Resistive Low-Temperature Sensor Based on the SiO2ZrO2 Film for Detection of High Concentrations of NO2 Gas
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
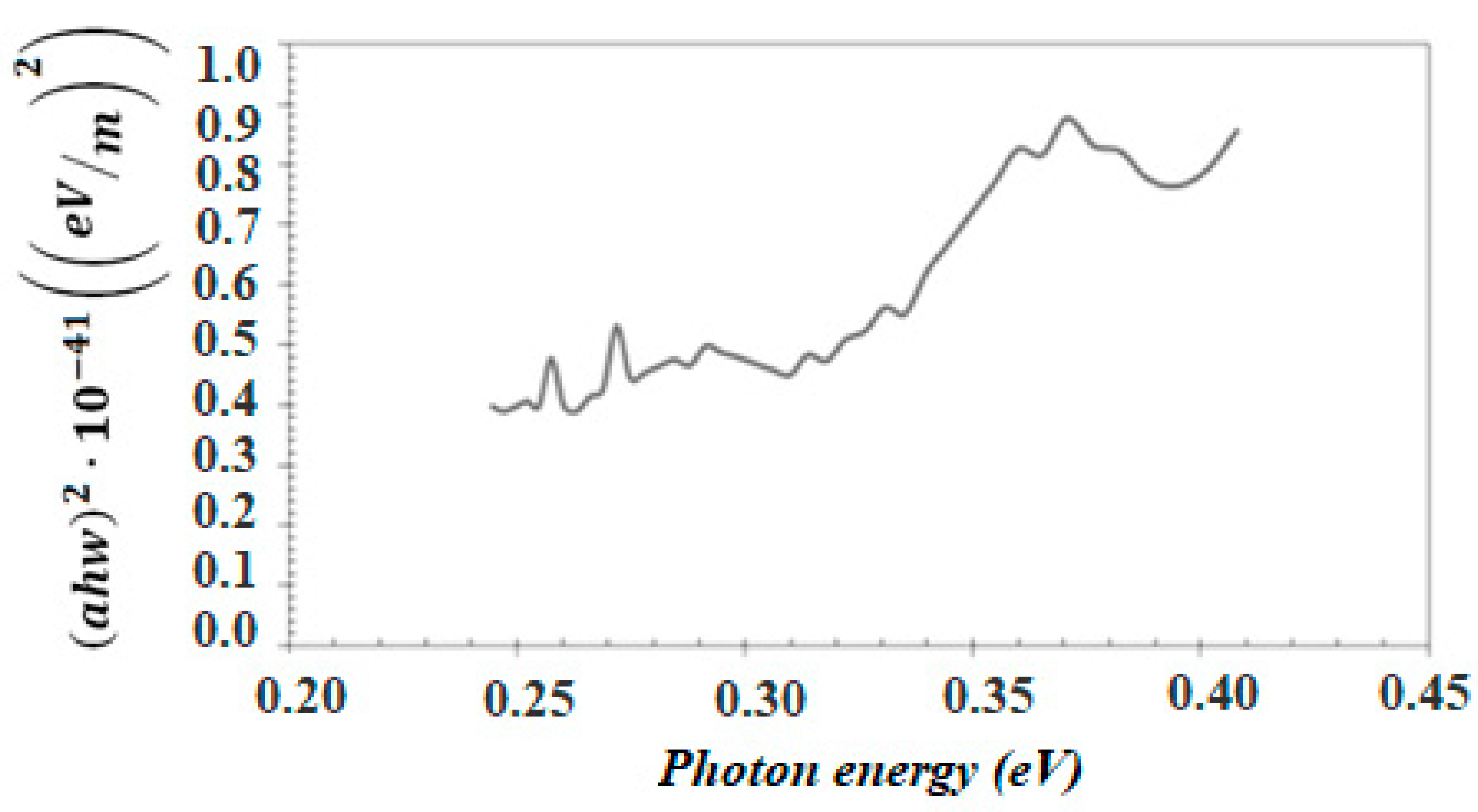
3.1. Characterization
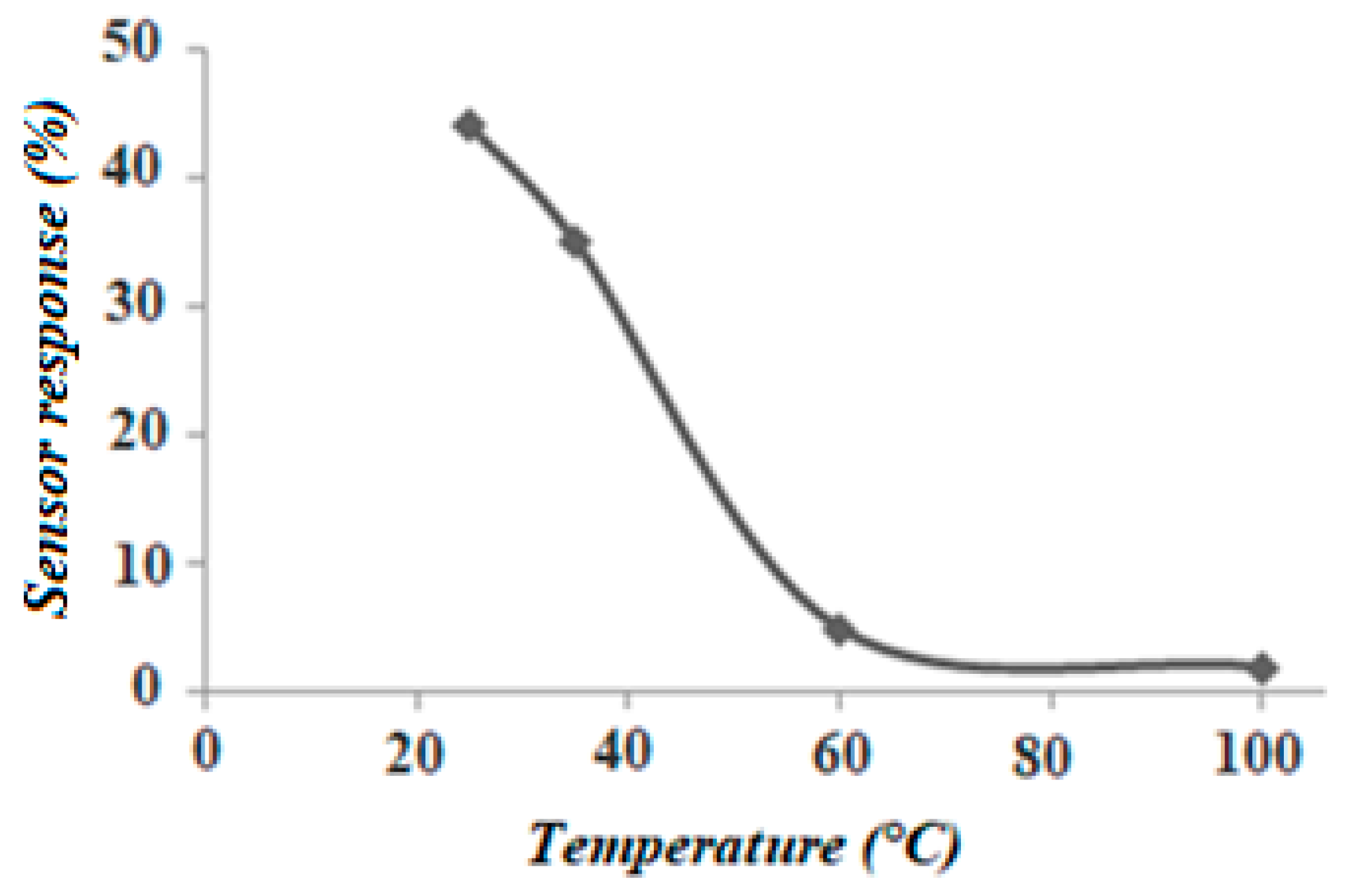
3.2. Gas Sensitivity Measurements
3.3. Gas Sensitivity Mechanism
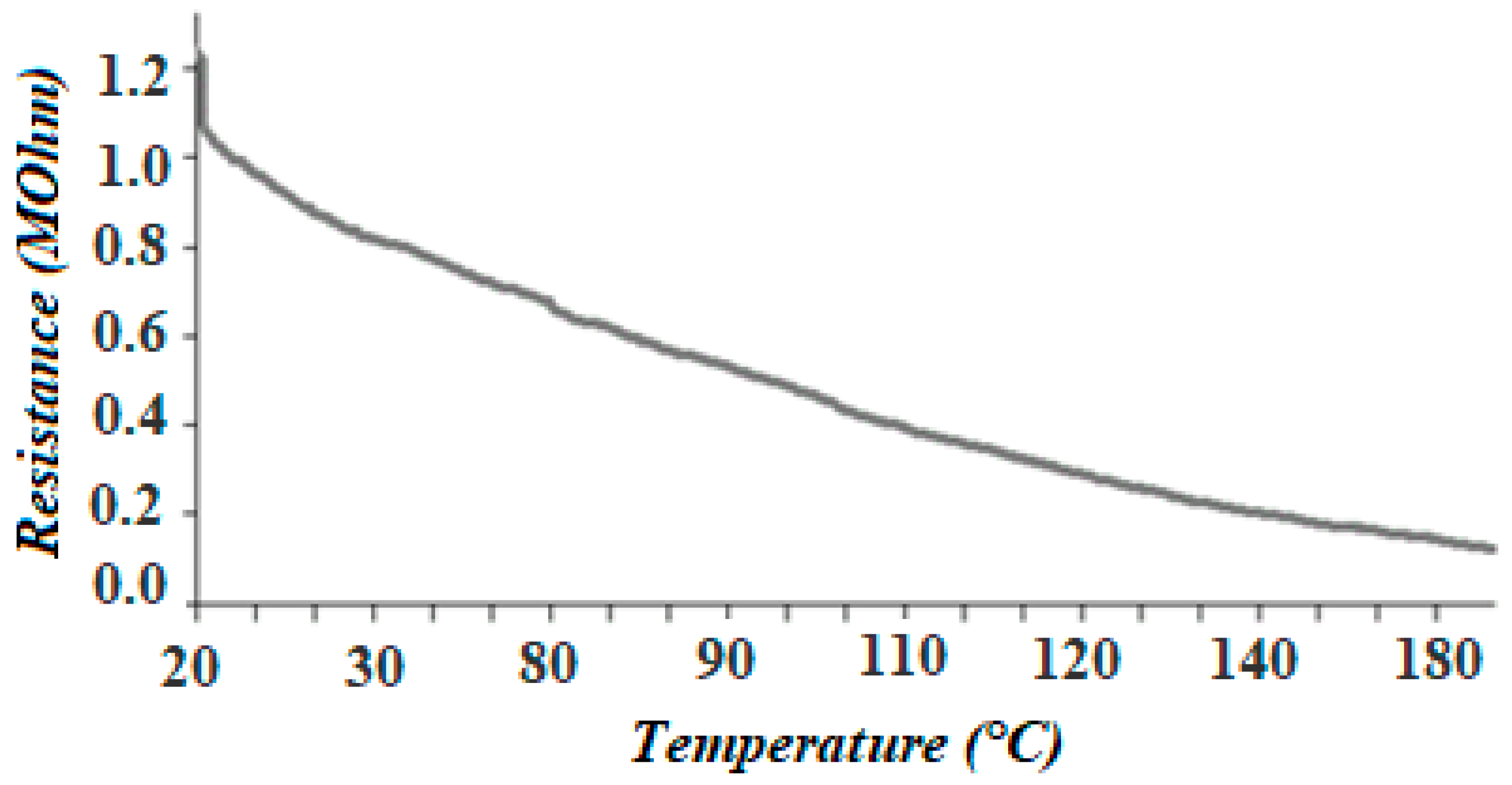
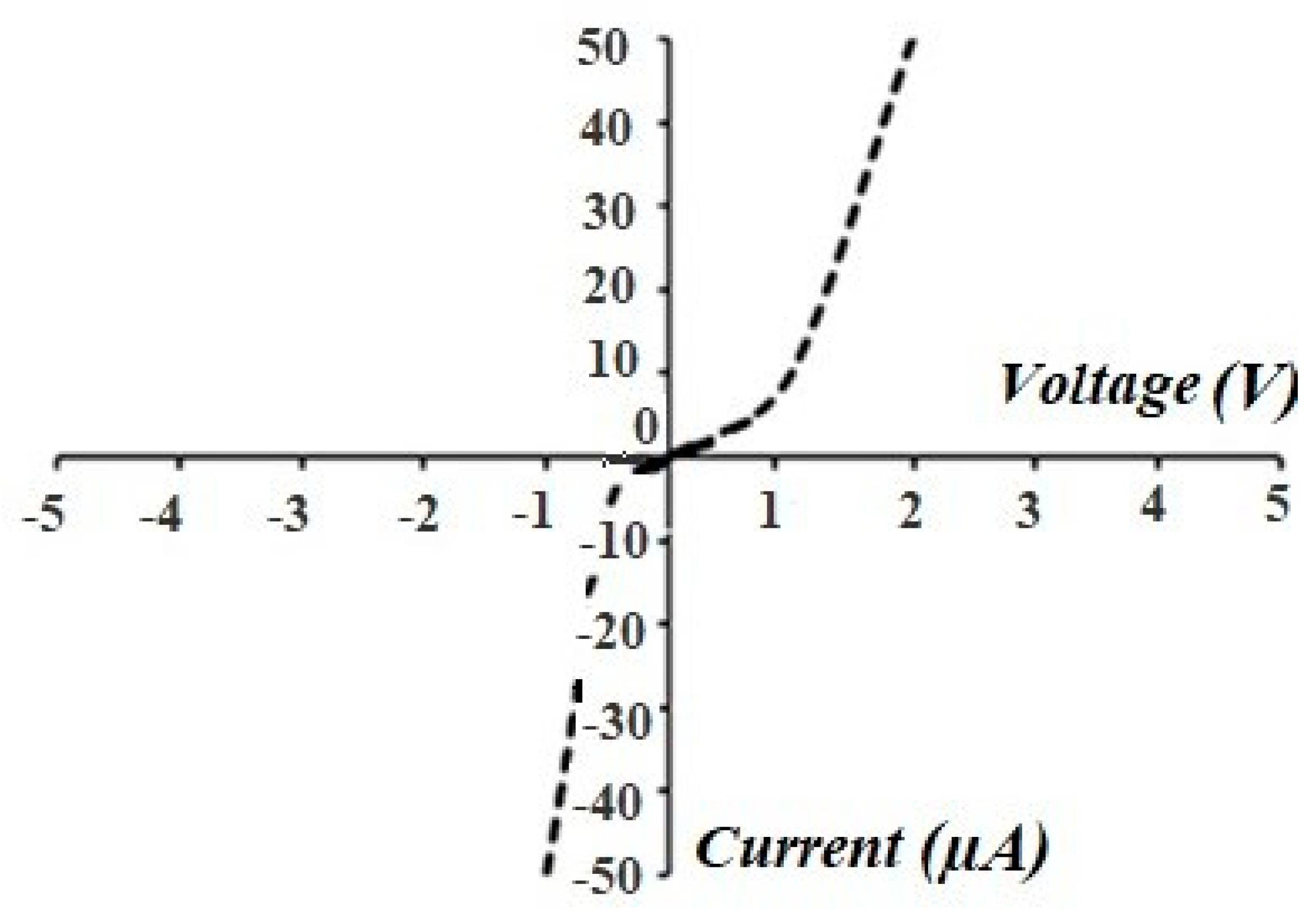
3.4. Electrophysical Measurements
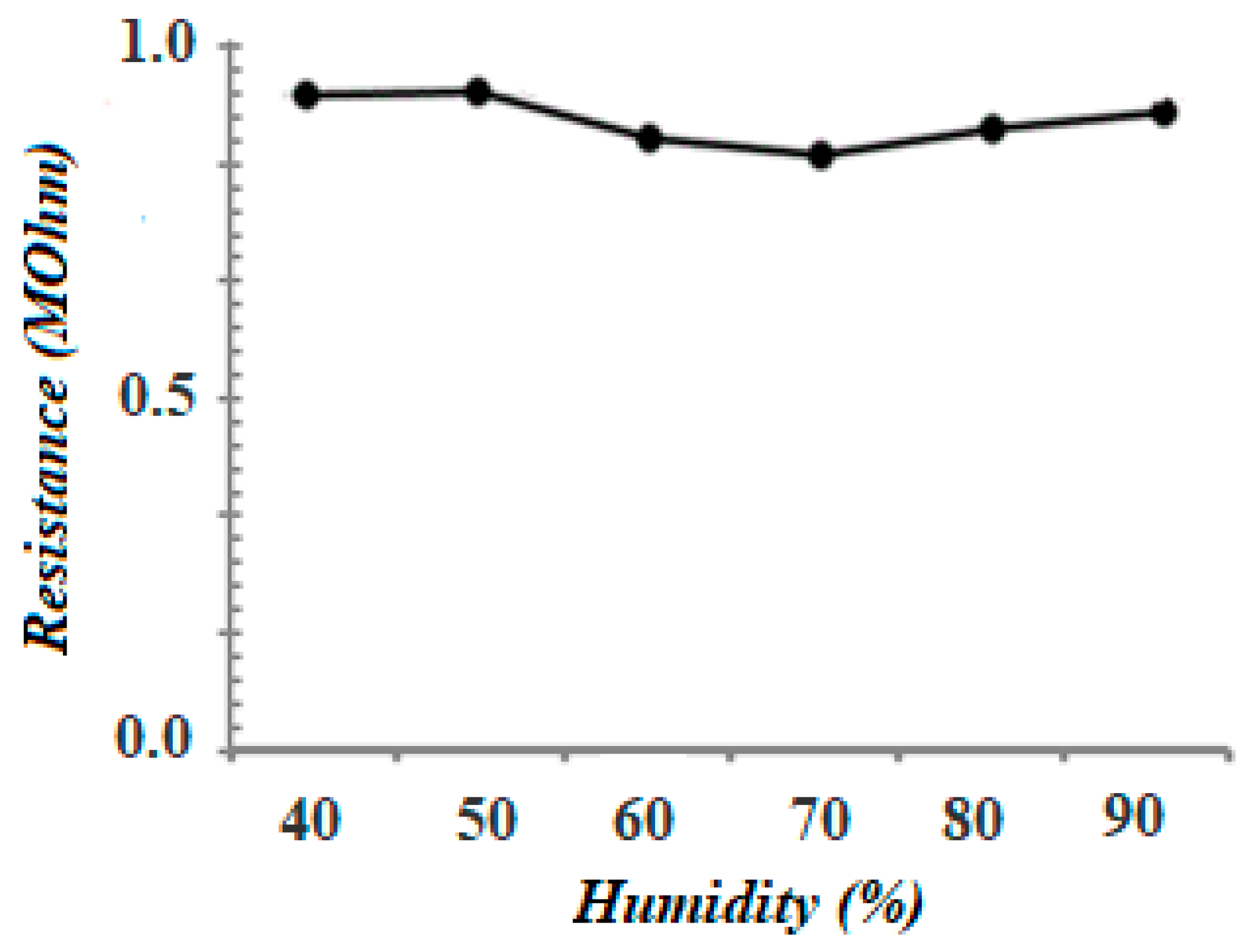
3.5. Stability Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mohsenin, V. Human exposure to oxides of nitrogen at ambient and supra-ambient concentrations. Toxicology 1994, 89, 301–312. [Google Scholar] [CrossRef]
- Mayorga, M.A. Overview of nitrogen dioxide effects on the lung with emphasis on military relevance. Toxicology 1994, 89, 175–192. [Google Scholar] [CrossRef] [Green Version]
- Baccile, N.; Babonneau, F.; Thomas, B.; Coradin, T. Introducing ecodesign in silica sol-gel materials. J. Mater. Chem. 2009, 19, 8537–8559. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, L.; Gao, C.; Zhao, Y. Synthesis of ZrO2–SiO2 mixed oxide by alcohol-aqueous heating method. J. Sol-Gel Sci. Technol. 2011, 58, 572–579. [Google Scholar] [CrossRef]
