Sodium Alginate Cross-Linkable Planar 1D Photonic Crystals as a Promising Tool for Pb2+ Detection in Water
Abstract
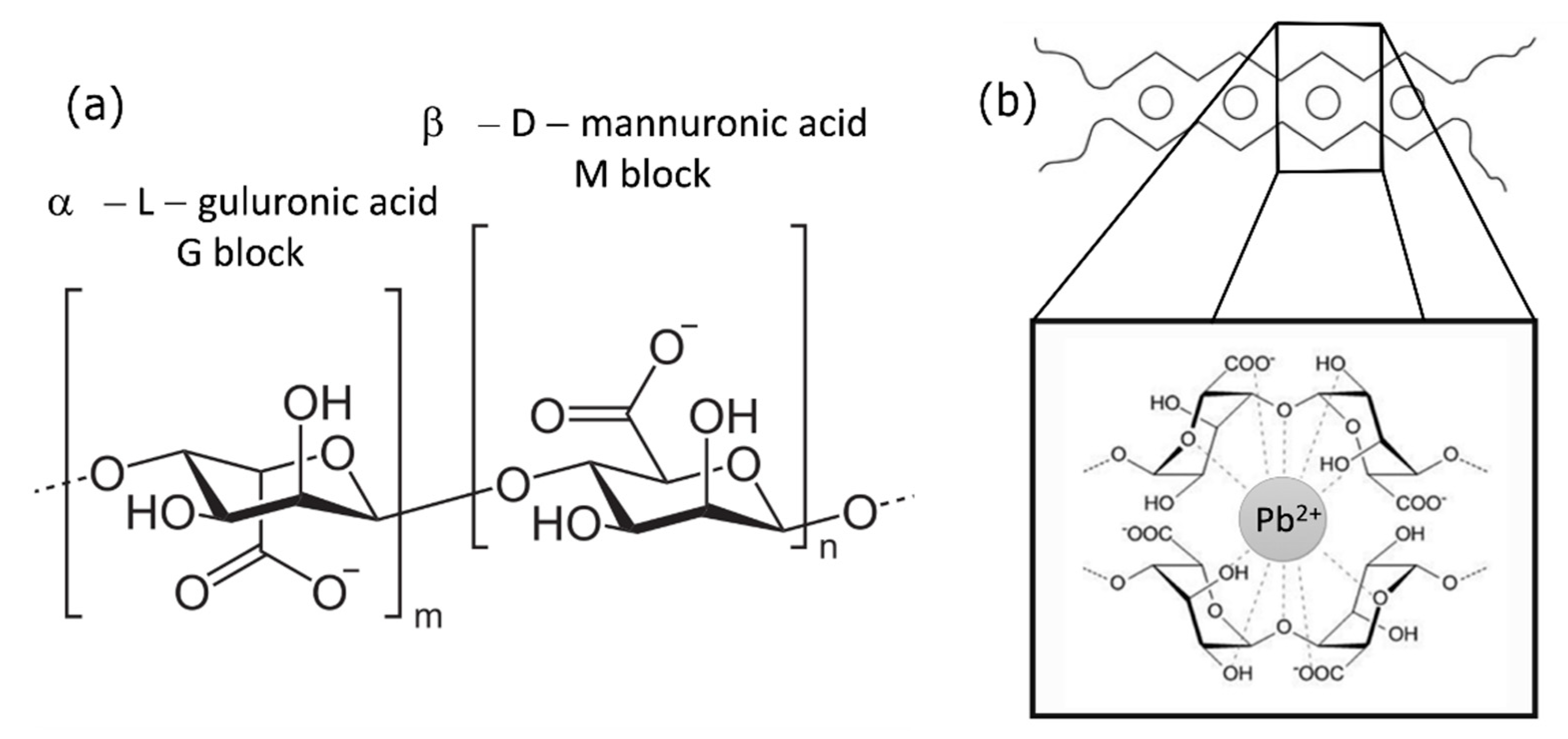
:1. Introduction
2. Materials and Methods
3. Results and Discussion
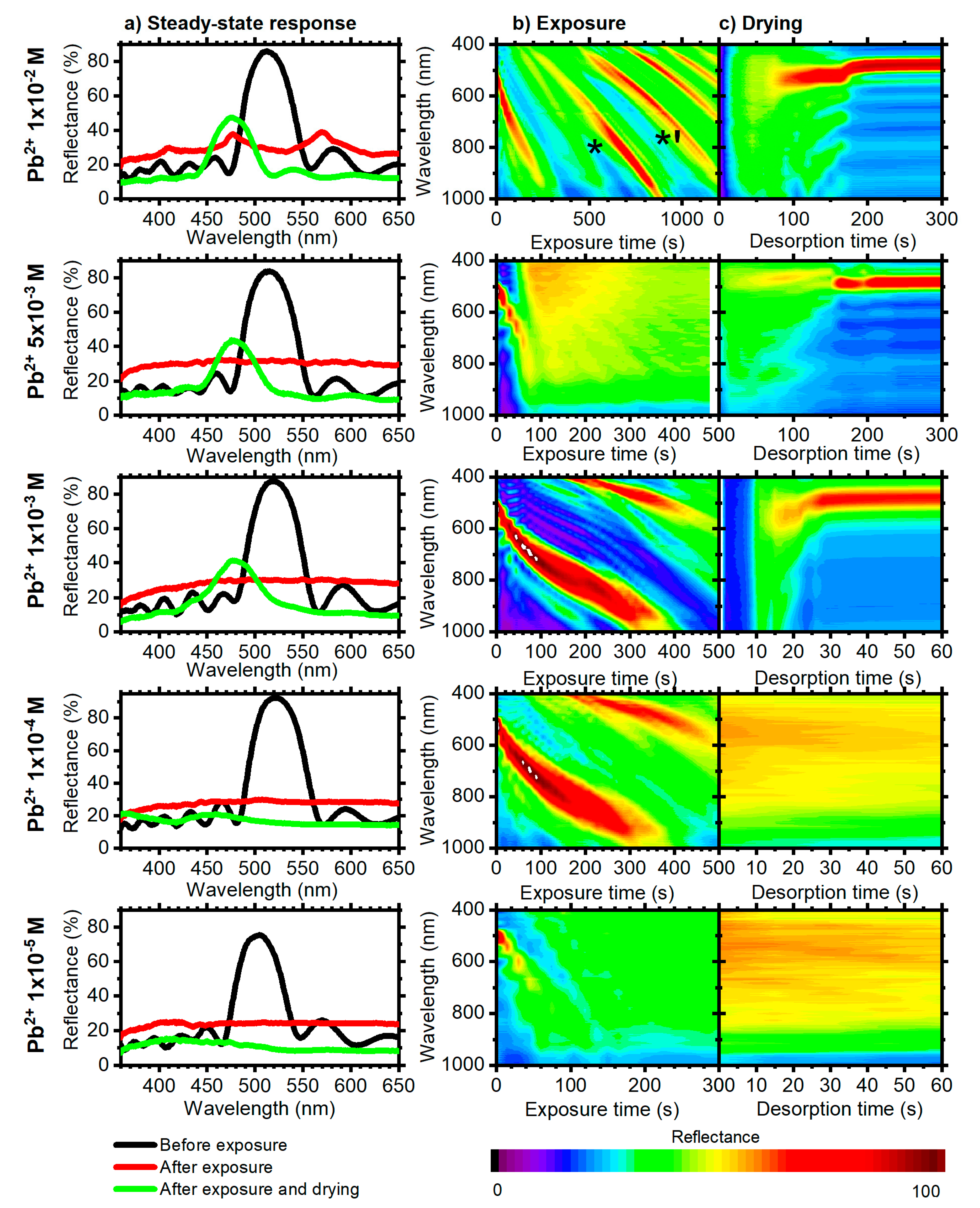
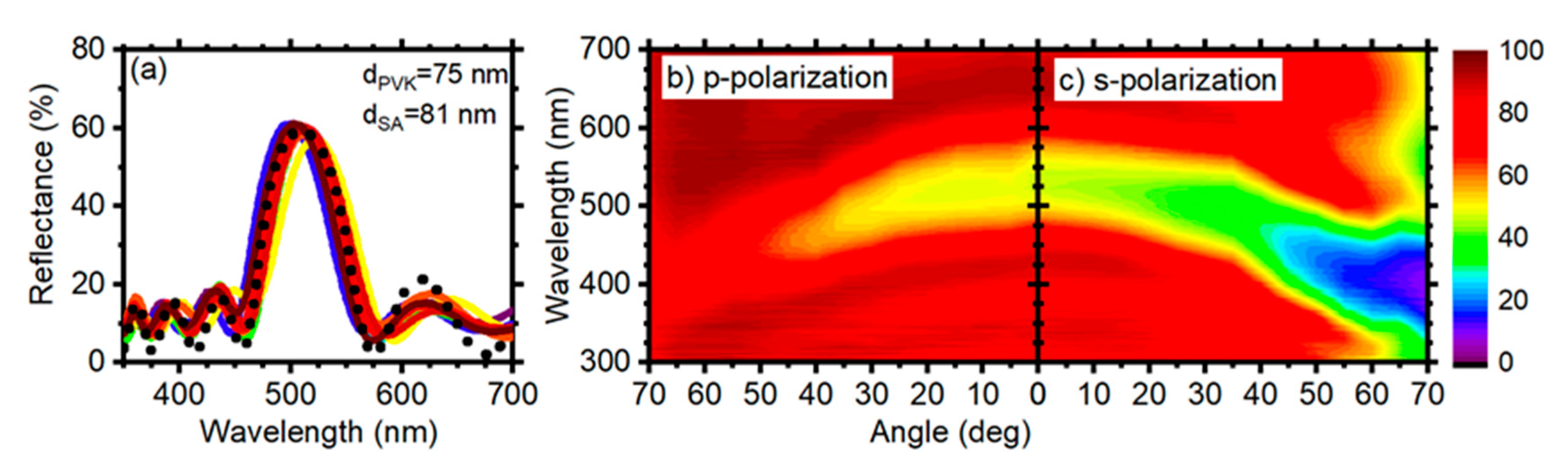
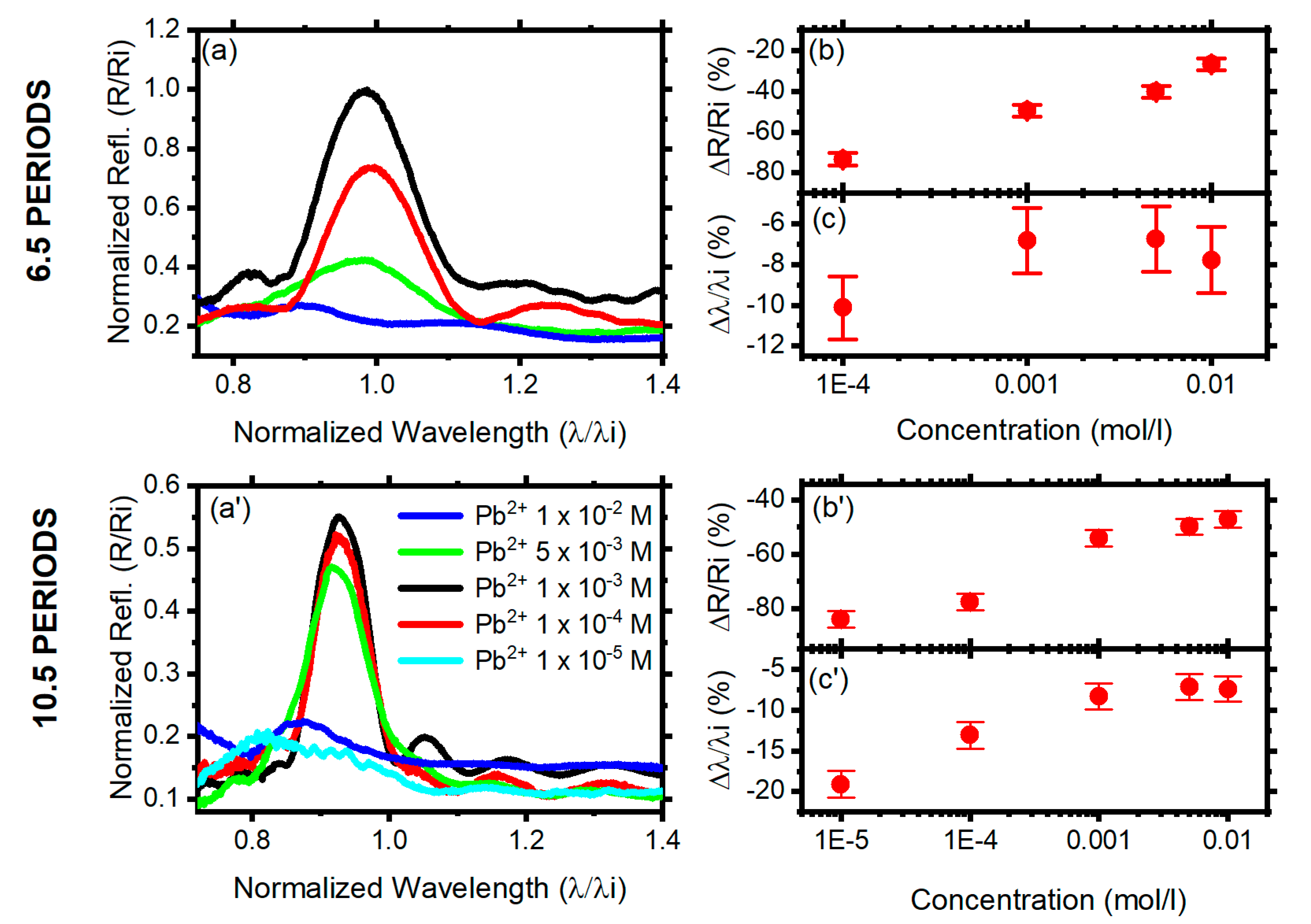
3.1. 10.5 Period DBRs
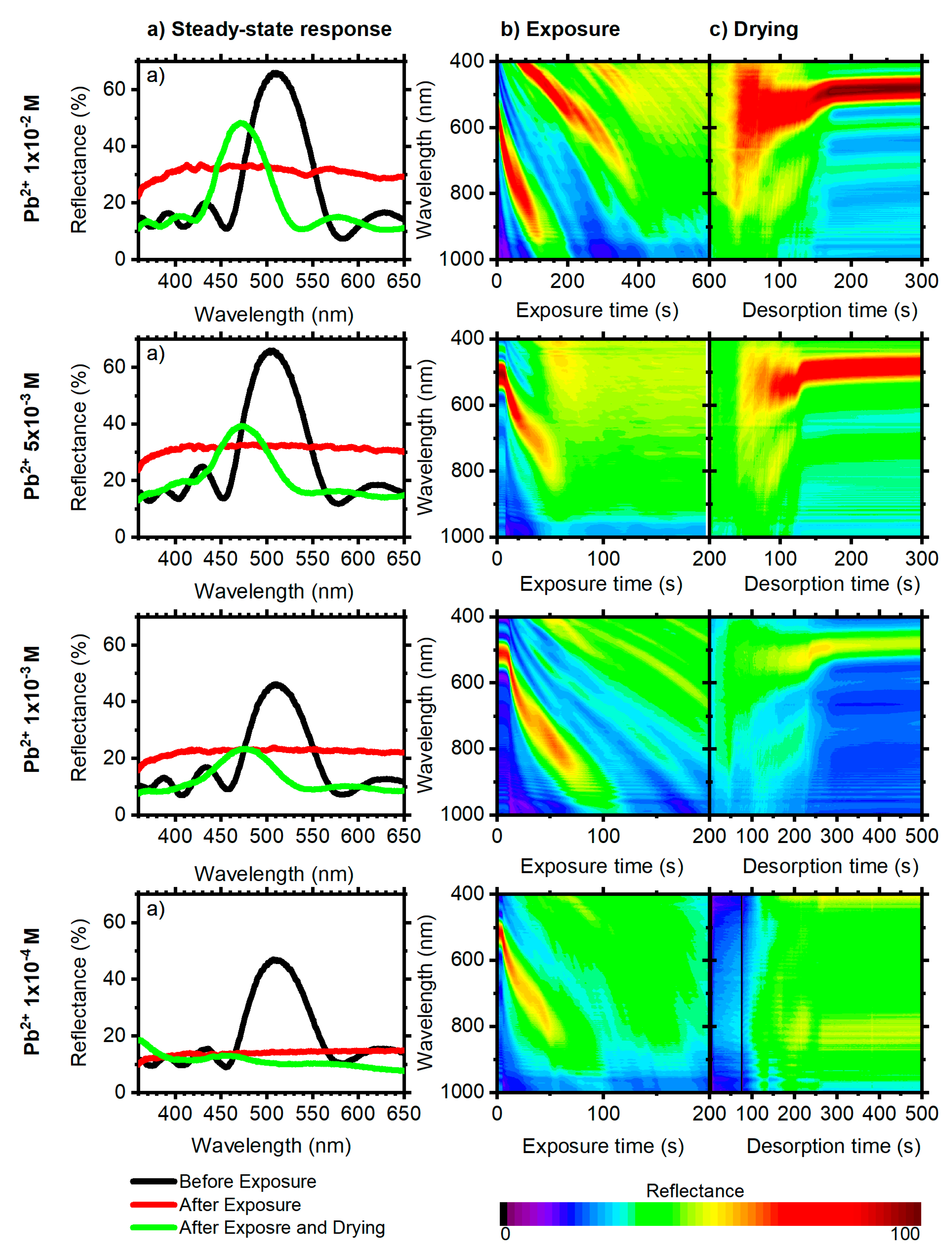
3.2. 6.5 Period DBRs
3.3. Quantitative DBR Response
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Halim, C.E.; Scott, J.A.; Natawardaya, H.; Amal, R.; Beydoun, D.; Low, G. Comparison Between Acetic Acid and Landfill Leachates for the Leaching of Pb(II), Cd(II), As(V), And Cr(VI) from Cementitious Wastes. Environ. Sci. Technol. 