real-time monitoring of universal cell-free DNA. Nat. Commun. 2022, 13, 3999. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wang, H.; Kong, J.; Fang, X. Long-term monitoring of ultratrace nucleic acids using tetrahedral nanostructure-based NgAgo on wearable microneedles. Nat. Commun. 2024, 15, 1936. [Google Scholar] [CrossRef]
- Yin, H.; Cao, Y.; Marelli, B.; Zeng, X.; Mason, A.J.; Cao, C. Soil sensors and plant wearables for smart and precision agriculture. Adv. Mater. 2021, 33, 2007764. [Google Scholar] [CrossRef]
- Coatsworth, P.; Gonzalez-Macia, L.; Collins, A.S.P.; Bozkurt, T.; Güder, F. Continuous monitoring of chemical signals in plants under stress. Nat. Rev. Chem. 2022, 7, 7–25. [Google Scholar] [CrossRef]
- Vulpe, G.; Liu, G.; Oakley, S.; Pletsas, D.; Yang, G.; Dutra, R.; Guy, O.; Liu, Y.; Waldron, M.; Neary, J.; et al. Wearable technology for one health: Charting the course of dermal biosensing. Biosens. Bioelectron. X 2024, 19, 100500. [Google Scholar] [CrossRef]
- Wang, B.; Lu, H.; Jiang, S.; Gao, B. Recent advances of microneedles biosensors for plants. Anal. Bioanal. Chem. 2024, 416, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Parrilla, M.; Steijlen, A.; Kerremans, R.; Jacobs, J.; den Haan, L.; De Vreese, J.; Van Noten Geron, Y.; Clerx, P.; Watts, R.; De Wael, K. Wearable platform based on 3D-printed solid microneedle potentiometric pH sensor for plant monitoring. Chem. Eng. J. 2024, 500, 157254. [Google Scholar] [CrossRef]
- Hu, Y.; Chatzilakou, E.; Pan, Z.; Traverso, G.; Yetisen, A.K. Microneedle Sensors for Point-of-Care Diagnostics. Adv. Sci. 2024, 11, 2306560. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Halima, H.; Lakard, B.; Jaffrezic-Renault, N. Microneedle-Based Sensors for Wearable Diagnostics. Chemosensors 2025, 13, 68. https://doi.org/10.3390/chemosensors13020068
Ben Halima H, Lakard B, Jaffrezic-Renault N. Microneedle-Based Sensors for Wearable Diagnostics. Chemosensors. 2025; 13(2):68. https://doi.org/10.3390/chemosensors13020068
Chicago/Turabian StyleBen Halima, Hamdi, Boris Lakard, and Nicole Jaffrezic-Renault. 2025. "Microneedle-Based Sensors for Wearable Diagnostics" Chemosensors 13, no. 2: 68. https://doi.org/10.3390/chemosensors13020068
APA StyleBen Halima, H., Lakard, B., & Jaffrezic-Renault, N. (2025). Microneedle-Based Sensors for Wearable Diagnostics. Chemosensors, 13(2), 68. https://doi.org/10.3390/chemosensors13020068






