Fluorescence-Based Detection of Benzene, Toluene, Ethylbenzene, Xylene, and Cumene (BTEXC) Compounds in Fuel-Contaminated Snow Environments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instruments and Reagents
2.2. Sample Collection Procedures
2.3. Details for Snow Characterization Experiments
2.3.1. GC-MS Experiments
2.3.2. pH Experiments
2.3.3. Conductivity Experiments
2.4. Details for Fluorescence Modulation Experiments
2.5. Details for Array Generation Experiments
2.6. Details for Limit-of-Detection Experiments
3. Results
3.1. Component Selection Details
3.1.1. Sampling Location Selection
3.1.2. Cyclodextrin Selection
3.1.3. Fluorophore Selection
3.1.4. Analyte Selection
3.2. Undoped Snow Sample Experiments
3.2.1. GC-MS Experiments on Undoped Snow Samples
3.2.2. pH and Conductivity Experiments on Undoped Snow Samples
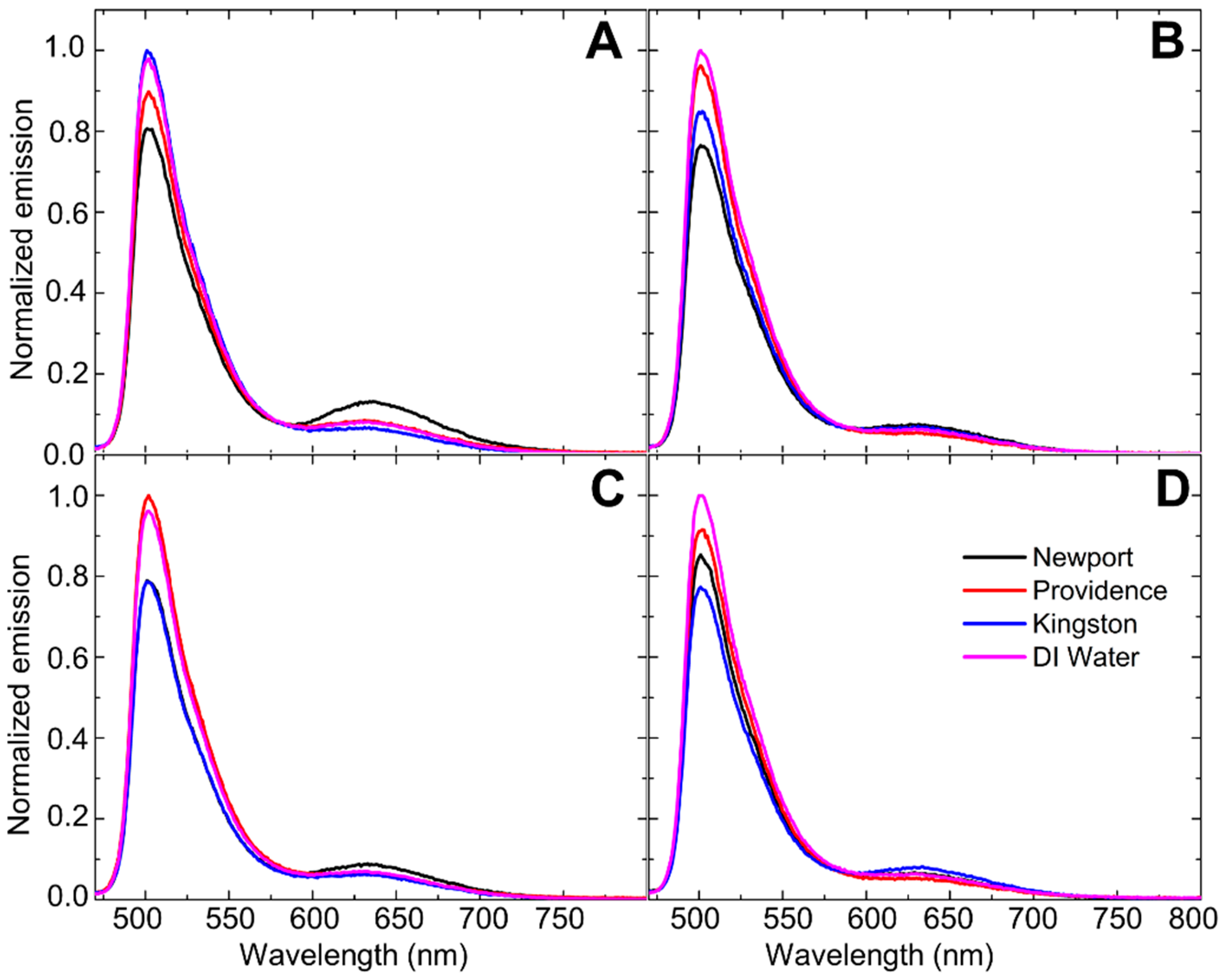
3.2.3. Fluorescence Experiments on Undoped Snow Samples
3.3. Analyte-Doped Snow Samples
3.3.1. Fluorescence Modulation of Analyte-Doped Snow Samples
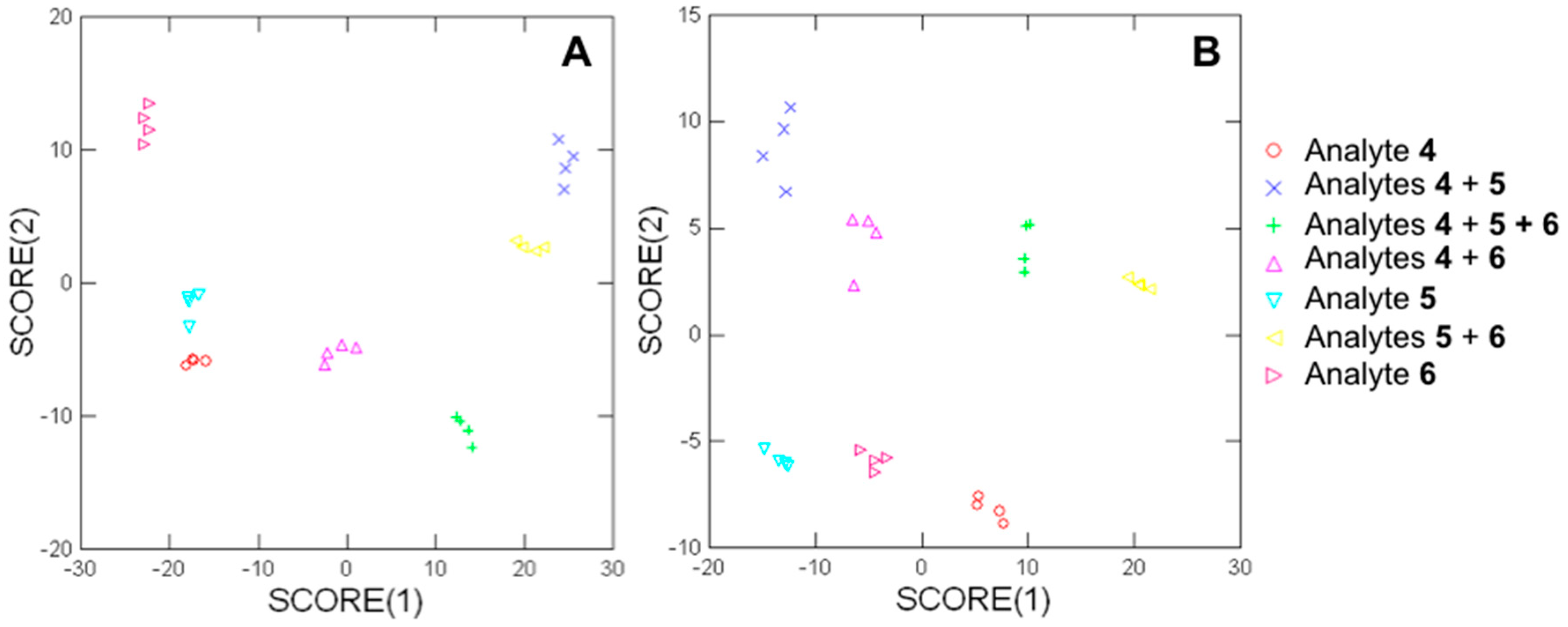
3.3.2. Array-Based Analysis of Analyte-Doped Snow Samples
3.3.3. Limit of Detection Experiments
3.4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Slominska, M.; Konieczka, P.; Namiesnik, J. The fate of BTEX compounds in ambient air. Critical Rev. Environ. Sci. Technol. 2014, 44, 455–472. [Google Scholar] [CrossRef]
- De Gennaro, G.; Dambruoso, D.M.; Di Gilio, A.; Marzoca, A.; Tutino, M. Indoor and outdoor volatile organic compounds monitoring in a multi-storey car park. Environ. Engineer. Manag. J. 2015, 14, 1563–1570. [Google Scholar] [CrossRef]
- Chambers, D.M.; Reese, C.M.; Thornburg, L.G.; Sanchez, E.; Rafson, J.P.; Blount, B.C.; Ruhl, J.R.E.; De Jesus, V.R. Distinguishing petroleum (crude oil and fuel) from smoke exposure within populations based on the relative blood levels of benzene, toluene, ethylbenzene, and xylenes (BTEX), styrene and 2,5-dimethylfuran by pattern recognition using artificial neural networks. Environ. Sci. Technol. 