2004, 38, 3977–3983. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.E.; Trainor, T.P.; Chaka, A.M. Hybridization-reactivity Relationship in Pb(II) Adsorption on α-Al2O3-water Interfaces: A DFT Study. J. Phys. Chem. C 2011, 115, 4008–4021. [Google Scholar] [CrossRef]
- Shukla, G.S.; Singhal, R.L. The Present Status of Biological Effects of Toxic Metals in the Environment: Lead, Cadmium, and Manganese. Can. J. Physiol. Pharmacol. 1984, 62, 1015–1031. [Google Scholar] [CrossRef] [PubMed]
- Aragay, G.; Pons, J.; Merkoçi, A. Recent Trends in Macro-, Micro-, and Nanomaterial-Based Tools and Strategies for Heavy-Metal Detection. Chem. Rev. 2011, 111, 3433–3458. [Google Scholar] [CrossRef]
- Taylor, D.H.; Steele, C.W.; Strickler-Shaw, S. Responses of green frog (Rana Clamitans) tadpoles to lead-polluted water. Environ. Toxicol. Chem. 1990, 9, 87–93. [Google Scholar] [CrossRef]
- Li, D. Effects of lead polluted water on activities of superoxide dismutase, peroxidase and ultrastructure in leaves of Trapa bicornis seedlings. China Environ. Sci. 2009, 29, 136–141. [Google Scholar]
- Scientific Committee on Health and Environmental Risks (SCHER). Lead Standard in Drinking Water. Available online: https://ec.europa.eu/health/scientific_committees/environmental_risks/docs/scher_o_128.pdf (accessed on 28 April 2020).