- Ponomareva, A.A.; Moshnikov, V.A.; Glöß, D.; Delan, A.; Kleiner, A.; Suchaneck, G. Metal-oxide-based nanocomposites comprising advanced gas sensing properties. J. Phys. Conf. Ser. 2012, 345, 012029. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Ping, Y.; Li, J.; Matras-Postolek, K.; Yue, Y.; Huang, B. Formation of SiO2-SnO2 Core-Shell Nanofibers and Their Gas Sensing Properties. RSC Adv. 2016, 6, 13371–13376. [Google Scholar] [CrossRef]
- Hirvonen, A.; Nowak, R.; Yamamoto, Y.; Sekino, T.; Niihara, K. Fabrication, structure, mechanical and thermal properties of zirconia-based ceramic nanocomposites. J. Eur. Ceram. Soc. 2006, 26, 1497–1505. [Google Scholar] [CrossRef]
- Ray, J.C.; Park, D.W.; Ahn, W.S. Chemical synthesis of stabilized nanocrystalline zirconia powders. J. Ind. Eng. Chem. 2006, 12, 142–148. [Google Scholar]
- Mokrushin, A.S.; Simonenko, E.P.; Simonenko, N.P.; Bukunov, K.A.; Sevastyanov, V.G.; Kuznetsov, N.T. Gas-sensing properties of nanostructured CeO2-xZrO2 thin films obtained by the sol-gel method. J. Alloys Compd. 2018, 773, 1023–1032. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Mokrushin, A.S.; Popov, V.S.; Vasiliev, A.A.; Sevastyanov, V.G.; Kuznetsov, N.T. Thin films of the composition 8% Y2O3–92% ZrO2 (8YSZ) as gas-sensing materials for oxygen detection. Russ. J. Inorg. Chem. 2017, 62, 695–701. [Google Scholar] [CrossRef]