2018, 52, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Sirotkin, A.V.; Harrath, A.H. Influence of oil-related environmental pollutants on female reproduction. Reprod. Toxicol. 2017, 71, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Miozzi, E.; Teodoro, M.; Briguglio, G.; Rapisadra, V.; Fenga, C. New insights on ‘old’ toxicants in occupational toxicology (Review). Mol. Med. Rep. 2017, 15, 3317–3322. [Google Scholar] [CrossRef] [PubMed]
- Mosallanejad, Z.; Fakhri, Y.; Ferrante, M.; Zandsalimi, Y.; Amirhajeloo, L.R.; Amanidaz, N.; Moradi, B.; Keramati, H. Association between benzene exposure and childhood leukemia: A systematic review and meta-analysis updated to July 2016. Int. J. Pharm. Technol. 2016, 8, 4640–4652. [Google Scholar]
- Hannigan, J.H.; Bowen, S.E. Reproductive toxicology and teratology of abused toluene. Syst. Biol. Reprod. Med. 2010, 56, 184–200. [Google Scholar] [CrossRef]
- Ikemoto, Y.; Motoba, K.; Suzuki, T.; Uchida, M. Quantitative structure-activity relationships of nonspecific and specific toxicants in several organism species. Environ. Toxicol. Chem. 1992, 11, 917–930. [Google Scholar] [CrossRef]
- Varona-Torres, E.; Carlton, D.D., Jr.; Hildebrand, Z.L.; Schug, K.A. Matrix-effect-free determination of BTEX in variable soil compositions using room temperature ionic liquid co-solvents in static headspace gas chromatography mass spectrometry. Anal. Chim. Acta. 2018, 1021, 41–50. [Google Scholar] [CrossRef]
- Beller, H.R. Analysis of benzylsuccinates in groundwater by liquid chromatography/tandem mass spectrometry and its use for monitoring in situ BTEX biodegradation. Environ. Sci. Technol. 2002, 36, 2724–2728. [Google Scholar] [CrossRef]
- Dilonardo, E.; Alvisi, M.; Rossi, R.; Cassano, G.; Di Palo, F.; Palazzo, G.; Penza, M. Sensing properties of MWCNTs layers electrodecorated with metal nanoparticles for detection of aromatic hydrocarbon compounds. MRS Adv. 2017, 2, 1009–1014. [Google Scholar] [CrossRef]