- Ullah, Z.; Khan, H.; Waseem, A.; Mahmood, Q.; Farooq, U. Water quality assessment of the River Kabul at Peshawar, Pakistan: Industrial and urban wastewater impacts. J. Water Chem. Technol. 2013, 35, 170–176. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Ahmad, I.; Shah, M.T.; Rehman, S.; Khaliq, A. Use of constructed wetland for the removal of heavy metals from industrial wastewater. J. Environ. Manag. 2009, 90, 3451–3457. [Google Scholar] [CrossRef]
- Tariq, S.R.; Shah, M.H.; Shaheen, N.; Khalique, A.; Manzoor, S.; Jaffar, M. Multivariate analysis of trace metal levels in tannery effluents in relation to soil and water: A case study from Peshawar, Pakistan. J. Environ. Manag. 2006, 79, 20–29. [Google Scholar] [CrossRef]
- Krishna, A.K.; Mohan, K.R. Risk assessment of heavy metals and their source distribution in waters of a contaminated industrial site. Environ. Sci. Pollut. Res. 2014, 21, 3653–3669. [Google Scholar] [CrossRef]
- Javaid, S.; Shah, S.G.S.; Chaudhary, A.J.; Khan, M.H. Assessment of Trace Metal Contamination of Drinking Water in the Pearl Valley, Azad Jammu and Kashmir. CLEAN–Soil Air Water 2008, 36, 216–221. [Google Scholar] [CrossRef]
- Paul, D. Research on heavy metal pollution of river Ganga: A review. Ann. Agrar. Sci. 2017, 15, 278–286. [Google Scholar] [CrossRef]
- Besmer, M.D.; Hammes, F. Short-term microbial dynamics in a drinking water plant treating groundwater with occasional high microbial loads. Water Res. 2016, 107, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Højris, B.; Kornholt, S.N.; Christensen, S.C.B.; Albrechtsen, H.-J.; Olesen, L.S. Detection of Drinking Water Contamination by an Optical Real-Time Bacteria Sensor. H2Open J. 2018, 1, 160–168. [Google Scholar] [CrossRef] [Green Version]
- Banna, M.H.; Imran, S.; Francisque, A.; Najjaran, H.; Sadiq, R.; Rodriguez, M.; Hoorfar, M. Online Drinking Water Quality Monitoring: Review on Available and Emerging Technologies. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1370–1421. [Google Scholar] [CrossRef]
- Daware, K.; Kasture, M.; Kalubarme, R.; Shinde, R.; Patil, K.; Suzuki, N.; Terashima, C.; Gosavi, S.; Fujishima, A. Detection of Toxic Metal Ions Pb2+ in Water Using SiO2@Au Core-Shell Nanostructures: A Simple Technique for Water Quality Monitoring. Chem. Phys. Lett. 2019, 732, 136635. [Google Scholar] [CrossRef]
- Chai, F.; Wang, C.; Wang, T.; Li, L.; Su, Z. Colorimetric Detection of Pb2+ Using Glutathione Functionalized Gold Nanoparticles. ACS Appl. Mater. Interfaces 2010, 2, 1466–1470. [Google Scholar] [CrossRef]
- Repetto, D.; Giordano, M.C.; Foti, A.; Gucciardi, P.G.; Mennucci, C.; Buatier de Mongeot, F. SERS amplification by ultra-dense plasmonic arrays on self-organized PDMS templates. Appl. Surf. Sci. 2018, 446, 83–91. [Google Scholar] [CrossRef]
- Li, M.; Zhou, X.; Guo, S.; Wu, N. Detection of Lead (Ii) with a “Turn-On” Fluorescent Biosensor Based on Energy Transfer from CdSe/ZnS Quantum Dots to Graphene Oxide. Biosens. Bioelectron. 2013, 43, 69–74. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y. A Highly Sensitive and Selective Catalytic DNA Biosensor for Lead Ions. JACS 2000, 122, 10466–10467. [Google Scholar] [CrossRef]
- Ju, H.; Lee, M.H.; Kim, J.; Kim, J.S.; Kim, J. Rhodamine-Based Chemosensing Monolayers on Glass as a Facile Fluorescent “Turn-On” Sensing Film for Selective Detection Of Pb2+. Talanta 2011, 83, 1359–1363. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Gupta, V.K.; Singh, L.P.; Raisoni, J.R. A Comparative Study of Pb2+ Selective Sensors Based on Derivatized Tetrapyrazole and Calix[4]Arene Receptors. Electrochim. Acta 2006, 51, 2547–2553. [Google Scholar] [CrossRef]
- Gao, C.; Yu, X.-Y.; Xu, R.-X.; Liu, J.-H.; Huang, X.-J. AlOOH-Reduced Graphene Oxide Nanocomposites: One-Pot Hydrothermal Synthesis and Their Enhanced Electrochemical Activity for Heavy Metal Ions. ACS Appl. Mater. Interfaces 2012, 4, 4672–4682. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef]