- Gao, X.J.; Fierro, L.G.; Wachs, I.E. Structural Characteristics and Catalytic Properties of Highly Dispersed ZrO2/SiO2 and V2O5/ZrO2/SiO2 Catalysts. Langmuir 1999, 15, 3169–3178. [Google Scholar] [CrossRef]
- Damyanova, S.; Grange, P.; Delmon, B. Surface Characterization of Zirconia-Coated Alumina and Silica Carriers. J. Catal. 1997, 168, 421–443. [Google Scholar] [CrossRef]
- Takada, T. Ozon detection by In2O3 thin films gas sensor. Chem. Sens. Technol. 1989, 2, 59–70. [Google Scholar]
- Traversa, E.; Sadaoka, Y.; Carotta, M.C.; Martinelli, G. Environmental monitoring field tests using screen-printed thick-film sensors based on semiconducting oxides. Sens. Actuators B Chem. 2000, 65, 181–185. [Google Scholar] [CrossRef]
- Schutze, A.; Pieper, N.; Zacheja, J. Quantitative ozone measurement using a phthalocyanine thin-film sensor and dynamic signal evaluation. Sens. Actuators B Chem. 1995, 23, 215–217. [Google Scholar] [CrossRef]
- Spirig, J.V.; Ramamoorthy, R.; Akbar, S.A.; Dutta, P.K. High temperature Zirconia Oxygen sensor with sealed metal/metal oxide internal reference. Sens. Actuators B Chem. 2007, 124, 192–201. [Google Scholar] [CrossRef]
- Zhuiykov, S. Electrochemistry of Zirconia Gas Sensors; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Myasoedova, T.N.; Yalovega, G.E.; Petrov, V.V.; Zabluda, O.V.; Shmatko, V.A.; Funik, A.O. Properties of SiO2CuOx Films for Nitrogen Dioxide Detection. Adv. Mater. Res. 2014, 834–836, 112–116. [Google Scholar]