- Colozza, N.; Kehe, K.; Popp, T.; Steinritz, D.; Moscone, D.; Arduini, F. Paper-based electrochemical sensor for on-site detection of the sulphur mustard. Environ. Sci. Poll. Res. 2018. [Google Scholar] [CrossRef]
- Al-Madhagi, S.; Joda, H.; Jauset-Rubio, M.; Ortiz, M.; Katakis, I.; O’Sullivan, C.K. Isothermal amplification using modified primers for rapid electrochemical analysis of coeliac disease associated DQB1*02 HLA Allele. Anal. Biochem. 2018, 556, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Gan, Y.; Liang, T.; Wan, H.; Wang, P. A Miniaturized electrochemical system for high sensitive determination of chromium (VI) by screen-printed carbon electrode with gold nanoparticles modification. Sens. Actuators B Chem. 2018, 272, 582–588. [Google Scholar] [CrossRef]
- Dutta, M.; Das, D. Recent developments in fluorescent sensors for trace-level determination of toxic-metal ions. Trends Anal. Chem. 2012, 32, 113–132. [Google Scholar] [CrossRef]
- Ding, H.; Chen, C.; Qi, S.; Han, C.; Yue, C. Smartphone-based spectrometer with high spectral accuracy for mHealth application. Sens. Actuators A Phys. 2018, 274, 94–100. [Google Scholar] [CrossRef]
- Kimura, K.; Onishi, S.; Moriyama, K. Fluorescence-based high-throughput salt screening. J. Pharm. Sci. 2018, 107, 1870–1878. [Google Scholar] [CrossRef]
- Thomas, S.W., III; Joly, G.D.; Swager, T.M. Chemical sensors based on amplifying fluorescent conjugated polymers. Chem. Rev. 2007, 107, 1339–1386. [Google Scholar] [CrossRef]
- Marks, P.; Cohen, S.; Levine, M. Highly efficient quenching of nanoparticles for the detection of electron-deficient nitroaromatics. J. Polym. Sci. A Polym. Chem. 2013, 51, 4150–4155. [Google Scholar] [CrossRef]
- Sokkalingam, P.; Kim, D.S.; Hwang, H.; Sessler, J.L.; Lee, C.-H. A dicationic calix[4]pyrrole derivative and its use for the selective recognition and displacement-based sensing of pyrophosphate. Chem. Sci. 2012, 3, 1819–1824. [Google Scholar] [CrossRef]
- Radaram, B.; Mako, T.; Levine, M. Sensitive and selective detection of cesium via fluorescence quenching. Dalton Trans. 2013, 42, 16276–16278. [Google Scholar] [CrossRef] [PubMed]