- Willemse, C.M.; Tlhomelang, K.; Jahed, N.; Baker, P.G.; Iwuoha, E.I. Metallo-Graphene Nanocomposite Electrocatalytic Platform for the Determination of Toxic Metal Ions. Sensors 2011, 11, 3970–3987. [Google Scholar] [CrossRef]
- Wen, Y.; Li, F.Y.; Dong, X.; Zhang, J.; Xiong, Q.; Chen, P. The Electrical Detection of Lead Ions Using Gold-Nanoparticle- and DNAzyme-Functionalized Graphene Device. Adv. Healthc. Mater. 2013, 2, 271–274. [Google Scholar] [CrossRef]
- Zhou, G.; Chang, J.; Cui, S.; Pu, H.; Wen, Z.; Chen, J. Real-Time, Selective Detection of Pb2+ in Water Using a Reduced Graphene Oxide/Gold Nanoparticle Field-Effect Transistor Device. ACS Appl. Mater. Interfaces 2014, 6, 19235–19241. [Google Scholar] [CrossRef]
- Niu, X.; Liu, Y.; Wang, F.; Luo, D. Highly sensitive and selective optical sensor for lead ion detection based on liquid crystal decorated with DNAzyme. Opt. Express 2019, 27, 30421–30428. [Google Scholar] [CrossRef]
- Reese, C.E.; Asher, S.A. Photonic Crystal Optrode Sensor for Detection of Pb2+ in High Ionic Strength Environments. Anal. Chem. 2003, 75, 3915–3918. [Google Scholar] [CrossRef]
- Yin, Y.; Li, S.; Wang, S.; Jia, S.; Ren, J.; Farrell, G.; Lewis, E.; Wang, P. Ultra-high-resolution detection of Pb 2+ ions using a black phosphorus functionalized microfiber coil resonator. Photonics Res. 2019, 7, 622–629. [Google Scholar] [CrossRef]
- Lova, P.; Manfredi, G.; Comoretto, D. Advances in Functional Solution Processed Planar One-Dimensional Photonic Crystals. Adv. Opt. Mater. 2018, 6, 1800730. [Google Scholar] [CrossRef]
- Lova, P.; Giusto, P.; Stasio, F.D.; Manfredi, G.; Paternò, G.M.; Cortecchia, D.; Soci, C.; Comoretto, D. All-Polymer Methylammonium Lead Iodide Perovskite Microcavity. Nanoscale 2019, 11, 8978–8983. [Google Scholar] [CrossRef] [PubMed]
- Robbiano, V.; Paternò, G.M.; La Mattina, A.A.; Motti, S.G.; Lanzani, G.; Scotognella, F.; Barillaro, G. Room-temperature low-Threshold Lasing from Monolithically Integrated Nanostructured Porous Silicon Hybrid Microcavities. ACS Nano 2018, 12, 4536–4544. [Google Scholar] [CrossRef] [PubMed]
- Paternò, G.M.; Iseppon, C.; D’Altri, A.; Fasanotti, C.; Merati, G.; Randi, M.; Desii, A.; Pogna, E.A.A.; Viola, D.; Cerullo, G.; et al. Solution Processable and Optically Switchable 1D Photonic Structures. Sci. Rep. 2018, 8, 3517. [Google Scholar] [CrossRef] [PubMed]
- Paternò, G.M.; Moscardi, L.; Kriegel, I.; Scotognella, F.; Lanzani, G. Electro-Optic and Magneto-Optic Photonic Devices Based on Multilayer Photonic Structures. SPIE Proc. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Yin, J.; Migas, D.B.; Panahandeh-Fard, M.; Chen, S.; Wang, Z.; Lova, P.; Soci, C. Charge Redistribution at GaAs/P3HT Heterointerfaces with Different Surface Polarity. J. Phys. Chem. Lett. 2013, 4, 3303–3309. [Google Scholar] [CrossRef]
- Sprengard, R.; Bonrad, K.; Daeubler, T.K.; Frank, T.; Hagemann, V.; Köhler, I.; Pommerehne, J.; Ottermann, C.R.; Voges, F.; Vingerling, B. OLED Devices for Signage Applications: A Review of Recent Advances and Remaining Challenges. In Proceedings of the Organic Light-Emitting Materials and Devices VIII, Denver, CO, USA, 2–4 August 2004; pp. 173–183. [Google Scholar]
- Torsi, L.; Magliulo, M.; Manoli, K.; Palazzo, G. Organic Field-Effect Transistor Sensors: A Tutorial Review. Chem. Soc. Rev. 2013, 42, 8612–8628. [Google Scholar] [CrossRef]
- Iasilli, G.; Francischello, R.; Lova, P.; Silvano, S.; Surace, A.; Pesce, G.; Alloisio, M.; Patrini, M.; Shimizu, M.; Comoretto, D.; et al. Luminescent Solar Concentrators: Boosted Optical Efficiency by Polymer Dielectric Mirrors. Mater. Chem. Front. 2019, 3, 429–436. [Google Scholar] [CrossRef]
- Manfredi, G.; Lova, P.; Di Stasio, F.; Rastogi, P.; Krahne, R.; Comoretto, D. Lasing From Dot-In-Rod Nanocrystals in Planar Polymer Microcavities. RSC Adv. 2018, 8, 13026–13033. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Pan, L.; Han, Y.; Ma, L.; Li, Y.; Xu, H.; Zhao, J. A visual water vapor photonic crystal sensor with PVA/SiO2 opal structure. App. Surf. Sci. 2017, 423, 421–425. [Google Scholar] [CrossRef]