- Wang, H.C.; Li, Y.; Yang, M.J. Fast response thin film SnO2 gas sensors operating at room temperature. Sens. Actuators B Chem. 2006, 119, 380–383. [Google Scholar] [CrossRef]
- Wei, B.Y.; Hsu, M.C.; Su, P.G.; Lin, H.M.; Wu, R.J.; Lai, H.J. A novel SnO2 gas sensor doped with carbon nanotubes operating at room temperature. Sens. Actuators B Chem. 2004, 101, 81–89. [Google Scholar] [CrossRef]
- Baratto, C.; Comini, E.; Faglia, G.; Sberveglieri, G.; Zha, M.; Zappettini, A. Metal oxide nanocrystals for gas sensing. Sens. Actuators B Chem. 2005, 109, 2–6. [Google Scholar] [CrossRef]
- Myasoedova, T.N.; Petrov, V.V.; Plugotarenko, N.K.; Sergeenko, D.V.; Yalovega, G.; Brzhezinskaya, M.M.; Shishlyanikova, E.N. SiO2ZrO2 Thin Films as Low Temperature NO2 and O3 Sensors. Adv. Mater. Res. 2015, 1088, 81–85. [Google Scholar] [CrossRef]
- Kaviyarasu, K.; Kotsedi, L.; Simo, A.; Fuku, X.; Mola, G.T.; Kennedy, J.; Maaza, M. Photocatalytic activity of ZrO2 doped lead dioxide nanocomposites: Investigation of structural and optical microscopy of RhB organic dye. Appl. Surf. Sci. 2017, 421, 234–239. [Google Scholar] [CrossRef]