- Saar-Reismaa, P.; Erme, E.; Vaher, M.; Kulp, M.; Kaljurand, M.; Mazina-Sinkar, J. In situ determination of illegal drugs in oral fluid by portable capillary electrophoresis with deep UV excited fluorescence detection. Anal. Chem. 2018, 90, 6253–6258. [Google Scholar] [CrossRef] [PubMed]
- Campos-Candel, A.; Llobat-Estelles, M.; Mauri-Aucejo, A. Comparative evaluation of liquid chromatography versus gas chromatography using a beta-cyclodextrin stationary phase for determination of BTEX in occupational environments. Talanta 2009, 78, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Serio, N.; Moyano, D.F.; Rotello, V.M.; Levine, M. Array-based detection of persistent organic pollutants via cyclodextrin-promoted energy transfer. Chem. Commun. 2015, 51, 11615–11618. [Google Scholar] [CrossRef] [PubMed]
- DiScenza, D.J.; Levine, M. Selective detection of non-aromatic pesticides via cyclodextrin-promoted fluorescence modulation. New J. Chem. 2016, 40, 789–793. [Google Scholar] [CrossRef] [Green Version]
- Serio, N.; Chanthalyma, C.; Prignano, L.; Levine, M. Cyclodextrin-promoted energy transfer for broadly applicable small-molecule detection. Supramol. Chem. 2014, 26, 714–721. [Google Scholar] [CrossRef] [Green Version]
- DiScenza, D.J.; Gareau, L.; Serio, N.; Roque, J.; Prignano, L.; Verderame, M.; Levine, M. Cyclodextrin-promoted detection of aromatic toxicants and toxicant metabolites in urine. Anal. Chem. Lett. 2016, 6, 345–355. [Google Scholar] [CrossRef]
- DiScenza, D.J.; Lynch, J.; Verderame, M.; Serio, N.; Prignano, L.; Gareau, L.; Levine, M. Efficient fluorescence detection of aromatic toxicants and toxicant metabolites in human breast milk. Supramol. Chem. 2018, 30, 267–277. [Google Scholar] [CrossRef]
- Serio, N.; Levine, M. Efficient extraction and detection of aromatic toxicants from crude oil and tar balls using multiple cyclodextrin derivatives. Marine Pollut. Bull. 2015, 95, 242–247. [Google Scholar] [CrossRef] [Green Version]