- Schauer, S.; Baumberg, J.J.; Hölscher, H.; Smoukov, S.K. Tuning of Structural Colors Like a Chameleon Enabled by Shape-Memory Polymers. Macromol. Rapid Commun. 2018, 39, 1800518. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Lu, Y.F.; Xiang, J.X.; Zhang, M.K.; Zhao, Y.J.; Xie, Z.Y.; Gu, Z.Z. A multifunctional wearable sensor based on a graphene/inverse opal cellulose film for simultaneous, in situ monitoring of human motion and sweat. Nanoscale 2018, 10, 2090–2098. [Google Scholar] [CrossRef] [PubMed]
- Fenzl, C.; Kirchinger, M.; Hirsch, T.; Wolfbeis, O. Photonic crystal-based sensing and imaging of potassium ions. Chemosensors 2014, 2, 207–218. [Google Scholar] [CrossRef]
- Fenzl, C.; Hirsch, T.; Wolfbeis, O. Photonic Crystal Based Sensor for Organic Solvents and for Solvent-Water Mixtures. Sensors 2012, 12, 16954–16963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolan, J.A.; Korzeb, K.; Dehmel, R.; Gödel, K.C.; Stefik, M.; Wiesner, U.; Wilkinson, T.D.; Baumberg, J.J.; Wilts, B.D.; Steiner, U.; et al. Controlling Self-Assembly in Gyroid Terpolymer Films By Solvent Vapor Annealing. Small 2018, 14, 1802401. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-H.; Koh, C.Y.; Singer, J.P.; Jeon, S.-J.; Maldovan, M.; Stein, O.; Thomas, E.L. 25th Anniversary Article: Ordered Polymer Structures for the Engineering of Photons and Phonons. Adv. Mater. 2013, 26, 532–569. [Google Scholar] [CrossRef] [Green Version]
- Lova, P.; Soci, C. Nanoimprint Lithography: Toward Polymer Photonic Crystals. In Organic and Hybrid Photonic Crystals, 1st ed.; Comoretto, D., Ed.; Springer: Cham, Switzerland, 2015; Volume 1, p. 493. [Google Scholar]
- Yan, X.; Li, B.; Cheng, T.; Li, S. Analysis of High Sensitivity Photonic Crystal Fiber Sensor Based on Surface Plasmon Resonance of Refractive Indexes of Liquids. Sensors 2018, 18, 2922. [Google Scholar] [CrossRef] [Green Version]
- Bing, P.; Huang, S.; Sui, J.; Wang, H.; Wang, Z. Analysis and Improvement of a Dual-Core Photonic Crystal Fiber Sensor. Sensors 2018, 18, 2051. [Google Scholar] [CrossRef] [Green Version]
- Lova, P.; Megahd, H.; Comoretto, D. Thin Polymer Films: Simple Optical Determination of Molecular Diffusion Coefficients. ACS Appl. Mater. Interfaces 2020, 2, 563–568. [Google Scholar] [CrossRef]
- Lova, P.; Manfredi, G.; Bastianini, C.; Mennucci, C.; Buatier de Mongeot, F.; Servida, A.; Comoretto, D. Flory-Huggins Photonic Sensors for the Optical Assessment of Molecular Diffusion Coefficients in Polymers. ACS Appl. Mater. Interfaces 2019, 11, 16872–16880. [Google Scholar] [CrossRef]
- Gao, S.; Tang, X.; Langner, S.; Osvet, A.; Harreiβ, C.; Barr, M.; Spiecker, E.; Bachmann, J.; Brabec, C.J.; Forberich, K. Time-Resolved Analysis of Dielectric Mirrors for Vapor Sensing. ACS Appl. Mater. Interfaces 2018, 10, 36398–36406. [Google Scholar] [CrossRef]
- Lim, H.S.; Lee, J.-H.; Walish, J.J.; Thomas, E.L. Dynamic Swelling of Tunable Full-Color Block Copolymer Photonic Gels via Counterion Exchange. ACS Nano 2012, 6, 8933–8939. [Google Scholar] [CrossRef]
- Lova, P. Selective Polymer Distributed Bragg Reflector Vapor Sensors. Polymers 2018, 10, 1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, H.; Singer, K.; Wu, Y.; Zhou, J.; Lott, J.; Andrews, J.; Hiltner, A.; Baer, E.; Weder, C.; Bunch, R.; et al. Layered Polymeric Optical Systems Using Continuous Coextrusion. Proc. SPIE 2009, 7467, 74670A. [Google Scholar]
- Singer, K.D.; Kazmierczak, T.; Lott, J.; Song, H.; Wu, Y.; Andrews, J.; Baer, E.; Hiltner, A.; Weder, C. Melt-processed All-polymer Distributed Bragg Reflector Laser. Opt. Express 2008, 16, 10358–10363. [Google Scholar] [CrossRef] [PubMed]
- Kazmierczak, T.; Song, H.; Hiltner, A.; Baer, E. Polymeric One-Dimensional Photonic Crystals by Continuous Coextrusion. Macromol. Rapid Commun. 2007, 28, 2210–2216. [Google Scholar] [CrossRef]
- 3M DICHROIC. Available online: https://www.3m.com/3M/en_US/company-us/all-3m-products/~/3M-Dichroic-Films-for-Architectural-Laminated-Glass/?N=5002385+3291680356&rt=rud (accessed on 27 January 2020).
- TORAY. Available online: http://www.toray.com/ (accessed on 27 January 2020).