- Rahulan, K.M.; Vinitha, G.; Devaraj, S.L.; Kanakam, C.C. Synthesis and optical limiting effects in ZrO2 and ZrO2-SiO2 core–shell nanostructures. Ceram. Int. 2013, 39, 5281–5286. [Google Scholar] [CrossRef]
- Petrov, V.V.; Nazarova, T.N.; Korolev, A.N.; Kopilova, N.F. Thin sol-gel SiO2-SnOx-AgOy films for low temperature ammonia gas sensor. Sens. Actuators B Chem. 2008, 133, 291–295. [Google Scholar] [CrossRef]
- Tanuma, T.; Okamoto, H.; Ohnishi, K.; Morikawa, S. Metal halide catalysts to synthesize dichloropentafluoropropanes by the reaction of dichlorofluoromethane with tetrafluoroethylene. Appl. Catal. A Gen. 2008, 348, 236–240. [Google Scholar] [CrossRef]
- Liao, W.-P.; Yang, R.; Kuo, W.-T.; Huang, J.-Y. The application of electrocoagulation for the conversion of MSWI flyash into nonhazardous materials. J. Environ. Manag. 2014, 137, 157–162. [Google Scholar]
- Yalovega, G.E.; Myasoedova, T.N.; Shmatko, V.A.; Brzhezinskaya, M.M.; Popov, Y.V. Influence of Cu/Sn mixture on the shape and structure of crystallites in copper-containing films: Morphological and X-ray spectroscopy studies. Appl. Surf. Sci. 2016, 372, 93–99. [Google Scholar] [CrossRef]
- Garadkar, K.M.; Shirke, B.S.; Pati, Y.B.; Patil, D.R. Nanostructured ZrO2 Thick film resistors operable at room temperature. Sens. Tranducers 2009, 110, 17–25. [Google Scholar]