- DiScenza, D.J.; Lynch, J.; Miller, J.; Verderame, M.; Levine, M. Detection of organochlorine pesticides in contaminated marine environments via cyclodextrin-promoted fluorescence modulation. ACS Omega 2017, 2, 8591–8599. [Google Scholar] [CrossRef]
- DiScenza, D.J.; Levine, M. Detection of benzene and alkylated benzene derivatives in fuel-contaminated environments. CLEAN Soil Air Water 2016, 44, 1621–1627. [Google Scholar] [CrossRef]
- Xue, B.-X.; Wei, L.; Li, C.-Y.; Li, T.-Y. A study of the distribution and composition of pollutants in snow collected from streets and a treatment system for recycling snow in winter cities. Desalin. Water Treat. 2015, 54, 1470–1478. [Google Scholar] [CrossRef]
- Hers, I.; Jourabchi, P.; Lahvis, M.A.; Dahlem, P.; Luo, E.H.; Devaull, G.E.; Mayer, K.U. Evaluation of seasonal factors on petroleum hydrocarbon vapor biodegradation and intrusion potential in a cold climate. Groundwater Monit. Remediat. 2014, 34, 60–78. [Google Scholar] [CrossRef]
- Nazarenko, Y.; Fournier, S.; Kurien, U.; Rangel-Alvarado, R.B.; Nepotchatykh, O.; Seers, P.; Ariya, P.A. Role of snow in the fate of gaseous and particulate exhaust pollutants from gasoline-powered vehicles. Environ. Pollut. 2017, 223, 665–675. [Google Scholar] [CrossRef]
- Martel, C.J.; Nadeau, B.M.J. Snow as an expedient sorbent for hazardous materials. Environ. Sci. Health A 1994, A29, 237–247. [Google Scholar] [CrossRef]
- Cheng, D.; Zhao, W.; Yang, H.; Huang, Z.; Liu, X.; Han, A. Detection of Hg2+ by a FRET ratiometric fluorescent probe based on a novel BODIPY-RhB system. Tetrahedron Lett. 2016, 57, 2655–2659. [Google Scholar] [CrossRef]
- Wenning, R.J.; Harris, M.A.; Ungs, M.J.; Paustenbach, D.J.; Bedbury, H. Chemometric comparisons of polychlorinated dibenzo-p-dioxin and dibenzofuran residues in surficial sediments from Newark Bay, New Jersey and other industrialized waterways. Arch. Environ. Contam. Toxicol. 1992, 22, 397–413. [Google Scholar] [CrossRef]
- Acid Rain Has Negative Impact on Rhode Island’s Lake Fish. Available online: http://turnto10.com/news/videos/acid-rains-impact-on-rhode-island-lakes (accessed on 25 June 2018).