- Giusto, P.; Lova, P.; Manfredi, G.; Gazzo, S.; Srinivasan, B.; Radice, S.V.; Comoretto, D. Colorimetric Detection of Perfluorinated Compounds by All-Polymer Photonic Transducers. ACS Omega 2018, 3, 7517–7522. [Google Scholar] [CrossRef]
- Dodero, A.; Vicini, S.; Alloisio, M.; Castellano, M. Sodium alginate solutions: Correlation between rheological properties and spinnability. J. Mater. Sci. 2019, 54, 8034–8046. [Google Scholar] [CrossRef]
- Dodero, A.; Alloisio, M.; Vicini, S.; Castellano, M. Preparation of Composite Alginate-Based Electrospun Membranes Loaded with Zno Nanoparticles. Carbohydr. Polym. 2020, 227, 115371. [Google Scholar] [CrossRef]
- Dodero, A.; Scarfi, S.; Pozzolini, M.; Vicini, S.; Alloisio, M.; Castellano, M. Alginate-Based Electrospun Membranes Containing ZnO Nanoparticles as Potential Wound Healing Patches: Biological, Mechanical, and Physicochemical Characterization. ACS Appl. Mater. Interfaces 2020, 12, 3371–3381. [Google Scholar] [CrossRef]
- Castellano, M.; Alloisio, M.; Darawish, R.; Dodero, A.; Vicini, S. Electrospun Composite Mats of Alginate with Embedded Silver Nanoparticles. J. Therm. Anal. Calorim. 2019, 137, 767–778. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive Compounds in Seaweed: Functional Food Applications and Legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Liu, L.S.; Kost, J.; Yan, F.; Spiro, R.C. Hydrogels from Biopolymer Hybrid for Biomedical, Food, and Functional Food Applications. Polymers 2012, 4, 997–1011. [Google Scholar] [CrossRef]
- Zia, K.M.; Zia, F.; Zuber, M.; Rehman, S.; Ahmad, M.N. Alginate Based Polyurethanes: A Review of Recent Advances and Perspective. Int. J. Biol. Macromol. 2015, 79, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Dodero, A.; Vicini, S.; Alloisio, M.; Castellano, M. Rheological properties of sodium alginate solutions in the presence of added salt: An application of Kulicke equation. Rheol. Acta 2020, 59, 365–374. [Google Scholar] [CrossRef]
- Bertasa, M.; Dodero, A.; Alloisio, M.; Vicini, S.; Riedo, C.; Sansonetti, A.; Scalarone, D.; Castellano, M. Agar Gel Strength: A Correlation Study Between Chemical Composition and Rheological Properties. Eur. Polym. J. 2020, 123, 109442. [Google Scholar] [CrossRef]
- Dodero, A.; Williams, R.; Gagliardi, S.; Vicini, S.; Alloisio, M.; Castellano, M. A Micro-Rheological and Rheological Study of Biopolymers Solutions: Hyaluronic Acid. Carbohydr. Polym. 2019, 203, 349–355. [Google Scholar] [CrossRef]
- Dodero, A.; Pianella, L.; Vicini, S.; Alloisio, M.; Ottonelli, M.; Castellano, M. Alginate-Based Hydrogels Prepared via Ionic Gelation: An Experimental Design Approach to Predict the Crosslinking Degree. Eur. Polym. J. 2019, 118, 586–594. [Google Scholar] [CrossRef]
- Salzano de Luna, M.; Castaldo, R.; Altobelli, R.; Gioiella, L.; Filippone, G.; Gentile, G.; Ambrogi, V. Chitosan Hydrogels Embedding Hyper-Crosslinked Polymer Particles as Reusable Broad-Spectrum Adsorbents for Dye Removal. Carbohydr. Polym. 2017, 177, 347–354. [Google Scholar] [CrossRef]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, Processing and Application of Hydrogels: A Review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef]
- Blackburn, R.S. Natural Polysaccharides and Their Interactions with Dye Molecules: Applications in Effluent Treatment. Environ. Sci. Technol. 2004, 38, 4905–4909. [Google Scholar] [CrossRef] [PubMed]
- Mittal, H.; Ray, S.S.; Okamoto, M. Recent Progress on the Design and Applications of Polysaccharide-Based Graft Copolymer Hydrogels as Adsorbents for Wastewater Purification. Macromol. Mater. Eng. 2016, 301, 496–522. [Google Scholar] [CrossRef]
- Guilherme, M.R.; Aouada, F.A.; Fajardo, A.R.; Martins, A.F.; Paulino, A.T.; Davi, M.F.T.; Rubira, A.F.; Muniz, E.C. Superabsorbent hydrogels based on polysaccharides for application in agriculture as soil conditioner and nutrient carrier: A review. Eur. Polym. J. 2015, 72, 365–385. [Google Scholar] [CrossRef] [Green Version]
- Okay, O. General Properties of Hydrogels. In Hydrogel Sensors and Actuators: Engineering and Technology; Gerlach, G., Arndt, K.-F., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–14. [Google Scholar] [CrossRef]
- Braccini, I.; Pérez, S. Molecular Basis of Ca2+-Induced Gelation in Alginates and Pectins: The Egg-Box Model Revisited. Biomacromolecules 2001, 2, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fang, Y.; Vreeker, R.; Appelqvist, I.; Mendes, E. Reexamining the Egg-Box Model in Calcium−Alginate Gels with X-ray Diffraction. Biomacromolecules 2007, 8, 464–468. [Google Scholar] [CrossRef]
- Sikorski, P.; Mo, F.; Skjåk-Bræk, G.; Stokke, B.T. Evidence for Egg-Box-Compatible Interactions in Calcium−Alginate Gels from Fiber X-ray Diffraction. Biomacromolecules 2007, 8, 2098–2103. [Google Scholar] [CrossRef]
- Borgogna, M.; Skjåk-Bræk, G.; Paoletti, S.; Donati, I. On the Initial Binding of Alginate by Calcium Ions. The Tilted Egg-Box Hypothesis. J. Phys. Chem. B 2013, 117, 7277–7282. [Google Scholar] [CrossRef]
- Plazinski, W.; Rudzinski, W. Molecular Modeling of Ca2+-oligo(α-l-guluronate) Complexes: Toward the Understanding of the Junction Zone Structure in Calcium Alginate Gels. Struct. Chem. 2012, 23, 1409–1415. [Google Scholar] [CrossRef]