- Nakanishi, K. Utilizing a phase separation mechanism as a pore forming principle. J. Porous Mater. 1997, 4, 67–112. [Google Scholar] [CrossRef]
- Barsan, N.; Simion, C.; Heine, T.; Pokhrel, S.; Weimar, U. Modeling of sensing and transduction for p-type semiconducting metal oxide based gas sensors. J. Electroceram. 2010, 25, 11–19. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, J.-H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Bochenkov, V.E.; Sergeev, G.B. Preparation and chemiresistive properties of nanostructured materials. Adv. Colloid Interface Sci. 2005, 116, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, T. Facet-dependent metal oxide semiconductor gas sensors: An overview. Sens. Actuators B Chem. 2018, 277, 604–633. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Conduction model of metal oxide gas sensors. J. Electroceram. 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Zaera, F. Use of molecular beams for kinetic measurements of chemical reactions on solid surfaces. Surf. Sci. Rep. 2017, 72, 59–104. [Google Scholar] [CrossRef]
- International Center for Diffraction Data. PDF 83-0940; JCPDS Database; International Center for Diffraction Data: Newtown Square, PA, USA, 2003. [Google Scholar]
- Borilo, L.P.; Spivakova, L.N. Synthesis and Characterization of ZrO2 Thin Films. Am. J. Mater. Sci. 2012, 2, 119–124. [Google Scholar] [CrossRef]