- Loftsson, T.; Jarho, P.; Masson, M.; Jarvinen, T. Cyclodextrins in drug delivery. Expert Opin. Drug Deliv. 2005, 2, 335–351. [Google Scholar] [CrossRef]
- Li, B.; Chen, J.B.; Xiong, Y.; Zhao, C.; Sun, J. Development of turn-on fluorescent probes for the detection of H2O2 vapor with high selectivity and sensitivity. Sens. Actuators B Chem. 2018, 268, 475–484. [Google Scholar] [CrossRef]
- Zhang, M.; Su, R.; Zhang, Q.; Hu, L.; Tian, X.; Chen, Y.; Zhou, H.; Wu, J.; Tian, Y. Ultra-bright intercellular lipids pseudo Di-BODIPY probe with low molecular weight, high quantum yield and large two-photon action cross-sections. Sens. Actuators B Chem. 2018, 261, 161–168. [Google Scholar] [CrossRef]
- Richards, G.J.; Gobo, Y.; Yamamura, M.; Nabeshima, T. Biphenyl appended BODIPY derivatives showing combined environmental polarity and heavy metal cation sensing functionality. New J. Chem. 2015, 39, 5886–5889. [Google Scholar] [CrossRef]
- Sarti, E.; Pasti, L.; Scaroni, I.; Casali, P.; Cavazzini, A.; Rossi, M. Determination of n-alkanes, PAHs, and nitro-PAHs in PM2.5 and PM1 sampled in the surroundings of a municipal waste incinerator. Atmos. Environ. 2017, 149, 12–23. [Google Scholar] [CrossRef]
- Cho, H.S.; Das, M.; Wang, H.; Dinh, H.N. The contamination mechanism and behaviour of amide bond containing organic contaminant PEMFC. J. Electrochem. Soc. 2015, 162, F427–F435. [Google Scholar] [CrossRef]
- Zare, A.; Nabi, M.N.; Bodisco, T.A.; Hossain, F.M.; Rahman, M.M.; Ristovski, Z.D.; Brown, R.J. The effect of triacetin as a fuel additive to waste cooking biodiesel on engine performance and exhaust emissions. Fuel 2016, 182, 640–649. [Google Scholar] [CrossRef]
- Dong, T.; Fei, Q.; Genelot, M.; Smith, H.; Laurens, L.M.L.; Watson, M.J.; Pienkos, P.T. A novel integrated biorefinery process for diesel fuel blendstock production using lipids from the methanotroph, methylmicrobium buryatense. Energy Convers. Manag. 2017, 140, 62–70. [Google Scholar] [CrossRef]
- Brewer, R.; Nagashima, J.; Kelley, M.; Heskett, M.; Rigby, M. Risk-based evaluation of total petroleum hydrocarbons in vapor intrusion studies. Int. J. Environ. Res. Public Health 2013, 10, 2441–2467. [Google Scholar] [CrossRef] [PubMed]
- Bosker, T.; Santoro, G.; Melvin, S.D. Salinity and sensitivity to endocrine disrupting chemicals: A comparison of reproductive endpoints in small-bodied fish exposed under different salinities. Chemosphere 2017, 183, 186–196. [Google Scholar] [CrossRef] [Green Version]
- Cai, W.; Sun, T.; Shao, X.; Chipol, C. Can the anomalous aqueous solubility of β-cyclodextrin be explained by its hydration free energy alone? Phys. Chem. Chem. Phys. 2008, 10, 3236–3243. [Google Scholar] [CrossRef]
- Nojavan, S.; Yazdanpanah, M. Micro-solid phase extraction of benzene, toluene, ethylbenzene and xylenes from aqueous solutions using water-insoluble β-cyclodextrin polymer as sorbent. J. Chromatogr. A. 2017, 1525, 51–59. [Google Scholar] [CrossRef]
- Nowicki, A.; Zhang, Y.; Leger, B.; Rolland, J.P.; Bricout, H.; Monifer, E.; Roucoux, A. Supramolecular shuttle and protective agent: A multiple role of methylated cyclodextrins in the chemoselective hydrogenation of benzene derivatives with ruthenium nanoparticles. Chem. Commun. 2006, 296–298. [Google Scholar] [CrossRef]
- Misawa, K.; Saito, Y.; Hashizaki, K.; Taguchi, H.; Ogawa, N.; Ueda, H. Stability constants for 1:1 complexes formed between 2-hydroxypropyl-β-cyclodextrin with an average substitution degree of 4.4 and benzene and alkylbenzenes as guests by modified static head-space gas chromatography method. J. Incl. Phenom. Macrocycl. Chem. 2005, 53, 237–240. [Google Scholar] [CrossRef]