- Liao, H.; Ai, W.; Zhang, K.; Nakauma, M.; Funami, T.; Fang, Y.; Nishinari, K.; Draget, K.I.; Phillips, G.O. Mechanisms of Oligoguluronate Modulating the Calcium-Induced Gelation of Alginate. Polymer 2015, 74, 166–175. [Google Scholar] [CrossRef]
- Agulhon, P.; Robitzer, M.; Habas, J.-P.; Quignard, F. Influence of Both Cation and Alginate Nature on The Rheological Behavior of Transition Metal Alginate Gels. Carbohydr. Polym. 2014, 112, 525–531. [Google Scholar] [CrossRef]
- Lova, P.; Grande, V.; Manfredi, G.; Patrin, M.; Herbst, S.; Würthner, F.; Comoretto, D. All-Polymer Photonic Microcavities Doped with Perylene Bisimide J-Aggregates. Adv. Opt. Mater. 2017, 5, 1700523–1700528. [Google Scholar] [CrossRef]
- Cathell, M.D.; Schauer, C.L. Structurally Colored Thin Films of Ca2+-Cross-Linked Alginate. Biomacromolecules 2007, 8, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zajforoushan Moghaddam, S.; Thormann, E. Structural Investigation of a Self-Cross-Linked Chitosan/Alginate Dialdehyde Multilayered Film with in Situ QCM-D and Spectroscopic Ellipsometry. ACS Omega 2019, 4, 2019–2029. [Google Scholar] [CrossRef] [PubMed]
- Comoretto, D. Organic and Hybrid Photonic Crystals; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Tavella, C.; Lova, P.; Marsotto, M.; Luciano, G.; Patrini, M.; Stagnaro, P.; Comoretto, D. High Refractive Index Inverse Vulcanized Polymers for Organic Photonic Crystals. Crystals 2020, 10, 154. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Kim, J.H.; Franco, C.M.M.; Middelberg, A.P.J. Characterisation of the Shrinkage of Calcium Alginate Gel Membrane with Immobilised Lactobacillus Rhamnosus. Appl. Microbiol. Biotechnol. 2000, 54, 28–32. [Google Scholar] [CrossRef]
- Guo, L.; Zheng, D.; Xu, J.; Guo, X.; Fu, X.; Zhang, Q. Effects of Ionic Crosslinking on Physical and Mechanical Properties of Alginate Mulching Films. Carbohydr. Polym. 2016, 136, 259–265. [Google Scholar]
- Mammarella, E.J.; Rubiolo, A.C. Crosslinking Kinetics of Cation-Hydrocolloid Gels. Chem. Eng. J. 2003, 94, 73–77. [Google Scholar] [CrossRef]
- Vargas, P.O.; Pereira, N.R.; Guimarães, A.O.; Waldman, W.R.; Pereira, V.R. Shrinkage and deformation during convective drying of calcium alginate. LWT-Food Sci. Technol. 2018, 97, 213–222. [Google Scholar] [CrossRef]
- Ogieglo, W.; Wormeester, H.; Eichhorn, K.-J.; Wessling, M.; Benes, N.E. In situ ellipsometry studies on swelling of thin polymer films: A review. Prog. Polym. Sci. 2015, 42, 42–78. [Google Scholar] [CrossRef]
- Lova, P.; Bastianini, C.; Giusto, P.; Patrini, M.; Rizzo, P.; Guerra, G.; Iodice, M.; Soci, C.; Comoretto, D. Label-free Vapor Selectivity in Poly(p-phenylene oxide) Photonic Crystal Sensors. ACS Appl. Mater. Interfaces 2016, 8, 31941–31950. [Google Scholar] [CrossRef] [Green Version]
- Lova, P.; Manfredi, G.; Boarino, L.; Comite, A.; Laus, M.; Patrini, M.; Marabelli, F.; Soci, C.; Comoretto, D. Polymer Distributed Bragg Reflectors for Vapor Sensing. ACS Photonics 2015, 2, 537–543. [Google Scholar] [CrossRef]
- Chamaleonlab. Available online: http://chameleonlab.nl/ (accessed on 11 December 2019).
- Buss, F.; Göcke, J.; Scharfer, P.; Schabel, W. From Micro to Nano Thin Polymer Layers: Thickness and Concentration Dependence of Sorption and the Solvent Diffusion Coefficient. Macromolecules 2015, 48, 8285–8293. [Google Scholar] [CrossRef]
- An, B.; Lee, H.; Lee, S.; Lee, S.-H.; Choi, J.-W. Determining the selectivity of divalent metal cations for the carboxyl group of alginate hydrogel beads during competitive sorption. J. Hazard. Mater. 2015, 298, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, S.K.; Katsaros, F.K.; Kouvelos, E.P.; Nolan, J.W.; Le Deit, H.; Kanellopoulos, N.K. Heavy metal sorption by calcium alginate beads from Laminaria digitata. J. Hazard. Mater. 2006, 137, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Fatin-Rouge, N.; Dupont, A.; Vidonne, A.; Dejeu, J.; Fievet, P.; Foissy, A. Removal of some divalent cations from water by membrane-filtration assisted with alginate. Water Res. 2006, 40, 1303–1309. [Google Scholar] [CrossRef]
- Sone, H.; Fugetsu, B.; Tanaka, S. Selective elimination of lead(II) ions by alginate/polyurethane composite foams. J. Hazard. Mater. 2009, 162, 423–429. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dodero, A.; Lova, P.; Vicini, S.; Castellano, M.; Comoretto, D. Sodium Alginate Cross-Linkable Planar 1D Photonic Crystals as a Promising Tool for Pb2+ Detection in Water. Chemosensors 2020, 8, 37. https://doi.org/10.3390/chemosensors8020037
Dodero A, Lova P, Vicini S, Castellano M, Comoretto D. Sodium Alginate Cross-Linkable Planar 1D Photonic Crystals as a Promising Tool for Pb2+ Detection in Water. Chemosensors. 2020; 8(2):37. https://doi.org/10.3390/chemosensors8020037
Chicago/Turabian StyleDodero, Andrea, Paola Lova, Silvia Vicini, Maila Castellano, and Davide Comoretto. 2020. "Sodium Alginate Cross-Linkable Planar 1D Photonic Crystals as a Promising Tool for Pb2+ Detection in Water" Chemosensors 8, no. 2: 37. https://doi.org/10.3390/chemosensors8020037