- Borhade, A.V.; Tope, D.R.; Agashe, J.A. Synthesis, characterization and gas sensing performance of nanocrystalline ZrO2, 5%Y/ZrO2 and Ag–5%Y/ZrO2 catalyst. J. Mater. Sci. Mater. Electron. 2018, 29, 7551–7561. [Google Scholar] [CrossRef]
- Deshmukh, S.B.; Bari, R.H.; Patil, G.E.; Kajale, D.D.; Jain, G.H.; Patil, L.A. Preparation and characterization of zirconia based thick film resistor as a ammonia gas sensor. Int. J. Smart Sens. Intell. Syst. 2012, 5, 540–558. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chang, K.-H. Temperature independent resistive oxygen sensor prepared using zirconia-doped ceria powders. Sens. Actuators B Chem. 2012, 162, 68–75. [Google Scholar] [CrossRef]








| O | Si | Cl | Zr | Total | |
|---|---|---|---|---|---|
| 1 | 41.67 | 10.70 | 0.87 | 46.76 | 100.00 |
| 2 | 47.67 | 47.13 | 5.19 | 100.00 |
| Material | Production Technique | Target Gas | Operating Temperature | Concentration Range | Source |
|---|---|---|---|---|---|
| ZrO2 5%Y/ZrO2 Ag–5%Y/ZrO2 | Hydrothermal synthesis | CO2 | 300–400 °C | 600–1000 ppm | [40] |
| CeO2-xZrO2 | Sol-gel | O2 | 400 °C | 0.4–20% | [9] |
| ZrO2 | Commercial ZrO2 powder with an organic binder (thick film resistor) | NH3 | 250–350 °C | 100 ppm | [41] |
| Zirconia-doped ceria | Thick film from the ceria–zirconia solid solution powder | O2 | 10−5–1 atm | [42] | |