- Gomez, E.C.; Anguiano Igea, S.; Gomez Amoza, J.L.; Ottero Espinar, F.J. Evaluation of the promoting effect of soluble cyclodextrins in drug nail penetration. Eur. J. Pharm. Sci. 2018, 117, 270–278. [Google Scholar] [CrossRef]
- Sen, C.P.; Shrestha, R.G.; Shrestha, L.K.; Ariga, K.; Valiyaveetil, S. Low-band-gap BODIPY conjugated copolymers for sensing volatile organic compounds. Chem. Eur. J. 2015, 21, 17344–17354. [Google Scholar] [CrossRef]
- Qiu, J.; Jiang, S.; Guo, H.; Yang, F. An AIE and FRET-based BODIPY sensor with large stokes shift: Novel pH probe exhibiting application in CO32− detection and living cell imaging. Dyes Pigm. 2018, 157, 351–358. [Google Scholar] [CrossRef]
- Ziessel, R.; Ulrich, G.; Harriman, A. The chemistry of bodipy: A new El Dorado for fluorescence tools. New J. Chem. 2007, 31, 496–501. [Google Scholar] [CrossRef]
- DiScenza, D.J.; Culton, E.; Verderame, M.; Lynch, J.; Serio, N.; Levine, M. Towards rational chemosensor design through improved understanding of experimental parameter variation and tolerance in cyclodextrin-promoted fluorescence detection. Chemosensors 2017, 5, 34. [Google Scholar] [CrossRef]
- Kfoury, M.; Landy, D.; Fourmentin, S. Characterization of cyclodextrin/volatile inclusion complexes: A review. Molecules 2018, 23, 1204. [Google Scholar] [CrossRef]
- Saha, S.; Agarwalla, H.; Gupta, M.; Suresh, S.K.; Ghosh, S.K.; Das, A. New chemodosimetric probe for the specific detection of Hg2+ in physiological condition and its utilisation for cell imagine studies. Dalton Trans. 2013, 42, 15097–15105. [Google Scholar] [CrossRef]
- DiScenza, D.J.; Lynch, J.; Feder, E.; Levine, M. Detection of bisphenol A and derivatives in human urine via cyclodextrin-promoted fluorescence modulation. Anal. Methods 2018, 10, 3783–3790. [Google Scholar] [CrossRef]
- Castronuovo, G.; Niccoli, M. Solvent effects on the complexation of 1-alkanols by parent and modified cyclodextrins. Calorimetric studies at 298 K. J. Thermal Anal. Calorim. 2011, 103, 641–646. [Google Scholar] [CrossRef]










| Sampling Location | pH a | Conductivity (µs/cm) b |
|---|---|---|
| Newport | 6.48 | 713 |
| Providence | 6.08 | 101 |
| Kingston | 6.46 | 584 |
| Compound | Binding Constant (M−1) |
|---|---|
| 1 | 121 |
| 2 | 287 |
| 3 | 435 |
| 4 | 305 |
| 5 | 210 |
| 6 | 353 |
| 7 | b |
| Heptanol | 1000 [61] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
DiScenza, D.J.; Intravaia, L.E.; Healy, A.; Dubrawski, S.B.; Levine, M. Fluorescence-Based Detection of Benzene, Toluene, Ethylbenzene, Xylene, and Cumene (BTEXC) Compounds in Fuel-Contaminated Snow Environments. Chemosensors 2019, 7, 5. https://doi.org/10.3390/chemosensors7010005
DiScenza DJ, Intravaia LE, Healy A, Dubrawski SB, Levine M. Fluorescence-Based Detection of Benzene, Toluene, Ethylbenzene, Xylene, and Cumene (BTEXC) Compounds in Fuel-Contaminated Snow Environments. Chemosensors. 2019; 7(1):5. https://doi.org/10.3390/chemosensors7010005
Chicago/Turabian StyleDiScenza, Dana J., Lauren E. Intravaia, Anna Healy, Sage B. Dubrawski, and Mindy Levine. 2019. "Fluorescence-Based Detection of Benzene, Toluene, Ethylbenzene, Xylene, and Cumene (BTEXC) Compounds in Fuel-Contaminated Snow Environments" Chemosensors 7, no. 1: 5. https://doi.org/10.3390/chemosensors7010005
APA StyleDiScenza, D. J., Intravaia, L. E., Healy, A., Dubrawski, S. B., & Levine, M. (2019). Fluorescence-Based Detection of Benzene, Toluene, Ethylbenzene, Xylene, and Cumene (BTEXC) Compounds in Fuel-Contaminated Snow Environments. Chemosensors, 7(1), 5. https://doi.org/10.3390/chemosensors7010005