| ZrO2 | Sol-gel assisted by microwave | H2 | Room temperature | 50 ppm | [29] |
| SiO2ZrO2 | Sol-gel | NO2 O3 | 60 °C 30 °C | 1 ppm 0.02 ppm | [22] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myasoedova, T.N.; Mikhailova, T.S.; Yalovega, G.E.; Plugotarenko, N.K. Resistive Low-Temperature Sensor Based on the SiO2ZrO2 Film for Detection of High Concentrations of NO2 Gas. Chemosensors 2018, 6, 67. https://doi.org/10.3390/chemosensors6040067
Myasoedova TN, Mikhailova TS, Yalovega GE, Plugotarenko NK. Resistive Low-Temperature Sensor Based on the SiO2ZrO2 Film for Detection of High Concentrations of NO2 Gas. Chemosensors. 2018; 6(4):67. https://doi.org/10.3390/chemosensors6040067
Chicago/Turabian StyleMyasoedova, Tatiana N., Tatiana S. Mikhailova, Galina E. Yalovega, and Nina K. Plugotarenko. 2018. "Resistive Low-Temperature Sensor Based on the SiO2ZrO2 Film for Detection of High Concentrations of NO2 Gas" Chemosensors 6, no. 4: 67. https://doi.org/10.3390/chemosensors6040067
APA StyleMyasoedova, T. N., Mikhailova, T. S., Yalovega, G. E., & Plugotarenko, N. K. (2018). Resistive Low-Temperature Sensor Based on the SiO2ZrO2 Film for Detection of High Concentrations of NO2 Gas. Chemosensors, 6(4), 67. https://doi.org/10.3390/chemosensors6040067







